Significance
We identify a mode of Neuregulin signaling through ErbB4, requiring the receptor but not its canonical tyrosine kinase activity, that selectively decreases fast synaptic GABAA currents on hippocampal interneurons. Neuregulin promotes the clustering and association of ErbB4 with α1-containing GABA receptors, and results in the selective internalization of α1-containing receptors via a mechanism that requires PKC activity and clathrin-dependent endocytosis. These findings emphasize the diverse modes of Neuregulin signaling that can regulate interneuron network activity and which may contribute to the pathophysiology of neuropsychiatric disorders and epilepsy.
Keywords: PKC, schizophrenia, hippocampus, parvalbumin, mIPSCs
Abstract
ErbB4 signaling in the central nervous system is implicated in neuropsychiatric disorders and epilepsy. In cortical tissue, ErbB4 associates with excitatory synapses located on inhibitory interneurons. However, biochemical and histological data described herein demonstrate that the vast majority of ErbB4 is extrasynaptic and detergent-soluble. To explore the function of this receptor population, we used unbiased proteomics, in combination with electrophysiological, biochemical, and cell biological techniques, to identify a clinically relevant ErbB4-interacting protein, the GABAA receptor α1 subunit (GABAR α1). We show that ErbB4 and GABAR α1 are robustly coexpressed in hippocampal interneurons, and that ErbB4-null mice have diminished cortical GABAR α1 expression. Moreover, we characterize a Neuregulin-mediated ErbB4 signaling modality, independent of receptor tyrosine kinase activity, that couples ErbB4 to decreased postsynaptic GABAR currents on inhibitory interneurons. Consistent with an evolving understanding of GABAR trafficking, this pathway requires both clathrin-mediated endocytosis and protein kinase C to reduce GABAR inhibitory currents, surface GABAR α1 expression, and colocalization with the inhibitory postsynaptic protein gephyrin. Our results reveal a function of ErbB4, independent of its tyrosine kinase activity, that modulates postsynaptic inhibitory control of hippocampal interneurons and may provide a novel pharmacological target in the treatment of neuropsychiatric disorders and epilepsy.
ErbB4 signaling regulates neuronal excitability (1, 2) and synaptic plasticity (3, 4) in the adult brain, and has been implicated in psychiatric disorders (5, 6) and epilepsy (2, 7). In the neocortex and hippocampus of rodents, monkeys, and humans, ErbB4 expression is restricted to GABAergic interneurons, and its expression is particularly high in parvalbumin-positive fast-spiking (PV+) interneurons (8, 9). Of note, targeted ablation of ErbB4 specifically in PV+ interneurons recapitulates behavioral abnormalities of full ErbB4-null mice, highlighting the importance of ErbB4 signaling in this GABAergic interneuron subclass (10). Moreover, gamma oscillations, a type of high-frequency network activity that depends on synchronization of local circuits by PV+ interneurons, are augmented by Neuregulin (NRG)1 in vitro in an ErbB4-dependent manner (11).
In the hippocampus, the GABAA receptor α1 subunit (GABAR α1), which imparts rapid decay kinetics (12), is also selectively expressed in subsets of inhibitory interneurons, especially in PV+ neurons (13, 14). Furthermore, a mutation in GABRA1 has been linked to absence seizures (15), and heterozygous Gabra1-null mice show cortical absence epileptiform activity (16). Additionally, genome-wide linkage analyses have repeatedly identified a cluster of GABAR subunits, which includes GABRA1, as a schizophrenia (SCZ) susceptibility locus (17). Therefore, identifying mechanisms that acutely regulate α1-containing GABARs on interneurons is important to understanding their role in neuronal network activity and their association with SCZ and epilepsy.
Numerous postmortem and functional imaging studies have implicated a selective loss of GABAergic interneuron function as a major deficit in SCZ (18). Interest has focused predominantly on PV+ basket and chandelier neurons in the dorsal lateral prefrontal cortex (DLPFC), because these interneurons target pyramidal neuron somata and axon initial segments to regulate excitatory–inhibitory balance and neuronal network activity important for numerous cognitive functions affected in SCZ (18, 19). However, there is mounting evidence for hippocampal dysfunction in SCZ (20), where PV+ interneurons (21) contribute to the altered gamma oscillations observed (18).
Although a fraction of ErbB4 receptors is tightly associated with PSD-95 at glutamatergic synapses (3, 22), the majority of the receptors are extrasynaptic (see below). To explore this largely overlooked pool of ErbB4 (i.e., outside the glutamatergic synapse), we used unbiased proteomics of detergent-soluble ErbB4 isolated from synaptic plasma membranes. Using this approach, here we report a unique interaction between ErbB4 and GABAR α1. We show that NRG2, a homolog of NRG1 that is highly expressed in the adult brain (23, 24), increases the association of ErbB4 with α1-containing GABARs, causes internalization of these receptors, and reduces the amplitude of miniature inhibitory postsynaptic currents (mIPSCs) on ErbB4+ interneurons. Unexpectedly, although the ErbB4 receptor is essential for reducing the mIPSCs in response to NRG2, its canonical receptor tyrosine kinase (RTK) activity is entirely dispensable. Our results are consistent with other studies suggesting a model for extrasynaptic GABAR trafficking (25–28), and they introduce NRG-ErbB4 signaling as a critical modulator of postsynaptic GABAR signaling in interneurons.
Results
GABAR α1 Is an ErbB4-Interacting Protein.
