Significance
Serotonergic innervation of sensory areas is seen ubiquitously across species and systems, but its functional role remains unclear to this day. We used a systems level approach to investigate the functional role of serotonergic input onto electrosensory pyramidal neurons in the weakly electric fish Apteronotus leptorhynchus. We found that serotonin selectively improved neuronal responses to stimuli associated with same-sex conspecifics by inducing increased excitability and burst firing. Further, serotonin enhanced perception of these stimuli while simultaneously inhibiting aggressive behaviors. Our results provide the first evidence that the serotonergic system acts as a “shut up and listen” system, thereby favoring covert behavior after an aggressive encounter together with enhanced perception of stimuli associated with dominant conspecifics.
Keywords: neuromodulation, neuroethology, excitability, neural coding
Abstract
Centrifugal serotonergic fibers innervating sensory brain areas are seen ubiquitously across systems and species but their function remains unclear. Here we examined the functional role of serotonergic innervation onto electrosensory neurons in weakly electric fish by eliciting endogenous release through electrical stimulation as well as exogenous focal application of serotonin in the vicinity of the cell being recorded from. Both approaches showed that the function of serotonergic input onto electrosensory pyramidal neurons is to render them more excitable by reducing the spike afterhyperpolarization amplitude and thereby promoting burst firing. Further, serotonergic input selectively improved neuronal responses to stimuli that occur during interactions between same-sex conspecifics but not to stimuli associated with either prey or that occur during interactions between opposite-sex conspecifics. Finally, we tested whether serotonin-mediated enhanced pyramidal neuron responses to stimuli associated with same-sex conspecifics actually increase perception by the animal. Our behavioral experiments show that exogenous injection and endogenous release of serotonin both increase the magnitude of behavioral responses to stimuli associated with same-sex conspecifics as well as simultaneously decrease aggressive behaviors. Thus, our data indicate that the serotonergic system inhibits aggressive behavior toward same-sex conspecifics, while at the same time increasing perception of stimuli associated with these individuals. This function is likely to be conserved across systems and species.
Animals must efficiently process natural sensory stimuli to successfully interact with their environment. It has become clear in recent years that sensory processing is not a passive process but instead actively depends on behavioral context (1). Adaptive control of sensory processing is in part achieved through neuromodulators such as serotonin (2). However, the functional role of serotonergic fibers emanating from the raphe nuclei innervating sensory brain areas remains largely unclear (3). This is in part because these fibers make diverse patterns of connectivity (4, 5), thereby causing a wide range of effects, such as response attenuation and gating (6, 7), as well as response enhancement (7). Thus, it is generally agreed that the function of serotonergic input onto sensory neurons is to enhance their responses to given stimulus features while attenuating responses to other features. As such, studies performed in model organisms well characterized anatomically, behaviorally, and physiologically are likely to speed progress toward a general understanding of how serotonin alters neuronal responses to natural stimuli as well as consequences on perception and behavior.
The weakly electric fish Apteronotus leptorhynchus generates a quasisinusoidal electric field through the electric organ discharge (EOD) (8). Electroreceptive neurons scattered on the skin surface monitor perturbations of this field caused by objects with conductivity different from that of the surrounding water and relay this information to pyramidal neurons within the electrosensory lateral line lobe (ELL). In particular, there are two important categories of behaviorally relevant electrosensory stimuli: those caused by prey are typically localized within a small region of the animal’s skin (9), whereas stimuli caused by conspecifics are typically diffuse and impinge on most if not all of the skin surface (8). In particular, when two fish come into contact (i.e., are within 1 m of one another), each animal will experience an amplitude modulation of its own EOD that oscillates at the difference between the two EOD frequencies (i.e., a beat). Because of a sexual dimorphism in EOD frequency, interactions between same-sex conspecifics tend to give rise to low frequency beats (<30 Hz), whereas interactions between opposite-sex conspecifics tend to give rise to higher frequency (30–400 Hz) beats (10). Moreover, these fish emit communication calls termed “chirps” during both agonistic and courtship behaviors that consist of brief modulations of their EOD. In particular, small chirps are preferentially elicited during encounters between same-sex conspecifics, whereas big chirps are preferentially elicited during encounters between opposite-sex conspecifics (10). The responses of ELL pyramidal neurons to such stimuli have been well characterized (8). Recent studies performed in vitro have shown that serotonin renders ELL pyramidal neurons more excitable by down-regulating potassium channels that contribute to the spike afterhyperpolarization (AHP) (11, 12). However, the effects of serotonin on the processing of behaviorally relevant stimuli by pyramidal neurons in vivo and its consequences on behavior have not been investigated to date.
Here, we used a systems level approach with behaviorally relevant stimuli to understand the functional role of serotonergic input onto ELL pyramidal neurons. We found that exogenous application of serotonin and endogenous release via electrical stimulation of serotonergic pathways both led to increased excitability and burst firing. Also, serotonin release enhanced pyramidal neuron responses to the low-frequency stimuli that occur during interactions between same-sex conspecifics as well as agonistic communication calls, but not to stimuli mimicking prey, opposite-sex conspecifics, or courtship communication calls. Finally, we found that both endogenous release and injection of serotonin caused enhanced behavioral responses to stimuli mimicking same-sex conspecific interactions, while simultaneously reducing aggressive behavior. We conclude that the functional role of serotonin is to enhance perception of stimuli associated with aggressive individuals, while at the same time promoting their avoidance through covert behavior. Our results thus demonstrate an important function for serotonergic input onto sensory neurons and its consequences at the organismal level.
Results
To determine the functional role of serotonergic input, we examined the effect of endogenous release as well as focal exogenous application of serotonin on electrosensory pyramidal neuron responses to behaviorally relevant stimuli recorded extracellularly and in some cases intracellularly (Materials and Methods).
