Significance
Although the relationship between endocrine and immune systems is well documented, few studies have been performed on autoimmune disorders other than those that are sex hormone-related. We studied a murine model of autoimmune diabetes, showing that growth hormone (GH) modifies the immune response to render diabetic mice resistant to disease development. The mechanism involves a GH-mediated effect on β-cell survival and/or proliferation and a direct effect on immune cells. GH triggers a cytokine environment that promotes anti-inflammatory macrophage polarization, maintains the activity of the suppressor T cells, and limits Th17 cell plasticity. This study provides evidence of the importance of endocrine control of immune functions and indicates that therapies based on GH analogs should be considered for treatment of autoimmune diabetes.
Keywords: beta cells, Tregs
Abstract
Evidence supports a relationship between the neuroendocrine and the immune systems. Data from mice that overexpress or are deficient in growth hormone (GH) indicate that GH stimulates T and B-cell proliferation and Ig synthesis, and enhances maturation of myeloid progenitor cells. The effect of GH on autoimmune pathologies has nonetheless been little studied. Using a murine model of type 1 diabetes, a T-cell–mediated autoimmune disease characterized by immune cell infiltration of pancreatic islets and destruction of insulin-producing β-cells, we observed that sustained GH expression reduced prodromal disease symptoms and eliminated progression to overt diabetes. The effect involves several GH-mediated mechanisms; GH altered the cytokine environment, triggered anti-inflammatory macrophage (M2) polarization, maintained activity of the suppressor T-cell population, and limited Th17 cell plasticity. In addition, GH reduced apoptosis and/or increased the proliferative rate of β-cells. These results support a role for GH in immune response regulation and identify a unique target for therapeutic intervention in type 1 diabetes.
Growth hormone (GH) is a pleiotropic hormone that affects a broad spectrum of physiological functions, from carbohydrate and lipid metabolism to the immune response (1). Several studies have linked GH with autoimmune diseases, although its effects on the immune system are still debated. Whereas some reports using GH-deficient mice indicate that it does not affect immune competence (2), others suggest that GH is necessary for correct immune system development (1, 3). The GH receptor (GHR) is expressed by several lymphocyte subpopulations (4). GH stimulates in vitro T and B-cell proliferation (5) and Ig synthesis (6); enhances human myeloid progenitor cell maturation (7); and modulates in vivo Th1/Th2 (8) and humoral immune responses (1). In addition, therapeutic activation of the GH/STAT5B axis is postulated as a target for restoring mucosal tolerance in Crohn disease (9, 10). A single point mutation in STAT5B limits its DNA binding activity as well as maintenance of FOXP3 expression by Treg cells in nonobese diabetic (NOD) mice (11). These mice develop type 1 diabetes, which is characterized by autoimmune destruction of pancreatic β-cells due to the effect of environmental factors on genetically predisposed individuals (12, 13). Although this murine model does not completely mimic the human disease, most steps in the pathogenesis, including prodromal and clinical symptoms, are closely comparable (14).
Despite the interdependence of GH and insulin regulation and the known effects of GH and insulin-like growth factor 1 (IGF1) on pancreatic β-cell survival, proliferation and neogenesis (15, 16), hormone influences have not been described in type 1 diabetes; no specific studies have addressed the consequences of long-term GH replacement therapy in this disease. Here we show the effects of long-term GH supplementation as a tool to modulate autoimmune attack on pancreatic β-cells. NOD mice transgenic for bovine GH (NOD-Tg bGH) do not develop type 1 diabetes, and show normal glycemia throughout their lives. Our histological analyses indicated that these mice develop periinsulitis, but show little or no islet infiltration or β-cell destruction. The mechanism involved specific GH-mediated effects on β-cells, where it influenced proliferation and apoptosis, and others that modulate the immune system. GH affected Th17/Th1 plasticity, M1/M2 macrophage differentiation, and Treg cell function. Our findings show an unanticipated GH effect on tolerization mechanisms that reduce type 1 diabetes development, and underline the importance of neuroendocrine regulation of the immune system.
Results
Sustained GH Expression Precludes Development of Overt Diabetes in NOD Mice.