ErbB4 has been shown to accumulate at interneuron glutamatergic synapses, where it associates with PSD-95 at excitatory postsynaptic densities (3, 22). However, coimmunofluorescence analysis in dissociated hippocampal neurons reveals a substantial fraction of ErbB4 immunoreactivity that does not overlap with PSD-95 (29) (Fig. S1A). In cortical membrane preparations, most ErbB4 can be extracted with 1% (vol/vol) Triton X-100, consistent with most ErbB4 protein not being tightly associated with glutamatergic postsynaptic densities (Fig. S1B). In an unbiased effort to identify proteins interacting with this population of ErbB4 receptors, a large-scale isolation of native ErbB4 from Triton X-100–extracted rat brain synaptic plasma membranes (24 mg of protein) was performed using monoclonal antibody mAb-10 (8). Immunoprecipitated ErbB4 complexes were resolved by SDS/PAGE and analyzed by tandem MS/MS (SI Materials and Methods); only proteins absent from the normal rabbit IgG control sample were considered for further investigation (Fig. S1C). Among proteins copurifying with ErbB4 was GABAR α1. Consistent with these findings, reciprocal immunoprecipitation of GABAR α1 also coprecipitates ErbB4 (see below).
ErbB4 and GABAR α1 Are Coexpressed in Hippocampal Interneurons in Vivo and in Vitro, and ErbB4−/− Mice Show Decreased Cortical GABAR α1 Expression.
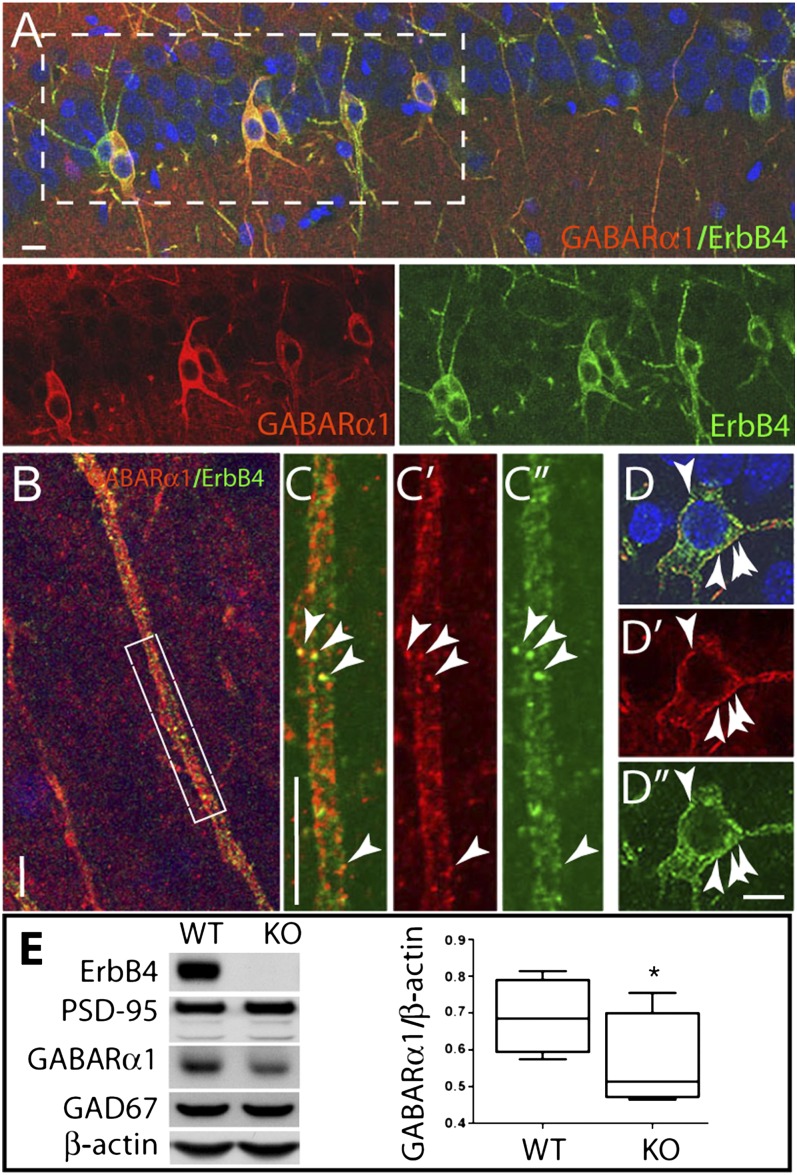
We evaluated ErbB4 and GABAR α1 expression in sections of adolescent rat hippocampus and primary hippocampal cultures. Double immunofluorescence analysis of hippocampal sections costained with antibodies to GABAR α1 and ErbB4 (Fig. 1A) show that the vast majority of ErbB4+ interneurons robustly coexpress GABAR α1 in their somatodendritic compartment (81.5 ± 4.2%, n = 3 animals, 1,359 cells). Furthermore, subsets of colocalizing puncta were evident in both dendrites (Fig. 1 B and C) and somata (Fig. 1 D–D′′) of hippocampal interneurons. In cultured hippocampal neurons, 98.1 ± 0.8% of ErbB4+ interneurons (n = 8 coverslips, 945 cells) coexpress GABAR α1 (Fig. S2 A and B). Furthermore, in these cells, the two proteins colocalize in a subset of ErbB4+ puncta (Fig. S2A).
Fig. 1.
GABAR α1 and ErbB4 are widely coexpressed in the hippocampus, and ErbB4-null mice have diminished cortical α1 expression. Sections from 4-wk-old rat hippocampi were stained for the GABAR α1 subunit and ErbB4; nuclei were stained with DAPI. (A) High level of GABAR α1 and ErbB4 coexpression in CA1 interneurons. (Lower, individual channels of boxed area.) (B) Representative hilar dendrite showing a high degree of dendritic coexpression. (C) Enlarged boxed area of B, showing discrete overlapping puncta for GABAR α1 (C′) and ErbB4 (C′′) (arrowheads). (D) Interneuron soma showing perisomal colocalization (arrowheads) of GABAR α1 (D′) and ErbB4 (D′′). (Scale bars, 10 µm.) (E) Representative Western blot of cortical P2 membranes from wild-type and ErbB4−/− mice; note the reduction in GABAR α1 in ErbB4−/− mice. Quantification of GABAR α1 normalized to β-actin is shown (Right) (n = 4 individual experiments; *P < 0.05, paired t test).