Serotonin Increases Pyramidal Neuron Excitability and Burst Firing.
How does serotonin alter spontaneous pyramidal cell activity? Our results show that endogenous release of serotonin (Fig. 1A, and Materials and Methods) gives rise to increased excitability (Fig. 1B). Indeed, we observed a large and significant increase in both firing rate (Fig. 1C) and burst fraction (Fig. 1D and Materials and Methods) when the stimulation electrodes were properly placed as verified by subsequent histology (Materials and Methods and Fig. S1). As a control, we did not observe significant increases in either firing rate (Fig. 1C) or burst fraction (Fig. 1D) when the stimulation electrodes were incorrectly placed and instead stimulated adjacent brain areas. To verify that the observed effects were due to serotonin release within the ELL, we instead focally applied serotonin in the vicinity of the recorded pyramidal cell (Fig. 1E, Fig. S2, and Materials and Methods). Focal serotonin application also caused increased excitability (Fig. 1F) as measured by large and significant increases in firing rate (Fig. 1G) and burst fraction (Fig. 1H). Note that focal saline application did not significantly alter either firing rate (Fig. 1G) or burst fraction (Fig. 1H).
Fig. 1.
Serotonin increases electrosensory pyramidal neuron excitability. (A) Schematic showing how stimulation of the raphe nuclei was achieved. Shown is a dorsal view of the animal’s brain with the recording pipette and the stimulation electrode (Materials and Methods). CCb, cerebellum; EGP, eminentia granularis posterior; Tel, telencephalon; TeO, optic tectum. (B) Extracellular recording (gray) from a typical pyramidal neuron showing spontaneous (i.e., in the absence of stimulation but in the presence of the animal’s EOD) activity before (Upper) and after (Lower) raphe nuclei stimulation. The bursts of action potentials are shown in black. (Inset) Expanded view showing a burst of action potentials. (C) Population-averaged firing rate before stimulation (baseline) (Left), when the stimulation electrode was incorrectly placed (control) in other brain areas (Center, n = 6), and after raphe stimulation as determined from histology (Right, n = 13). (D) Population-averaged burst fraction (i.e., the fraction of interspike intervals < 10 ms) before stimulation (Left), when the stimulation electrode was stimulating adjacent brain areas (Center, n = 6), and after raphe stimulation (Right, n = 13). (E) Schematic showing the setup used to apply serotonin focally. Shown are the recording electrode that is positioned near a pyramidal neuron and the double barrel pharmacology pipette containing glutamate and serotonin that is positioned close to this neuron’s dendritic tree. (F) Extracellular recording (gray) showing spontaneous activity from a typical pyramidal neuron before (Upper) and after (Lower) exogenous serotonin application. The bursts of action potentials are shown in black. (Inset) Expanded view showing a burst of action potentials. (G) Population-averaged firing rate before application (Left), after saline application (Center, n = 8), and after serotonin exogenous application (Right, n = 38). (H) Population-averaged burst fraction before application (Left), after saline application (Center, n = 8), and after exogenous application (Right, n = 38). Stars indicate statistical significance at the P = 0.05 level using a sign-rank test.
Serotonin Selectively Enhances Pyramidal Neuron Responses to Stimuli Associated with Same-Sex Conspecifics.
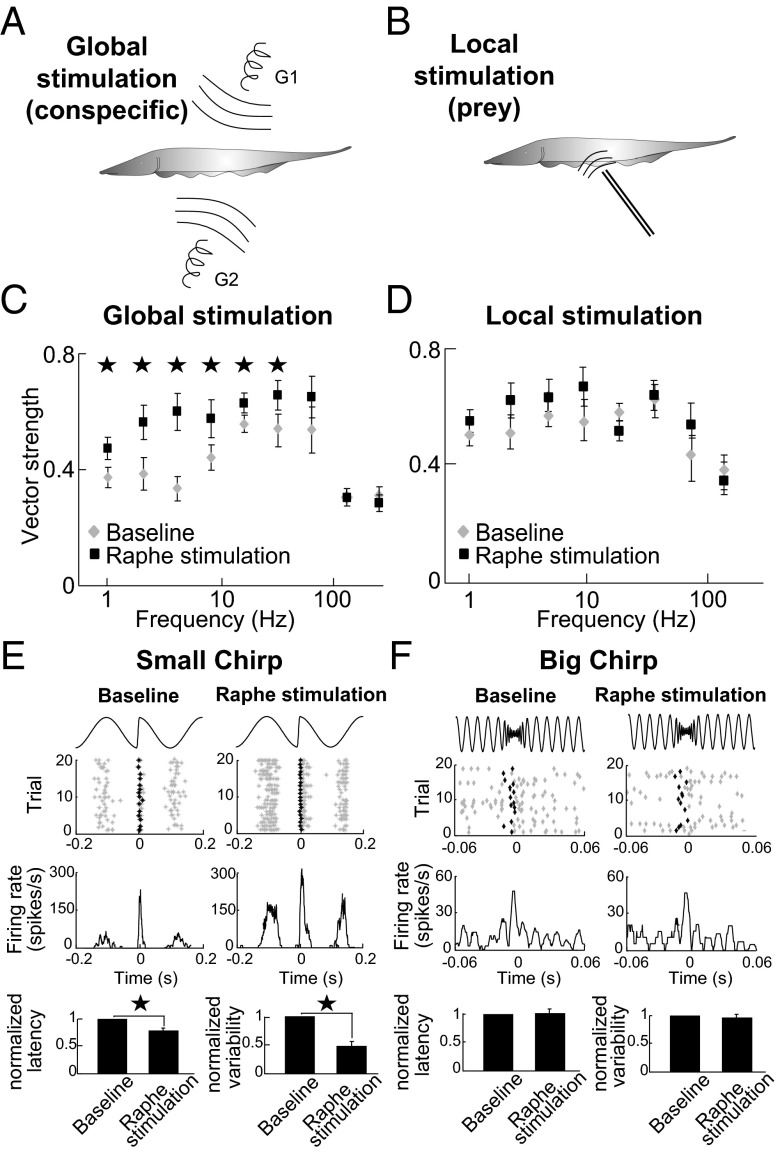
To understand how increased excitability due to activation of serotonergic input can affect pyramidal neuron responses to sensory input, we recorded their spiking activity before and after endogenous release of serotonin. Our stimuli were designed to mimic two important behavioral contexts. First, signals mimicking conspecifics were delivered “globally” through electrodes positioned far from and on either side of the animal (13) (Fig. 2A and Materials and Methods). Second, signals mimicking prey were delivered “locally” through a small dipole located close to the animal (Fig. 2B). We initially used sinusoidal waveforms of varying frequencies. When delivered globally, these waveforms mimic the beats that occur when two conspecific individuals come into close proximity to one another. As mentioned above, same-sex interactions tend to give rise to low frequency signals (<30 Hz), whereas opposite-sex interactions tend to give rise to higher frequency signals.