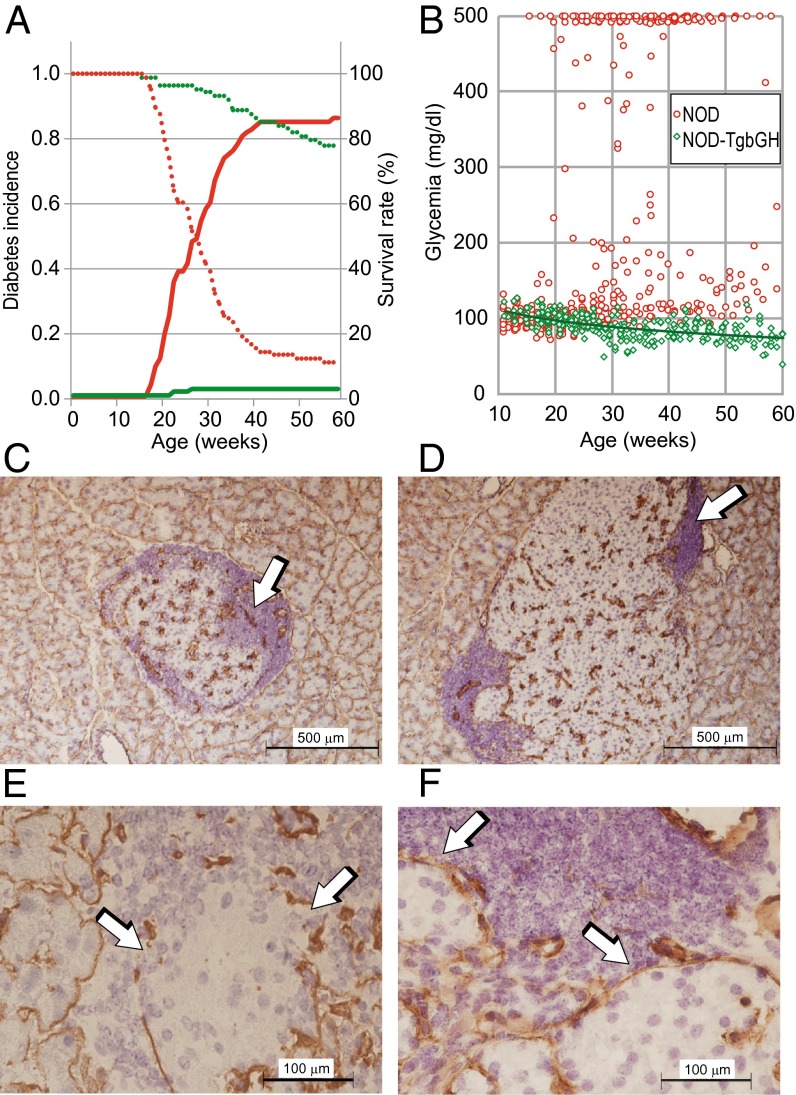
In our animal facility, >85% of virgin female NOD mice develop overt diabetes before 40 wk of age (Fig. 1A). To study the effects of sustained high levels of circulating GH, we obtained a mouse strain transgenic for bGH under the control of the rat phosphoenolpyruvate carboxykinase (PEPCK) promoter (17), on the NOD background. In this strain, as in the parental C57BL/6-Tg bGH strain, circulating GH levels are constant (∼5 μg/mL). Our mice were healthy and showed no external signs of other autoimmune diseases or tumor development throughout their lives. Histological examination showed mild sialitis. We monitored glycemia in female NOD-Tg bGH mice and control littermates for 60 wk, and found that the transgenic mice were almost completely resistant to diabetes development (Fig. 1A), as reflected by their higher survival rate compared with NOD mice (Fig. 1A). The results indicated significant lowering of circulating glucose levels in the transgenic mice with age (Fig. 1B), resulting in relative hypoglycemia. This observation contrasts with the susceptibility of C57BL/6-Tg bGH mice to type 2 diabetes in a high-fat diet study (18). In histological analyses, NOD-Tg bGH mice showed giant islets with anomalous morphology and a periinsular mononuclear cell infiltrate that characterizes the initial stages of diabetes (Fig. 1 C–F). The irregular islet morphology suggests β-cell hyperproliferation and islet coalescence (Fig. 1D), in agreement with the β-cell hyperproliferation observed in the presence of placental lactogen (19). Although the results were not statistically significant, the number of apoptotic β-cells was reduced and Ki-67+ cells were increased in NOD Tg-bGH pancreas (Fig. S1). These data suggest a role for GH in protection of β-cells from apoptosis and stimulation of their proliferative capacity.
Fig. 1.
NOD-Tg bGH mice are protected against type 1 diabetes development. (A) Cumulative diabetes incidence in female NOD (continuous red line, n = 90) and NOD-Tg bGH mice (continuous green line, n = 89). Mice were maintained and glucose levels determined as described in Materials and Methods. The survival rate of transgenic mice after 60 wk in the absence of diabetes was 76% (dotted green line). The NOD mouse survival curve (dotted red line) reflects mouse sacrifice at disease onset. (B) Tendency to lower blood glucose levels with age in NOD-Tg bGH mice (green line; n = 10 mice per group, Pearson’s correlation coefficient, r = −0.61, P < 0.01). Each glucose measurement is plotted (twice monthly per mouse). Red, NOD mice; green, NOD-Tg bGH mice. (C–F) Immunohistochemistry of pancreas from 12-wk-old NOD (C and E) and NOD-Tg bGH (D and F) mice stained for laminin and counterstained with hematoxylin. (C and D) Low-magnification images (6×) showing mononuclear infiltrates (arrows) surrounding islets. (Scale bar: 500 μm.) (E and F) High-magnification (40×) images showing damage induced in the NOD mouse in the laminin sheet (arrows) surrounding islets and compared with its preservation in NOD-Tg bGH mice. (Scale bar: 100 μm.)