Because of the extensive coexpression of ErbB4 and GABAR α1 in tissue and cultured neurons, we reasoned that ErbB4 deficiency might affect cortical GABAR α1 expression. We therefore analyzed cortical P2 membrane fractions from ErbB4−/−,Δheart mice [rescued from embryonic death by selective receptor expression in heart (30), and hereafter referred to as ErbB4−/−] and evaluated GABAR α1 levels. As shown in Fig. 1E, GABAR α1 expression is reduced by 18.5% in ErbB4−/− vs. wild-type mice (n = 4; *P < 0.05), with no changes in expression of either GAD67, PSD-95, or β-actin. Thus, ErbB4-null mice have concomitant deficiencies in GABAR α1 expression.
NRG2 Increases ErbB4–GABAR α1 Association.
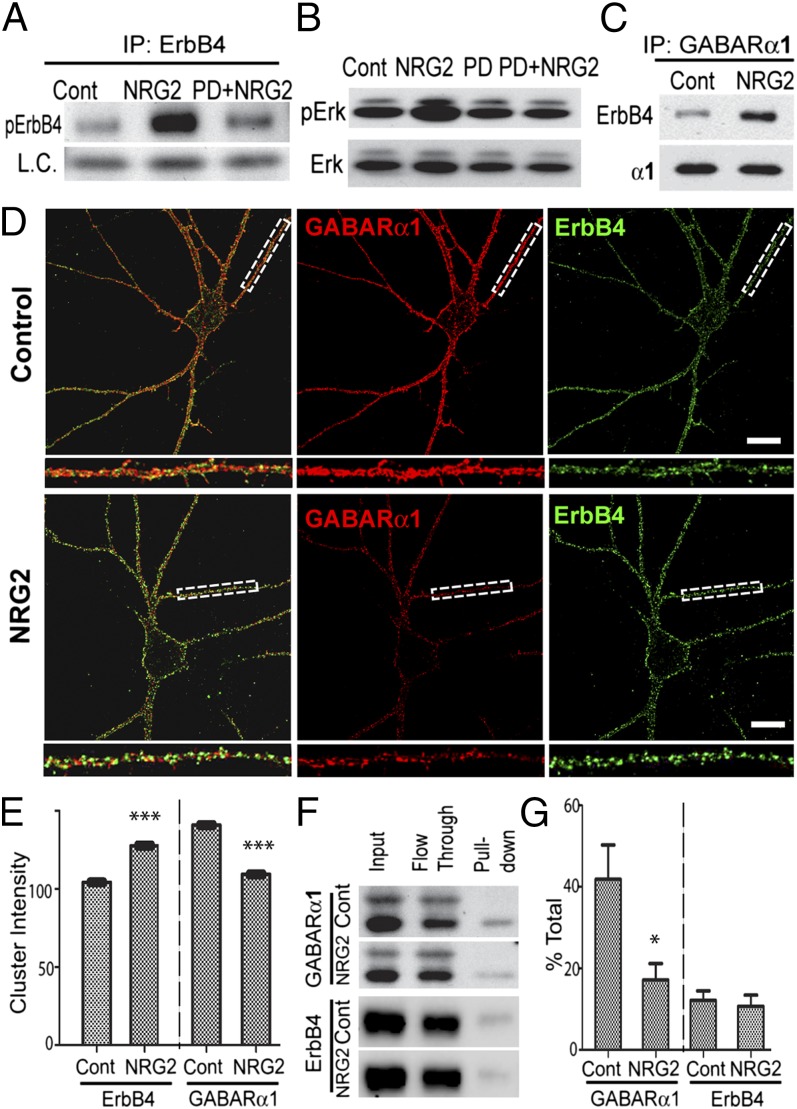
Next, we sought to determine whether stimulation by NRG acutely modulates the interaction between ErbB4 and GABAR α1. To this end, we treated dissociated hippocampal cultures with the extracellular domain of NRG2β, the major Ig-like domain-containing NRG expressed in the adult hippocampus (23, 24). ErbB4 receptor autophosphorylation (Fig. 2A) and canonical MAPK activation (Fig. 2B) were robustly elevated 10 min after addition of 10 nM NRG2, and both responses were blocked by the general ErbB kinase inhibitor PD158780 [4-6-(methyl-amino)-pyrido[3,4-d]pyrimidine] (10 μM). Importantly, NRG2 dramatically increased the association of ErbB4 and GABAR α1, as shown by coimmunoprecipitation of ErbB4 with an antibody against GABAR α1 (Fig. 2C).
Fig. 2.
NRG2 clusters ErbB4 receptors and reduces surface GABAR α1. (A) NRG2 functions as a canonical ErbB4 ligand. Hippocampal cultures were vehicle-treated (Cont), treated for 10 min with 10 nM NRG2, or pretreated for 10 min with PD158780 (10 µM) before the addition of NRG2 for 10 min (PD+NRG2). ErbB4 immunoprecipitates were probed for phosphotyrosine (pErbB4). NRG2 increases of pErbB4 (Center) are fully blocked by PD158780 (Right). IP, immunoprecipitation; L.C., IgG light chain. (B) NRG2 activates canonical ErbB4 downstream signaling pathways in hippocampal neurons, as shown by pronounced Erk1/2 phosphorylation (NRG2) that is blocked by PD158780 pretreatment (PD+NRG2). (C) NRG2 increases ErbB4–GABAR α1 association. Hippocampal cultures were vehicle-treated (Cont) or treated for 10 min with NRG2; GABAR α1 immunoprecipitates were probed for ErbB4 and the α1 subunit. (D and E) NRG2 treatment increases ErbB4, but reduces α1, mean surface cluster intensity. (D) Representative images of surface-labeled ErbB4/α1-expressing hippocampal interneurons following vehicle (Upper) or NRG2 (Lower) treatment; boxed areas are enlarged below. (Scale bars, 10 μm). (E) Quantification of ErbB4 (Left) and GABAR α1 (Right) mean surface cluster intensities (n = 10 neurons per condition; ***P ≤ 0.0001, t test). (F) Biotinylation analyses for surface (pull-down) and intracellular (flow through) GABAR α1 and ErbB4 expression following vehicle (Cont) or NRG2 treatment. (G) Densitometric quantification of relative surface biotinylation: % total = (surface/input) × 100 (n = 6; *P < 0.05, t test).