Fig. 2.
Serotonin selectively increases responses to stimuli associated with same-sex conspecifics. (A) Schematic showing global stimulation geometry for which the stimulus was delivered through two electrodes, G1 and G2, located lateral to the animal. Global stimuli impinge upon a large fraction of the sensory epithelium and mimic stimuli generated by conspecifics (Materials and Methods). (B) Schematic showing local stimulation geometry for which the stimulus was instead delivered through a small dipole located close to the animal. Local stimuli impinge upon a small fraction of the sensory epithelium and mimic stimuli caused by prey (Materials and Methods). (C) Population-averaged vector strength values as a function of global stimulus frequency before (gray, n = 6) and after (black, n = 6) raphe stimulation. (Inset) Segment from a global 4-Hz stimulus waveform (Top) and extracellular recording from a typical pyramidal neuron before (Middle) and after (Bottom) raphe nuclei stimulation. Note the increased burst firing (black) and that the neuron phase locks much better to the stimulus after raphe stimulation. (D) Population-averaged vector strength values as a function of local stimulus frequency before (gray, n = 5) and after (black, n = 5) raphe stimulation. (Inset) Segment from a local 4-Hz stimulus waveform (Top) and extracellular recording from a typical pyramidal neuron before (Middle) and after (Bottom) raphe nuclei stimulation. Note the similarity between this neuron’s activity before and after raphe stimulation. (E) Stimulus waveform delivered globally showing a small chirp at the center (Top), raster plot (Middle) showing spike times (gray), and the first spike occurring immediately after the small chirp (black) and peristimulus time histogram (PSTH) (Bottom) before (Left) and after (Right) raphe stimulation. Bar graphs show the population-averaged normalized first spike latency (Left, n = 13) and the normalized SD of the first spike latency (Right, n = 13) before and after raphe stimulation. (F) Stimulus waveform delivered globally showing a big chirp at the center (Top), raster plot (Middle) showing spike times (gray), and the first spike occurring immediately after the big chirp (black), and PSTH (Bottom) before (Left) and after (Right) raphe stimulation. Bar graphs show the normalized first spike latency (Left, n = 10) and its variability (Right, n = 10) before and after raphe stimulation. Stars indicate statistical significance at the P = 0.05 level using a sign-rank test.
Fig. 2C shows that endogenous serotonin release significantly increased phase locking to low- (<32 Hz) but not high-frequency global stimuli. Importantly, similar results were obtained when we instead focally applied serotonin (Fig. S3 A and B), confirming that these effects are primarily if not exclusively due to serotonin released within the ELL. No significant changes in phase locking were observed when applying saline focally (P > 0.13, sign-rank tests, n = 8). In contrast, endogenous serotonin release did not alter phase locking to local stimuli (Fig. 2D). We found no significant differences between the firing rates, burst fractions, or vector strength of pyramidal neurons when driven by sinusoidal local or global stimuli for all frequencies (P > 0.4, sign-rank tests), indicating that the differential effects of serotonin on local vs. global stimuli are most likely not due to differences in how strong pyramidal neurons were driven by the stimuli. Together, these findings suggest that the function of serotonin is to selectively enhance pyramidal neuron responses to stimuli that are generated by interactions between same-sex conspecifics.
To test this hypothesis, we used mimics of both small (Fig. 2E, Upper traces) and big chirps (Fig. 2F, Upper traces) that occur preferentially during agonistic interactions between same-sex conspecifics and courtship interactions between opposite-sex conspecifics, respectively. If our hypothesis is true, then serotonin should enhance pyramidal neuron responses to the former but not the latter. Confirming our prediction, endogenous serotonin release decreased spiking latency and increased spiking reliability to small chirps (Fig. 2E). We observed similar effects when serotonin was instead focally applied (Fig. S3 C and D). Note that no significant differences in spike latency (P > 0.6, sign-rank test, n = 8) or spiking reliability (P > 0.9, sign-rank test, n = 8) were observed when saline was focally applied. In contrast, both endogenous release (Fig. 2F) as well as focal exogenous application (Fig. S3E) of serotonin did not alter responses to big chirps. Thus, our results show an important functional role for serotonin: to selectively enhance neural responses to stimuli that occur during interactions between same-sex conspecifics.
Serotonin Increases Pyramidal Cell Excitability by Reducing the Spike Afterhyperpolarization.