ErbB4 activation causes surface internalization of ionotropic receptors (31, 32); we therefore asked whether NRG2 might reduce GABAR α1 surface expression as well. We performed quantitative immunofluorescence analysis of surface-labeled GABAR α1 and ErbB4 using antibodies recognizing their extracellular domains under nonpermeabilizing conditions. As shown in Fig. 2D, 10 min of NRG2 resulted in a dramatic and distinct redistribution of both proteins. Compared with vehicle-treated controls, ErbB4 puncta were notably brighter and their mean intensity increased by 22.3% (Fig. 2E; ***P < 0.0001), likely indicating ligand-mediated clustering of surface ErbB4; by contrast, GABAR α1 surface cluster intensity decreased by 22.4% (Fig. 2E; ***P < 0.0001). These findings were confirmed using surface protein biotinylation assays of cultured hippocampal neurons (Fig. 2F), which showed that NRG2 decreases surface GABAR α1 by 59% (Fig. 2G; *P < 0.05) with no changes in surface ErbB4 levels. These results suggest that the internalization of GABARs following NRG2 treatment results from transient interactions between ErbB4 and α1-containing GABARs.
NRG2 Decreases Synaptic GABAR Currents.
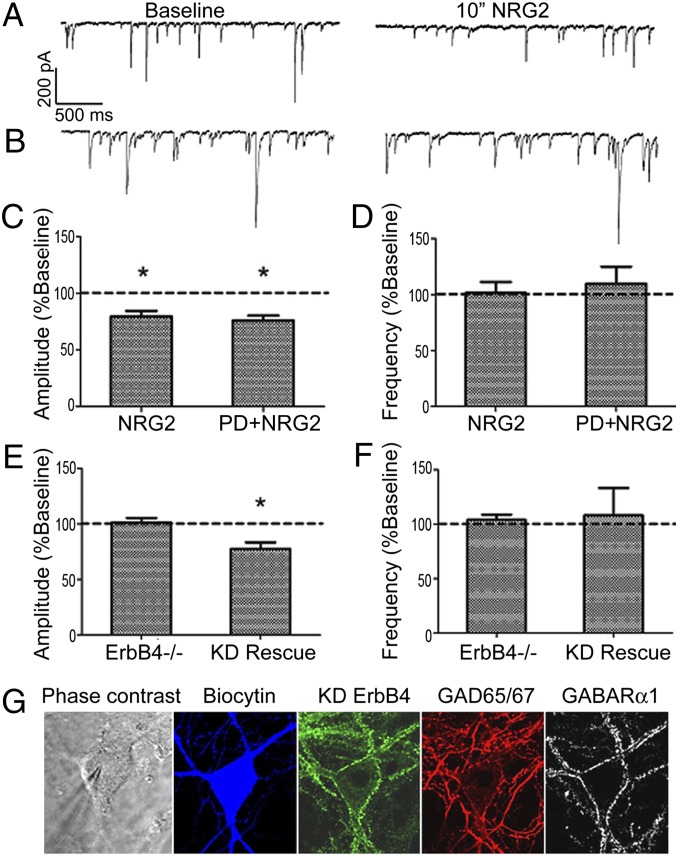
Because hippocampal cultures robustly respond to NRG2, we reasoned they constitute a good model in which to further explore functional interactions between ErbB4 and GABAR α1 in response to NRG2. ErbB4+ interneurons were identified in hippocampal cultures by live labeling with antibody Ab-77 at concentrations that do not affect receptor activity (1) and analyzed by whole-cell voltage-clamp recordings. Under baseline conditions, mIPSC frequency (2–16 Hz) and amplitude (28–142 pA) were highly variable, and responses to NRG2 were normalized and expressed as percent baseline within each cell (see Table S1 for raw data). As shown in Fig. 3A, 10 min of NRG2 (2 nM) significantly decreased the bicuculline-sensitive mIPSC amplitude to 79.6 ± 4.8% of baseline (Fig. 3C, NRG2), whereas frequency remained unaffected at 101.7 ± 9.5% (Fig. 3D, NRG2). Similar effects were observed with 5 min of NRG2 (87.8 ± 4.7% baseline; n = 8; P < 0.05). This response was specific to GABAR currents, as NRG2 did not affect miniature excitatory postsynaptic currents amplitude or frequency (Table S1).
Fig. 3.
NRG2-mediated reductions in mIPSC frequency are ErbB4- but not receptor tyrosine kinase-dependent. (A) Representative whole-cell voltage-clamp mIPSC recording from an identified wild-type ErbB4+ interneuron at baseline and following NRG2 application (10 min, 2 nM), showing a NRG2-mediated decrease in mIPSC amplitude. (B) Same as A but the recording is from an ErbB4−/− interneuron. Summary of mIPSC (C) amplitude and (D) frequency analyses for hippocampal rat neurons treated with NRG2 (n = 8) or PD+NRG2 (n = 5; *P < 0.05, t test). Summary of mIPSC (E) amplitude and (F) frequency data following NRG2 treatment of either mouse ErbB4−/− cultures (n = 6) or ErbB4−/− cultures infected with a kinase-dead ErbB4 AAV vector (KD Rescue; n = 6; *P < 0.05, t test). (G) Representative post hoc immunofluorescence analysis illustrating inclusion criteria for kinase-dead ErbB4 rescue experiments. Neurons were filled with biocytin for recordings from mouse ErbB4 KO hippocampal cultures. Post hoc immunohistochemistry was used to confirm expression of KD ErbB4 in infected neurons, inhibitory phenotype (GAD67), and GABAR α1 expression as indicated. GAD67-negative neurons were excluded from the analysis.
NRG2 Requires ErbB4, but Not Its Tyrosine Kinase Activity, to Reduce Synaptic GABAR Currents.
In additional experiments, we unexpectedly found that the effect of NRG2 on mIPSCs does not require canonical ErbB4 kinase activity. Although PD158780, a competitive inhibitor of the catalytic ATP-binding domain of ErbB receptors, completely blocks canonical receptor phosphorylation and MAPK signaling (shown in Fig. 2 A and B), mIPSC amplitudes were significantly reduced in both the presence and absence of 10 µM PD158780, applied for 10 min before and throughout recordings [Fig. 3C; PD+NRG2: 75.9 ± 4.2% baseline vs. control (NRG2): 79.6 ± 4.8% baseline].
Although NRG2’s effects on mIPSC amplitude are independent of canonical ErbB4 RTK activity, ErbB4 may still be required for NRG2’s effects on GABAergic currents. To test this possibility, we prepared hippocampal cultures from ErbB4−/− mice. Biocytin (1% wt/vol) was added to internal solutions for post hoc identification of GAD67 immunoreactivity to confirm GABAergic interneuron characterization (Fig. 3G). Importantly, over 88% of cultured hippocampal interneurons are also ErbB4+ in wild-type cultures (29). In these ErbB4−/− cultured interneurons, NRG2 was without effect (Fig. 3B), as mIPSC amplitude (Fig. 3E; 101.5 ± 4% baseline, ErbB4−/−) and frequency (Fig. 3F; 104 ± 4% baseline, Erb4−/−) were unaltered by NRG2 application, indicating that the ErbB4 receptor is critical for NRG2’s actions.
To further confirm that ligand binding of ErbB4, but not stimulation of its tyrosine kinase activity, is required for reduced GABAR currents, we infected hippocampal cultures from ErbB4−/− mice with an adeno-associated virus (AAV) harboring a kinase-dead (K751M) ErbB4 receptor variant (ErbB4-KD) (33). Interneurons rescued with ErbB4-KD regained the capacity for reducing mIPSC amplitude upon NRG2 treatment (Fig. 3E; 77.6 ± 5.7% baseline in infected GABAR α1+ interneurons; KD Rescue), without altering frequency (Fig. 3F; KD Rescue). A representative infected GABAR α1+ interneuron used for recording is shown (Fig. 3G). Thus, an ErbB4 mutant that lacks RTK activity is capable of rescuing the decrease in mIPSC amplitude by NRG2. Taken together, these results show that the NRG2-dependent decrease of mIPSC amplitude on ErbB4+ hippocampal interneurons requires ErbB4, but its canonical RTK activity is entirely dispensable.
NRG2 Decreases Synaptic α1, but Not α2, Subunit-Containing GABARs and Prolongs mIPSC Decay Kinetics.
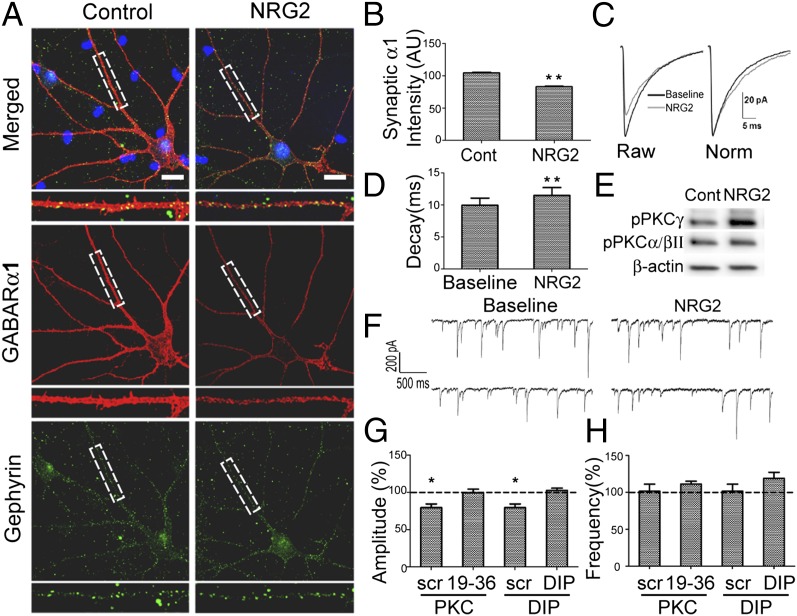
Immunofluorescence and electrophysiological data described above are consistent with the notion that NRG2 selectively reduces mIPSCs on cultured ErbB4+ interneurons by promoting GABAR α1 association with ErbB4 and subsequent receptor internalization. However, GABAR α1 surface labeling appears ubiquitous on the somatodendritic membrane of cultured hippocampal interneurons (Fig. S2B), and thus does not distinguish between synaptic and extrasynaptic receptor pools. To determine whether NRG2 affects synaptic GABAR α1 expression, we first surface-labeled neurons for the α1 subunit, permeabilized the cells, and then colabeled with an antibody for the inhibitory postsynaptic protein gephyrin. As shown in Fig. 4 A and B, NRG2 reduced the mean GABAR α1 signal intensity associated with gephyrin-positive puncta by 20.5%, compared with vehicle-treated controls (see SI Materials and Methods for details on quantitation methodology). These results are consistent with the decreases in mIPSC amplitude observed with NRG2. Importantly, in additional experiments, we observed that NRG2 does not decrease synaptic levels of GABAR α2 on ErbB4+ neurons (Fig. S3), indicating a selective effect of NRG2 in reducing synaptic α1-containing GABARs on inhibitory interneurons.
Fig. 4.