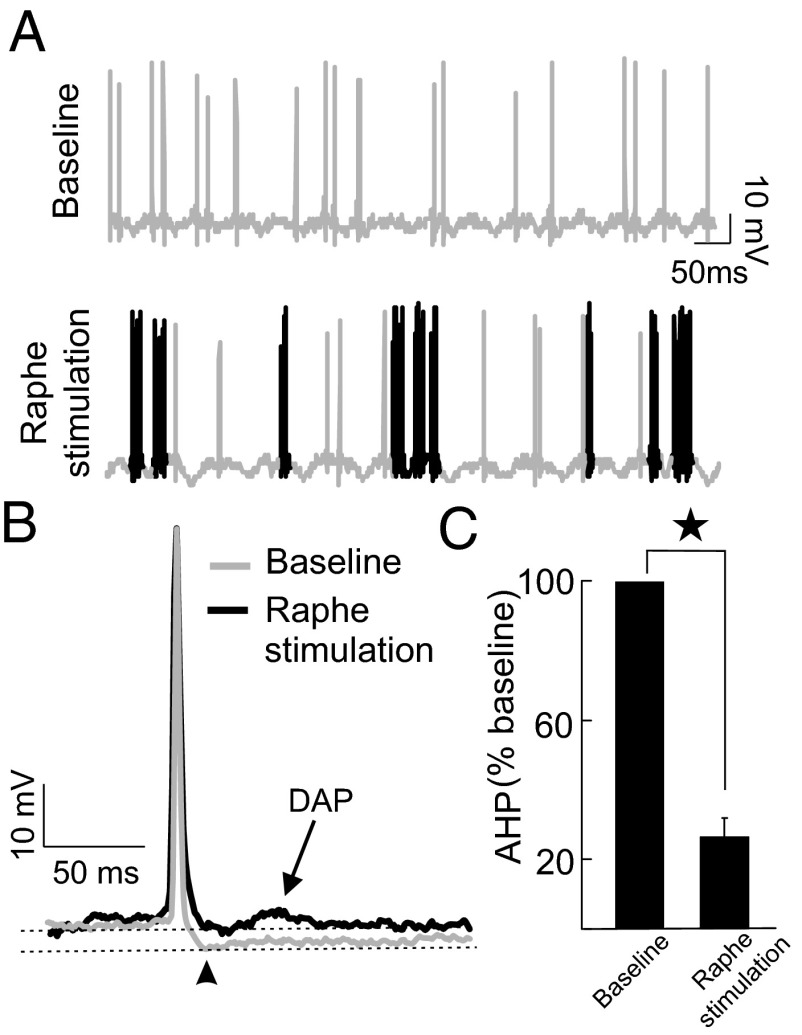
By what mechanism(s) does serotonin enhance pyramidal cell excitability and alter responses to stimuli associated with same-sex conspecifics? To investigate this important question, we performed intracellular recordings from pyramidal cells before and after electrical stimulation of serotonergic pathways. As expected, electrical stimulation gave rise to increased excitability and burst firing (Fig. 3A, black spikes). Superimposing the spike shapes before and after electrical stimulation showed a significant decrease in the AHP (Fig. 3 B and C), thereby revealing a depolarizing afterpotential (DAP) (Fig. 3B) that promotes burst firing.
Fig. 3.
Serotonin alters pyramidal neuron excitability by reducing the spike afterhyperpolarization (AHP). (A) Intracellular recording (gray) from a typical pyramidal neuron before (Upper) and after (Lower) endogenous serotonin release with the bursts shown in black. (B) Average action potential waveforms from this same neuron before (gray) and after (black) endogenous serotonin release showing a reduced AHP (arrowhead) as well as a depolarizing afterpotential (DAP, arrow). (C) Measured AHP (Materials and Methods) before (Left, n = 8) and after (Right, n = 8) endogenous serotonin release.
Serotonin Enhances Behavioral Responses to Stimuli Generated by Same-Sex Conspecifics.
Information transmitted by neurons is only useful to an organism if it is decoded by downstream neurons and leads to behavior. To test whether the increased pyramidal cell responses to stimuli generated by same-sex conspecifics are behaviorally relevant, we recorded behavioral responses of restrained fish to mimics of such stimuli before and after serotonin injection (Fig. 4A and Materials and Methods). We focused on two behaviors. First, the jamming avoidance response (JAR), a behavior by which the animal changes the frequency of its self-generated electric field to increase the frequency difference of jamming signals. This complex behavior requires organization, identification, and interpretation of sensory information in the brain (14, 15), and is typically used as a measure of perception in weakly electric fish (16). Furthermore, it is preferentially elicited by low-frequency sinusoidal stimuli that are associated with same-sex conspecific interactions. The second behavior was small chirp production, which was used as a control because previous studies have shown that this behavior associated with aggression is inhibited by serotonin (17).
Fig. 4.