NRG2 decreases synaptic α1 subunit expression, prolongs mIPSC decay, and requires clathrin and PKC. (A) Representative confocal images of vehicle (Control) and NRG2-treated hippocampal interneurons surface-labeled for GABAR α1, permeabilized, and stained for gephyrin. (Scale bars, 10 μm.) (B) Quantification of mean synaptic GABAR α1 in ErbB4+ interneurons following vehicle or NRG2 treatment (n = 12 neurons per condition; **P < 0.001, t test). AU, arbitrary units. (C) Averaged mIPSC traces at baseline and after NRG2 treatment (Left) and scaled to peak amplitude for decay kinetics comparison (Right). (D) Single-exponential decay fittings of mIPSCs (n = 8; **P < 0.005, t test). (E) NRG2 causes PKCγ phosphoactivation (Thr514) without altering PKCα/βII phosphorylation (Thr638/641). (F) Representative whole-cell recordings from ErbB4+ interneurons, with PKC(19–36) inhibitory peptide in the internal solution, taken at baseline (Left) and following NRG2 treatment (Right). (G and H) Summary of mIPSC amplitude (G) and frequency (H) analyses following NRG2 application with intracellular application of either PKC(19–36) (n = 5) or dynamin inhibitory peptide (n = 6); a scrambled peptide was used as control (*P < 0.05, t test).
A selective reduction in α1 subunit-containing GABARs is further supported by mIPSC single-exponential decay kinetics. NRG2 prolonged mIPSC decay kinetics to a 116.1 ± 2.6% baseline (Fig. 4 C and D) in ErbB4+ interneurons, consistent with a reduction of rapidly decaying synaptic currents. Because GABAR α1 confers rapid decay kinetics (12, 34, 35), our results indicate that NRG2–ErbB4 association preferentially targets synaptic α1 subunit-containing GABARs.
NRG2 Alters GABAR Currents Through Clathrin-Dependent Endocytosis and PKC Activity.
NRG2 selectively reduces mIPSC amplitude, but not frequency, indicating NRG2’s effects are likely postsynaptic. As GABAR surface expression is regulated by clathrin-dependent endocytosis in hippocampal neurons (27, 36), we asked whether NRG2 decreases postsynaptic GABARs through a similar mechanism. To test this, we supplemented the internal solution with dynamin inhibitory peptide (DIP; 50 µM), which blocks amphiphysin and dynamin binding, thus blocking clathrin-dependent endocytosis. NRG2 was added only after allowing the intracellular peptide to dialyze the cell for at least 10 min. Summarized data in Fig. 4G show that NRG2 failed to affect mIPSC amplitude (102.6 ± 3% baseline) when dynamin/clathrin-dependent endocytosis was blocked with DIP. By contrast, in cells dialyzed with a scrambled control peptide (scr; 50 µM), NRG2 continued to decrease mIPSC amplitude (85.6 ± 3.6% baseline). NRG2 was also without effect on mIPSC amplitude when the cell-permeable dynamin inhibitor dynasore (40 µM) was added to the extracellular bath (104 ± 2.7% baseline; Table S1). Together, these data suggest that NRG2 decreases GABAergic currents by activating clathrin-dependent receptor endocytosis.
PKC has a well-established role in governing GABAR surface expression and receptor turnover (27, 37). Therefore, we sought to determine whether PKC activity is required for NRG2-mediated decreases in GABAR current density. As shown in Fig. 4E, NRG2 results in phosphoactivation of PKCγ but not PKCα/βII. To selectively block PKC activation, we included an inhibitory PKC(19–36) peptide (10 µM) in the intracellular solution. As illustrated in Fig. 4F and summarized in Fig. 4 G and H, intracellular delivery of the PKC peptide prevented an NRG2-mediated mIPSC amplitude decrease (99.4 ± 5% baseline), suggesting that NRG2-ErbB4 acts through a PKC-dependent mechanism to decrease inhibitory synaptic current in hippocampal interneurons. Intracellular application of the control peptide was without effect (scr; 79.6 ± 3.1% baseline). Furthermore, bath application of the cell-permeable PKC inhibitor chelerythrine chloride (3 µM) also prevented NRG2’s actions on inhibitory currents (102.3 ± 2.5% baseline; Table S1). Thus, NRG2 mediates its effects on postsynaptic GABAR currents through ErbB4 via a mechanism requiring both clathrin-dependent endocytosis of α1 subunit-containing receptors and PKC activity.
Discussion
Much of what is currently known about ErbB4 signaling stems from its well-documented interaction with PSD-95. However, as we show here, the bulk of the receptor is extrasynaptic. By isolating ErbB4-interacting proteins from this biochemically defined fraction, we have found a unique and clinically relevant target of ErbB4 receptor signaling, α1-containing GABARs.
NRG-ErbB signaling has a unique degree of complexity among classical RTK signaling pathways. Whereas there is ample evidence for the proteolytic processing and release of soluble NRG ligands resulting in paracrine activation of ErbB receptors (38), there is additional evidence in the peripheral nervous system (39) and other models (40) for stable juxtacrine NRG–ErbB interactions. In addition, both NRG1 (41) and ErbB4 (42, 43) undergo reverse signaling and, in this fashion, bear similarities to both canonical and noncanonical Notch signaling (44). Our present data reveal yet another layer of complexity in the NRG-ErbB signaling network: NRG-ErbB4 signaling in GABAergic interneurons independent of receptor tyrosine kinase activity that alters postsynaptic inhibitory drive onto GABAergic interneurons. This finding is reminiscent of the established role of the ErbB1 receptor acting via a tyrosine kinase-independent mechanism (for other RTK-independent mechanisms, see ref. 45) to stabilize the sodium/glucose cotransporter (46), and it reveals a unique and exciting mode of NRG-ErbB4 signaling.
Ion-channel motility into and out of the synapse regulates synaptic and homeostatic plasticity at excitatory (47, 48) and inhibitory synapses (49, 50). Accumulation of GABARs at synaptic sites is governed by lateral migration within the membrane, rather than insertion of newly synthesized receptors (26), where interactions with scaffolding proteins such as gephyrin stabilize postsynaptic receptors (51). Furthermore, GABAR insertion and endocytosis via AP2 adaptin occur predominantly at extrasynaptic sites, indicating that lateral diffusion and trafficking of receptors are critically important for regulating synaptic strength at GABAergic synapses (25). Our data show that NRG2 causes a robust somatodendritic-wide decrease of GABAR α1 surface labeling and colocalization with gephyrin-positive puncta, consistent with the reduction of mIPSC amplitude. In addition, the residual synaptic α1- and α2-containing GABARs may contribute to the prolonged decay kinetics (12, 34). As NRG2 effects require PKC activity and clathrin-dependent endocytosis (Fig. 4 F–H), it is plausible that NRG2 functions to selectively recruit α1 subunit-containing GABARs to endocytic machinery via a transient interaction with ErbB4, resulting in lateral diffusion out of the synapse and decreasing mIPSC amplitude (see the proposed model in Fig. S4). Because NRG2 elicits its effects on GABA neurotransmission independent of ErbB4 tyrosine kinase activity—contrasting it with other RTK ligands such as BDNF (52), PDGF (53), and insulin (54) that require tyrosine kinase activity of their cognate receptors to modulate GABAergic transmission—it is plausible that different structural/conformational or cytoskeletal mechanisms are used by ErbB4 to regulate GABAR trafficking.
Genetic linkage and association studies identify ERBB4 as an at-risk gene for SCZ and potentially bipolar disorder (BiP). Interestingly, the human chromosome region 5q34–5q35, which harbors a cluster of genes encoding GABAR subunits (α1, α6, β2, and γ2), has been repeatedly shown as an SCZ and BiP susceptibility locus (reviewed in ref. 17). Genome-wide linkage scans of Portuguese families identified a genomic region that includes GABRA1 (55), and haplotypes and SNPs in the gene are also associated with risk for SCZ, as well as alterations in the expression of GABAR subunits (56). Moreover, GABAR α1 transcript levels are lower in the postmortem brain of persons diagnosed with SCZ (19) and depression (57), suggesting a functional role of GABAR α1-containing receptors in the etiology of these disorders. Whereas reductions in GABAR α1 transcripts in the postmortem DLPFC are reported to be restricted mostly to excitatory neurons (58), in the hippocampus—another anatomical structure affected in SCZ (59, 60) that exhibits interneuron deficits (21) and whose dysfunction may spread to the DLPFC (20, 61)—GABAR α1 expression is mostly restricted to interneurons (13). Additionally, hippocampal synapses between PV+ neurons have 3.2 times more α1 subunit than PV+-to-pyramidal neuron synapses (14), underscoring the importance of GABAR α1 in inhibitory network connectivity. Finally, GABAR α1 has been genetically linked to absence seizures (15), and heterozygous Gabra1-null mice show cortical absence epileptiform activity (16). Taken together, our studies implicate GABAR α1 as a protein regulated by ErbB4 in GABAergic interneurons, and as a potential downstream target of ErbB4 that is altered in SCZ and epilepsy. Importantly, because ErbB4 has functions entirely independent of its canonical RTK activity and by virtue of its robust interneuron-specific expression, the receptor (independent of its activity) constitutes an enticing target for novel drug discovery.
Materials and Methods
See SI Materials and Methods for detailed protocols.
Electrophysiology.
Whole-cell voltage-clamp recordings of mIPSCs from ErbB4+ interneurons were performed at 32 °C with borosilicate glass microelectrodes (3–5 MΩ) using standard conditions (1). Miniature IPSCs were isolated by bath application of tetrodotoxin (1 µM), 6-cyano-7-nitroquinoxaline-2,3-dione (10 µM), and D-2-amino-5-phosphonopentanoic acid (25 µM), and were confirmed with the GABA antagonist bicuculline methobromide (25 µM). All cells were held at −70 mV throughout the experiment. Access resistance was monitored during recordings, and experiments with change >15% were discarded.
Histology.
Four- to 6-wk-old Sprague–Dawley rats were transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M PBS (pH 7.4). Brains were postfixed overnight in the same fixative, and 50-µm free-floating sections were stained and analyzed as described (9, 10). All animal protocols were approved by National Institute of Child Health and Human Development (NICHD) Animal Care and Use Committee.
Immunocytochemistry and Image Analysis.
Following NRG2 treatment, hippocampal cultures were fixed in 4% PFA in PBS containing 4% sucrose at 37 °C for 20 min followed by blocking and antibody incubations. Laser scanning confocal images were acquired using an LSM 510 microscope (Zeiss). Acquired images were analyzed using Volocity 6.1.1 (PerkinElmer).
Immunoprecipitation and Biotinylation Assays.
Triton X-100–solubilized synaptic plasma membranes or hippocampal culture lysates were normalized for total protein, and immunoprecipitation was performed essentially as described (8). Biotinylation reactions of hippocampal cultures were performed at 4 °C, washed, and processed essentially as described (29) using NeutrAvidin UltraLink Resin (Pierce).
In-Gel Digestion and Mass Spectroscopy-Based Proteomics.
Gel lanes were manually excised top to bottom into 20 ∼2-mm bands. In-gel tryptic digestion and peptide extraction were performed using a standard protocol (62), followed by one-dimensional liquid chromatography tandem mass spectrometry.
Supplementary Material
Acknowledgments
We thank Dr. Carolyn Smith from the National Institute of Neurological Disorders and Stroke microscopy core facilities for expert assistance with confocal microscopy and Volocity software. We also thank Dr. Jean-Marc Fritschy for providing GABAA receptor α1 and α5 subunit-specific polyclonal antibodies. We are grateful to Daniel Abebe for expert assistance with rodent husbandry, Dr. Jody Martin for adeno-associated virus production, Dr. Swagata Roychowdhury for assistance with electrophysiological recordings, and Mr. Christopher R. Parrino for assistance with immunocytochemical experiments. This work was kindly supported by the NICHD and NIMH Intramural Research Programs.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
1R.M.M. and M.J.J. contributed equally to this work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312791110/-/DCSupplemental.