Serotonin simultaneously enhances behavioral responses to stimuli generated by same-sex conspecifics and inhibits aggressive behaviors. (A) Schematic of the experimental setup in which a fish is restrained in a tube (chirp chamber) in an otherwise empty tank. The animal’s electric field is monitored by a pair of electrodes E1, E2 (straight lines) while the stimulus (i.e., the EOD AM; Materials and Methods) is delivered using global stimulation geometry S1, S2 (spirals). (B, Left) Electric organ discharge (EOD) frequency as a function of time before (blue) and after (red) saline injection. The shaded region shows the time when the stimulus was on. Arrows indicate the JAR magnitude. (Right) EOD frequency as a function of time before (blue) and after (red) serotonin injection. (C) EOD spectrogram (i.e., EOD power spectrum as a function of time) with the vertical bands corresponding to the black vertical bars representing chirps before injection (Left), after saline injection (Center), and after serotonin injection (Right). (D, Left) Chirp rate before injection (Left, n = 6), after saline injection (Center, n = 6), and after serotonin injection (Right, n = 6). (Right) JAR magnitude before injection (Left, n = 6), after saline injection (Center, n = 6), and after serotonin injection (Right, n = 6). Stars indicate statistical significance at the P = 0.05 level using a sign-rank test. (E) Schematic of the experimental setup used for bilateral exogenous serotonin application in the ELL. Two electrodes containing serotonin were placed in each ELL (Materials and Methods). (F) EOD frequency from an example specimen as a function of time before (blue) and after (red) serotonin injection. (G) Chirp rate before application (Left, n = 6), after saline application (Center, n = 6), and after serotonin application (Right, n = 6). (H) JAR magnitude before application (Left, n = 6), after saline application (Center, n = 6), and after serotonin application (Right, n = 6). Stars indicate statistical significance at the 0.05 level using a sign-rank test.
Fig. 4 B and C show that serotonin injection both increased the frequency excursion during the JAR and decreased aggression, as quantified by the rate at which small chirps occurred. Similar results were seen across our dataset (Fig. 4D). We also took advantage of the fact that electrosensory behaviors do not require movement and can thus be elicited in immobilized animals (15) and tested whether endogenous serotonin release by electrical stimulation would lead to similar changes in behavior. Confirming our prediction, we found that stimulation of serotonergic pathways also led to an increased JAR response and a significant reduction in small chirp rate (Fig. S4 A–C). Critically, bilateral exogenous serotonin application in the ELL (Fig. 4E and Materials and Methods) also led to a significant increase in the JAR and a significant reduction in chirp rate (Fig. 4 F–H), thereby showing that these changes in behavior are indeed primarily due to serotonin directly acting on pyramidal neuron activity. Thus, these results demonstrate that increased pyramidal cell responses mediated by serotonin do indeed lead to improved perception at the behavioral level. Further, they show that serotonin can simultaneously enhance perception of and inhibit aggressiveness in response to stimuli that occur during interactions between same-sex conspecifics.
Discussion
We investigated how serotonergic inputs onto electrosensory pyramidal neurons alter their responses to sensory input and its consequences on behavior. We found that activation of serotonergic pathways led to increased pyramidal neuron excitability and burst firing by reducing the AHP. Further, such activation selectively enhanced neuronal responses to low-frequency sinusoidal as well as agonistic communication signals that are associated with same-sex conspecifics. We also found that endogenous release of serotonin can inhibit behavioral responses associated with aggression while at the same time enhancing behavioral responses to stimuli associated with same-sex conspecifics. Thus, our results demonstrate an important function for serotonergic inputs onto electrosensory pyramidal neurons as well as the underlying mechanism by which it is achieved.
Our results have shown that serotonin increases excitability in vivo by reducing the AHP and thereby promoting burst firing. The similar effects observed for both endogenous release and focal application suggest that serotonin acts directly on serotonergic receptors located on pyramidal neurons. Further, the remarkable similarity between the effects of serotonin in vivo and in vitro (11) strongly suggests that the mode of action found in the latter (i.e., down-regulation of potassium currents that contribute to the AHP) also applies to the former. Previous results have shown that electrosensory pyramidal neurons can fire bursts of action potentials through a somatodendritic interaction involving a DAP (18), which is similar to that seen in hippocampal pyramidal neurons. Such burst firing in electrosensory pyramidal neurons is extensively regulated (19, 20). In particular, the strong AHP seen in vivo counteracts the DAP and generally opposes burst firing (13). However, recent studies have shown that small chirps can elicit burst firing (21). Together with previous results showing that burst firing in ELL pyramidal cells codes for low-frequency stimuli (22), our results suggest that the DAP-mediated burst firing seen in pyramidal neurons primarily serves to signal the occurrence of stimuli associated with same-sex conspecifics.
Why does serotonin selectively affect pyramidal neuron responses to stimuli associated with interactions between same-sex conspecifics? Pyramidal neurons receive massive projections from higher brain centers (23) whose function is to attenuate responses to low-frequency (<32 Hz) global stimuli by providing a negative image of the stimulus (24). It is important to note that neither local stimuli (24, 25) nor high-frequency (>32 Hz) global stimuli (26) elicit this feedback input and that small chirp stimuli typically occur on top of low-frequency beats (21). Further, previous findings have shown that serotonergic fibers are mostly colocalized with feedback input onto pyramidal neurons (11). Therefore, we propose that serotonin selectively enhances responses to stimuli that occur during interactions between same-sex conspecifics because only these elicit feedback input onto pyramidal neurons that can be altered by serotonin in the first place.
It is likely that the mechanisms by which serotonin alters pyramidal neuron activity will be found in other systems. This is because: (i) many neurons display burst firing similar to ELL pyramidal neurons (27) and such burst firing also encodes similar stimulus features (28); (ii) potassium channels in weakly electric fish display over 90% functional homology with their mammalian counterparts (29); and (iii) reduction of potassium conductances by serotonin has been shown to decrease first spike latency and increase excitability in mammalian sensory neurons (30), thereby giving rise to greater signal detection (31).
Whereas our results have shown that changes in pyramidal neuron activity due to serotonin are sufficient to elicit changes in behavior, it is likely that other brain areas contribute to this effect under natural conditions. Indeed, other higher order sensory areas have been shown to receive serotonergic innervation (32); for example, neurons within the midbrain torus semicircularis (TS) that receive synaptic input from pyramidal neurons receive serotonergic innervation. Whereas previous studies have shown that TS neurons can respond selectively to behaviorally relevant stimuli such as chirps (33), the effects of serotonin on these responses are unknown. It is likely that serotonin will selectively improve the responses of some TS neurons, whereas reducing those of others, as observed in homologous midbrain areas (3). However, based on the results presented here, we might expect that only the TS neurons that respond selectively to either small chirps or low-frequency beats would have their responses enhanced. Further studies are needed to understand how serotonin alters the responses of TS neurons.
Finally, both endogenous release as well as injection of serotonin led to increased perception of low-frequency stimuli that occur during interactions between same-sex conspecifics while simultaneously inhibiting aggression. Thus, we propose that the serotonergic system acts as a “shut up and listen” system, thereby favoring more cautious behavior after an aggressive encounter together with enhanced perception of stimuli associated with dominant individuals. Indeed, serotonin is one of the main modulatory systems of aggression (34). It is well known that serotonin will decrease aggressive behaviors across species (35) as well as promote avoidance and escape from dangerous situations (36). Importantly, our results provide a functional role linking perception to behavior for the commonly observed larger levels of serotonin in submissive individuals with respect to those observed in dominant ones (37). Specifically, we propose that these larger levels of serotonin selectively enhance sensory neuron responses to stimuli associated with aggressive conspecifics, thereby leading to enhanced perception and better avoidance by more submissive individuals.
In summary, we have shown that the function of serotonergic inputs onto ELL pyramidal cells is to selectively enhance their responses to stimuli associated with same-sex conspecifics through a reduction of the AHP, which leads to their enhanced perception at the organismal level. This important and function is likely to be shared among organisms, given that the serotonergic system is remarkably conserved across vertebrates (38).
Materials and Methods
Animals.
We used 58 weakly electric fish A. leptorhynchus of both sexes in the present experiments. Animals were purchased from tropical fish suppliers and were exclusively used in these experiments. Fish were acclimated to laboratory conditions and housed in groups of 8–10. Water conductivity was between 400 and 800 μS, the pH was maintained between 6.8 and 7.2, and the temperature was kept between 27 and 29 °C (15). McGill University’s Animal Care Committee approved all procedures.
Surgical procedures were explained in detail previously (13). Briefly, 0.1–0.5 mg of tubocurarine (Sigma) was injected intramuscularly to immobilize the fish. The fish was then transferred to a recording tank and respirated via a mouth tube at a flow rate of 10 mL/min. To stabilize the head during recording, a metal post was glued to the exposed area of the skull, rostral to the opening. We drilled a small hole of ∼2 mm2 over the cerebellum and the ELL area, caudal to the border between hindbrain and midbrain in order to access the pyramidal neurons. In some experiments, an opening in the midline, 1 mm rostral to border between hindbrain and midbrain was made to gain access to the ipsilateral serotonergic fibers originating from raphe nucleus for electrical stimulation.
Recordings.
We used well-established techniques to record extracellularly and intracellular from pyramidal cells within the lateral segment (LS) of the ELL (13), which receive the greatest amount of serotonergic innervation (11). We used CED 1401-plus hardware and Spike II software to detect both extracellular and intracellular spikes at a resolution of 0.1 ms (Cambridge Electronic Design).
Pharmacology.
Glutamate (Sigma) and serotonin (Sigma) were dissolved in saline for application. Drug application electrodes were two-barrel KG-33 glass micropipettes (OD 1.5 mm, Garner Glass Co.) pulled by a micropipette puller (David Kopf Instruments) to a fine tip and subsequently broken to attain a tip diameter of ∼10 μm as done previously (13). The two barrels were used for separate application of serotonin (1 mM) and glutamate (1 mM). During recordings, we first used excitatory responses to glutamate application to verify that we were in the correct location (Fig. S2). Serotonin was then applied as done previously (13). Note that previous studies have shown that saline applied in this manner does not significantly alter pyramidal neuron activity (39).
Stimulation.
As the electric organ discharge of A. leptorhynchus is neurogenic, it is not affected by immobilization with curare-like drugs. All stimuli consisted of amplitude modulations (AMs) of the animal’s own EOD and were produced by applying a train of sinusoidal waveforms to the fish. Each sinusoid was triggered at the zero crossing of each EOD cycle and had a period slightly less than that of the EOD waveform; hence the train remains synchronized to the animal’s discharge and, depending on its polarity, either adds to or subtracts from the animal’s own discharge. A modulation waveform was then multiplied with the train of sinusoidal waveforms (MT3 multiplier; Tucker Davis Technologies) and the resulting signal was first isolated from ground (A395 linear stimulus isolator; World Precision Instruments) before being delivered using either global or local stimulation geometry. For global stimulation, signals were delivered through pairs of chloridized silver wire electrodes positioned 15 cm away from the fish in either side of the recording tank. In contrast, for local stimulation, we used a small local dipole electrode that was located 1–3 mm from the skin. The intensities of local and global stimuli were similar to those used previously and were adjusted such as to give rise to similar changes in EOD amplitude as measured by a small dipole close to the animal’s skin (40). Four different types of stimuli were applied: sinusoids with frequencies 1, 2, 4, 8, 16, 32, 64, 128, and 256 Hz that were applied either globally or locally, as well as small and big chirps that were applied globally. All stimuli were generated as done previously (33).
Electrical Stimulation of Serotonergic Fibers onto ELL Pyramidal Neurons.
A bipolar tungsten electrode was positioned over the serotonergic fibers traveling to ELL, 1 mm rostral to T0 at the midline level of the brain surface and 3.5 mm in depth. The electrical stimulating electrode was positioned so that the stimulation affected the chirps produced by fish. Stimuli consisted of 10 pulses (200–300 pA) at 40 Hz with each pulse lasting 0.3 ms.
Histology.