References
- 1.Janssen MJ, Leiva-Salcedo E, Buonanno A. Neuregulin directly decreases voltage-gated sodium current in hippocampal ErbB4-expressing interneurons. J Neurosci. 2012;32(40):13889–13895. doi: 10.1523/JNEUROSCI.1420-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li KX, et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci. 2012;15(2):267–273. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- 3.Huang YZ, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26(2):443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 4.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25(41):9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11(3):287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 6.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan GH, et al. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci. 2012;15(2):258–266. doi: 10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- 8.Vullhorst D, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29(39):12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neddens J, et al. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: Implications for schizophrenia. Biol Psychiatry. 2011;70(7):636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamir A, et al. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32(9):2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: Implications for schizophrenia. Cereb Cortex. 2009;19(3):612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein PA, et al. Prolongation of hippocampal miniature inhibitory postsynaptic currents in mice lacking the GABA(A) receptor alpha1 subunit. J Neurophysiol. 2002;88(6):3208–3217. doi: 10.1152/jn.00885.2001. [DOI] [PubMed] [Google Scholar]
- 13.Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6(5):837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 14.Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22(7):2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maljevic S, et al. A mutation in the GABA(A) receptor alpha(1)-subunit is associated with absence epilepsy. Ann Neurol. 2006;59(6):983–987. doi: 10.1002/ana.20874. [DOI] [PubMed] [Google Scholar]
- 16.Arain FM, Boyd KL, Gallagher MJ. Decreased viability and absence-like epilepsy in mice lacking or deficient in the GABAA receptor α1 subunit. Epilepsia. 2012;53(8):e161–e165. doi: 10.1111/j.1528-1167.2012.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charych EI, Liu F, Moss SJ, Brandon NJ. GABA(A) receptors and their associated proteins: Implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology. 2009;57(5–6):481–495. doi: 10.1016/j.neuropharm.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13(2):147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schobel SA, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konradi C, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1–3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci USA. 2000;97(7):3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carraway KL, III, et al. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387(6632):512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 24.Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J Comp Neurol. 2004;472(2):156–172. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- 25.Bogdanov Y, et al. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25(18):4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8(7):889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- 27.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9(5):331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70(3):385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longart M, Chatani-Hinze M, Gonzalez CM, Vullhorst D, Buonanno A. Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons. Brain Res Bull. 2007;73(4–6):210–219. doi: 10.1016/j.brainresbull.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gassmann M, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 31.Chang Q, Fischbach GD. An acute effect of neuregulin 1 beta to suppress alpha 7-containing nicotinic acetylcholine receptors in hippocampal interneurons. J Neurosci. 2006;26(44):11295–11303. doi: 10.1523/JNEUROSCI.1794-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25(20):4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen WS, et al. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. Nature. 1987;328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- 34.Eyre MD, Renzi M, Farrant M, Nusser Z. Setting the time course of inhibitory synaptic currents by mixing multiple GABA(A) receptor α subunit isoforms. J Neurosci. 2012;32(17):5853–5867. doi: 10.1523/JNEUROSCI.6495-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABA(A) receptor alpha subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci. 2000;20(6):2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kittler JT, et al. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20(21):7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connolly CN, et al. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem. 1999;274(51):36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- 38.Esper RM, Pankonin MS, Loeb JA. Neuregulins: Versatile growth and differentiation factors in nervous system development and human disease. Brain Res Brain Res Rev. 2006;51(2):161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Fricker FR, et al. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J Neurosci. 2011;31(9):3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguilar Z, Slamon DJ. The transmembrane heregulin precursor is functionally active. J Biol Chem. 2001;276(47):44099–44107. doi: 10.1074/jbc.M103442200. [DOI] [PubMed] [Google Scholar]
- 41.Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161(6):1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127(1):185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 43.Ni CY, Murphy MP, Golde TE, Carpenter G. Gamma-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294(5549):2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 44.Fortini ME. Notch signaling: The core pathway and its posttranslational regulation. Dev Cell. 2009;16(5):633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Rauch J, Volinsky N, Romano D, Kolch W. The secret life of kinases: Functions beyond catalysis. Cell Commun Signal. 2011;9(1):23. doi: 10.1186/1478-811X-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weihua Z, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13(5):385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 48.Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006;2006(356):re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- 49.Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012;75(6):951–962. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91(3):1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacob TC, et al. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25(45):10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17(9):2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenzuela CF, et al. Platelet-derived growth factor receptor is a novel modulator of type A gamma-aminobutyric acid-gated ion channels. Mol Pharmacol. 1995;48(6):1099–1107. [PubMed] [Google Scholar]
- 54.Wan Q, et al. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388(6643):686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 55.Sklar P, et al. Genome-wide scan in Portuguese Island families identifies 5q31–5q35 as a susceptibility locus for schizophrenia and psychosis. Mol Psychiatry. 2004;9(2):213–218. doi: 10.1038/sj.mp.4001418. [DOI] [PubMed] [Google Scholar]
- 56.Petryshen TL, et al. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol Psychiatry. 2005;10(12):1074–1088. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- 57.Yamada K, Watanabe A, Iwayama-Shigeno Y, Yoshikawa T. Evidence of association between gamma-aminobutyric acid type A receptor genes located on 5q34 and female patients with mood disorders. Neurosci Lett. 2003;349(1):9–12. doi: 10.1016/s0304-3940(03)00611-6. [DOI] [PubMed] [Google Scholar]
- 58.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor α1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36(10):2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison PJ. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174(1):151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 60.Schobel SA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464(7289):763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.