Following completion of recordings, fish (n = 17) were deeply anesthetized with Tricaine methanesulfonate (MS-222). The brain was then extracted and moved to paraformaldehyde (0.04 g/mL) at 4 °C for 72 h and then transferred to ethanol (0.592 g/mL). Prussian blue staining for iron was performed to localize stimulation sites, as described by previous studies (41). Brain slices of 5-μm thickness were made and immersed for 20 min in a solution of 0.22 g/mL hydrochloric acid and 0.185 g/mL potassium ferrocyanide. After washing three times in distilled water, slices were counterstained with Nuclear Fast Red for 5 min and rinsed two times in distilled water. Next, they were dehydrated through ethanol (0.592 and 0.789 g/mL) and were cleared in 860 g/mL xylene solution (for 3 min, twice). We then verified the exact location of the electrical stimulation (Fig. S1).
Data Analysis.
Burst fraction was computed as the fraction of interspike intervals that are less than 10 ms (11, 13). Average action potential waveforms were computed to measure the AHP following each action potential (42). The vector strength was computed as done previously (33). The latency (in milliseconds) of the first spike generated by neurons after the beginning of small and big chirp stimuli was measured and the SD of these values was used as a measure of the variability of this latency. All quantities are reported as mean ± SE.
Behavior.
For some behavioral experiments, animals were placed in a “chirp chamber” (15). The animals were injected i.m. with 0.1 mL of saline solution. Additionally, the animals were injected i.m. with 0.01–0.06 mL of 10 mM serotonin hydrochloride solution to achieve a dose of 10 μg/1 g of body mass. Another set of behavioral experiments was performed in immobilized animals in response to 4-Hz sinusoidal AMs of the EOD after either bilateral injection of serotonin or saline in the ELL molecular layer (Fig. 4E) or after endogenous release of serotonin following electrical stimulation of serotonergic pathway (see above) (Fig. S4).
Sinusoidal waveforms with frequency of 4 Hz below the animal’s baseline EOD frequency and with intensity of 1.39 mV/cm with duration at least 15 s were presented. Previous studies have shown that such stimuli will reliably elicit JAR and chirp responses in A. leptorhynchus (15). The stimuli were repeated five times with a rest period of 50 s between each presentation. The EOD frequency was estimated as before (15). The JAR magnitude was defined as the maximum frequency elicited during stimulation minus the baseline (i.e., without stimulation) value.
Supplementary Material
Acknowledgments
The authors thank Dr. Leonard Maler for useful discussions and Dr. Ana Giassi for assistance with histology. This work was supported by grants from the Canadian Institutes of Health Research and the Fonds de recherche du Québec - Nature et technologies (to M.J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314008110/-/DCSupplemental.
References
- 1.Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol. 2007;17(4):423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marder E. Neuromodulation of neuronal circuits: Back to the future. Neuron. 2012;76(1):1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: Monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14(4):488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Foehring RC, van Brederode JF, Kinney GA, Spain WJ. Serotonergic modulation of supragranular neurons in rat sensorimotor cortex. J Neurosci. 2002;22(18):8238–8250. doi: 10.1523/JNEUROSCI.22-18-08238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson AM, Hurley LM. Dense serotonergic innervation of principal nuclei of the superior olivary complex in mouse. Neurosci Lett. 2004;356(3):179–182. doi: 10.1016/j.neulet.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009;12(6):784–791. doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- 7.Hurley LM, Pollak GD. Serotonin shifts first-spike latencies of inferior colliculus neurons. J Neurosci. 2005;25(34):7876–7886. doi: 10.1523/JNEUROSCI.1178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chacron MJ, Longtin A, Maler L. Efficient computation via sparse coding in electrosensory neural networks. Curr Opin Neurobiol. 2011;21(5):752–760. doi: 10.1016/j.conb.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson ME, Maciver MA. Prey capture in the weakly electric fish Apteronotus albifrons: Sensory acquisition strategies and electrosensory consequences. J Exp Biol. 1999;202(Pt 10):1195–1203. doi: 10.1242/jeb.202.10.1195. [DOI] [PubMed] [Google Scholar]
- 10.Zakon HH, Oestreich J, Tallarovic S, Triefenbach F. EOD modulations of brown ghost electric fish: JARs, chirps, rises, and dips. J Physiol Paris. 2002;96(5-6):451–458. doi: 10.1016/S0928-4257(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 11.Deemyad T, Maler L, Chacron MJ. Inhibition of SK and M channel-mediated currents by 5-HT enables parallel processing by bursts and isolated spikes. J Neurophysiol. 2011;105(3):1276–1294. doi: 10.1152/jn.00792.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Márquez BT, Krahe R, Chacron MJ. Neuromodulation of early electrosensory processing in gymnotiform weakly electric fish. J Exp Biol. 2013;216(Pt 13):2442–2450. doi: 10.1242/jeb.082370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toporikova N, Chacron MJ. SK channels gate information processing in vivo by regulating an intrinsic bursting mechanism seen in vitro. J Neurophysiol. 2009;102(4):2273–2287. doi: 10.1152/jn.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiligenberg W. Neural Nets in Electric Fish. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- 15.Hitschfeld ÉM, Stamper SA, Vonderschen K, Fortune ES, Chacron MJ. Effects of restraint and immobilization on electrosensory behaviors of weakly electric fish. ILAR J. 2009;50(4):361–372. doi: 10.1093/ilar.50.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson BA, Kawasaki M. Ambiguous encoding of stimuli by primary sensory afferents causes a lack of independence in the perception of multiple stimulus attributes. J Neurosci. 2006;26(36):9173–9183. doi: 10.1523/JNEUROSCI.1513-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith GT, Combs N. Serotonergic activation of 5HT1A and 5HT2 receptors modulates sexually dimorphic communication signals in the weakly electric fish Apteronotus leptorhynchus. Horm Behav. 2008;54(1):69–82. doi: 10.1016/j.yhbeh.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Lemon N, Turner RW. Conditional spike backpropagation generates burst discharge in a sensory neuron. J Neurophysiol. 2000;84(3):1519–1530. doi: 10.1152/jn.2000.84.3.1519. [DOI] [PubMed] [Google Scholar]
- 19.Mehaffey WH, Doiron B, Maler L, Turner RW. Deterministic multiplicative gain control with active dendrites. J Neurosci. 2005;25(43):9968–9977. doi: 10.1523/JNEUROSCI.2682-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastian J, Nguyenkim J. Dendritic modulation of burst-like firing in sensory neurons. J Neurophysiol. 2001;85(1):10–22. doi: 10.1152/jn.2001.85.1.10. [DOI] [PubMed] [Google Scholar]
- 21.Marsat G, Proville RD, Maler L. Transient signals trigger synchronous bursts in an identified population of neurons. J Neurophysiol. 2009;102(2):714–723. doi: 10.1152/jn.91366.2008. [DOI] [PubMed] [Google Scholar]
- 22.Oswald AMM, Chacron MJ, Doiron B, Bastian J, Maler L. Parallel processing of sensory input by bursts and isolated spikes. J Neurosci. 2004;24(18):4351–4362. doi: 10.1523/JNEUROSCI.0459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman NJ, Maler L. Neural architecture of the electrosensory lateral line lobe: Adaptations for coincidence detection, a sensory searchlight and frequency-dependent adaptive filtering. J Exp Biol. 1999;202(Pt 10):1243–1253. doi: 10.1242/jeb.202.10.1243. [DOI] [PubMed] [Google Scholar]
- 24.Bastian J, Chacron MJ, Maler L. Plastic and nonplastic pyramidal cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron. 2004;41(5):767–779. doi: 10.1016/s0896-6273(04)00071-6. [DOI] [PubMed] [Google Scholar]
- 25.Chacron MJ, Maler L, Bastian J. Feedback and feedforward control of frequency tuning to naturalistic stimuli. J Neurosci. 2005;25(23):5521–5532. doi: 10.1523/JNEUROSCI.0445-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bol K, Marsat G, Harvey-Girard E, Longtin A, Maler L. Frequency-tuned cerebellar channels and burst-induced LTD lead to the cancellation of redundant sensory inputs. J Neurosci. 2011;31(30):11028–11038. doi: 10.1523/JNEUROSCI.0193-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5(1):13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- 28.Lesica NA, Stanley GB. Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. J Neurosci. 2004;24(47):10731–10740. doi: 10.1523/JNEUROSCI.3059-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis LD, Maler L, Dunn RJ. Differential distribution of SK channel subtypes in the brain of the weakly electric fish Apteronotus leptorhynchus. J Comp Neurol. 2008;507(6):1964–1978. doi: 10.1002/cne.21597. [DOI] [PubMed] [Google Scholar]
- 30.Stefani A, Surmeier DJ, Kitai ST. Serotonin enhances excitability in neostriatal neurons by reducing voltage-dependent potassium currents. Brain Res. 1990;529(1-2):354–357. doi: 10.1016/0006-8993(90)90851-2. [DOI] [PubMed] [Google Scholar]
- 31.Massaux A, Dutrieux G, Cotillon-Williams N, Manunta Y, Edeline JM. Auditory thalamus bursts in anesthetized and non-anesthetized states: Contribution to functional properties. J Neurophysiol. 2004;91(5):2117–2134. doi: 10.1152/jn.00970.2003. [DOI] [PubMed] [Google Scholar]
- 32.Johnston SA, Maler L, Tinner B. The distribution of serotonin in the brain of Apteronotus leptorhynchus: an immunohistochemical study. J Chem Neuroanat. 1990;3(6):429–465. [PubMed] [Google Scholar]
- 33.Vonderschen K, Chacron MJ. Sparse and dense coding of natural stimuli by distinct midbrain neuron subpopulations in weakly electric fish. J Neurophysiol. 2011;106(6):3102–3118. doi: 10.1152/jn.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montoya ER, Terburg D, Bos PA, van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv Emot. 2012;36(1):65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fachinelli C, Sargo S, Bataller R, Rodríguez Echandía EL. Effect of 5-HTP and ketanserine on the aggressive reaction induced by food competition in dominant and submissive pigeons (Columba livia) Behav Brain Res. 1989;35(3):265–270. doi: 10.1016/s0166-4328(89)80146-9. [DOI] [PubMed] [Google Scholar]
- 36.Tops M, Russo S, Boksem MA, Tucker DM. Serotonin: Modulator of a drive to withdraw. Brain Cogn. 2009;71(3):427–436. doi: 10.1016/j.bandc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Larson ET, Summers CH. Serotonin reverses dominant social status. Behav Brain Res. 2001;121(1-2):95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- 38.Parent A. Comparative anatomy of the serotoninergic systems. J Physiol (Paris) 1981;77(2-3):147–156. [PubMed] [Google Scholar]
- 39.Bastian J. The role of amino acid neurotransmitters in the descending control of electroreception. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1993;172(4):409–423. doi: 10.1007/BF00213523. [DOI] [PubMed] [Google Scholar]
- 40.Bastian J, Chacron MJ, Maler L. Receptive field organization determines pyramidal cell stimulus-encoding capability and spatial stimulus selectivity. J Neurosci. 2002;22(11):4577–4590. doi: 10.1523/JNEUROSCI.22-11-04577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrington JK, et al. Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB J. 2011;25(12):4150–4161. doi: 10.1096/fj.11-185140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faber ESL, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22(5):1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.