NOD-Tg bGH Mice Have Normal Delayed Type Hypersensitivity Responses.
Some reports indicate a role for GH as an immune response activator (5, 20); we thus characterized immune cell populations in blood, spleen, and peripheral lymph nodes of NOD and NOD-Tg bGH mice. Flow cytometry analysis showed similar immune cell populations in both mouse lines (Table S1). No differences were observed in activation markers (CD25, CD69, CD44, and CD62L) in CD3+ cells isolated from NOD and NOD Tg-bGH lymph nodes (Fig. S2).
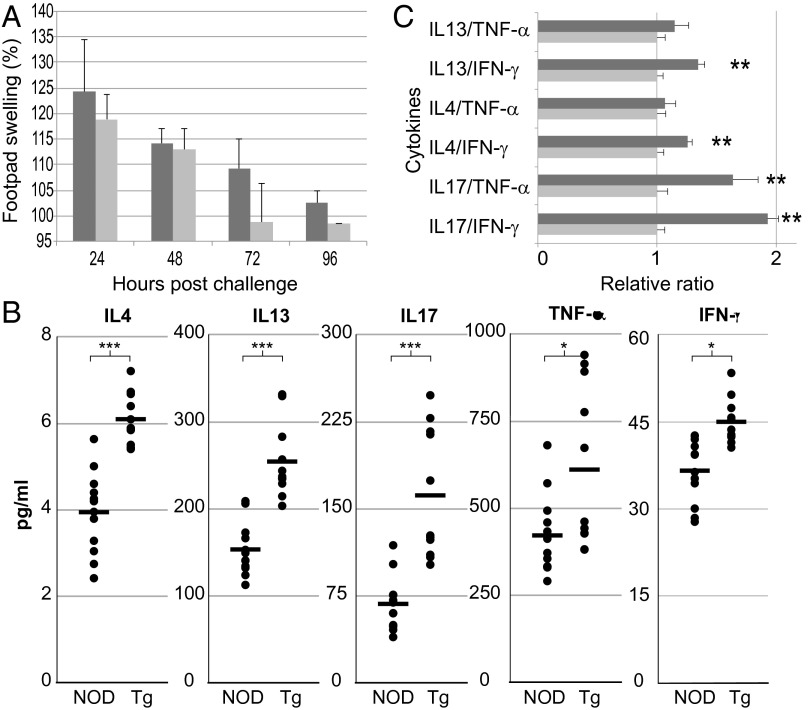
To obtain an overview of immune function in NOD-Tg bGH mice, we evaluated T-cell responses in a delayed type hypersensitivity (DTH) assay of sensitization and challenge with allogeneic splenocytes. NOD-Tg bGH mice and control littermates were sensitized by i.v. injection of C57BL/6 splenocytes and challenged 6 d later by inoculating splenocytes into the right hind footpad. Inflammation was measured every 24 h until remission by comparing thickness of the inoculated to the contralateral, vehicle-inoculated footpad. Both mouse groups reacted similarly, with no significant differences in inflammation grade or resolution time, with only a slight remission delay in the transgenic mice (Fig. 2A).
Fig. 2.
T-cell response in NOD-Tg bGH mice. (A) NOD-Tg bGH mice show standard DTH responses. Footpad swelling at several times postchallenge in NOD-Tg bGH (dark gray, n = 6) and NOD mice (light gray, n = 6). Swelling was calculated as the percentage of footpad thickness compared with the baseline at t = 0. Values shown are mean ± SD. Student t test showed no significant differences at any time. (B) Circulating cytokine levels in 4-mo-old NOD (n = 12) and NOD-Tg bGH (Tg) (n = 11) mice. Individual and mean values are shown. Student t test, *P < 0.05, ***P < 0.001. (C) Ratios between Th2/Th1 and Th17/Th1 profiles, assessed by circulating cytokines quantification, in NOD (light gray bars, n = 12) and NOD-Tg bGH (dark gray, n = 11) mice. Values have been normalized to NOD mice data. Student t test, **P < 0.01.
NOD-Tg bGH Mice Have Altered Serum Cytokine Levels.
In type 1 diabetes, a Th1-to-Th2 shift in the immune response is postulated to be protective for pancreatic islets (21). Indeed, the conversion of Th17 to Th1 is necessary to induce diabetes efficiently (22). To determine whether the GH-protective effect is associated with changes in circulating cytokines, we used a Luminex assay and detected higher peripheral blood levels of IFN-γ, IL-4, IL-13, IL-17A, and TNF-α in sera from NOD-Tg bGH mice than from NOD littermates (Fig. 2B). The Th2/Th1 and Th17/Th1 cytokine ratios suggested a GH-mediated bias against a Th1 response in the transgenic mice (Fig. 2C).
Preclinical Type 1 Diabetes Symptoms in NOD-Tg bGH Mice.
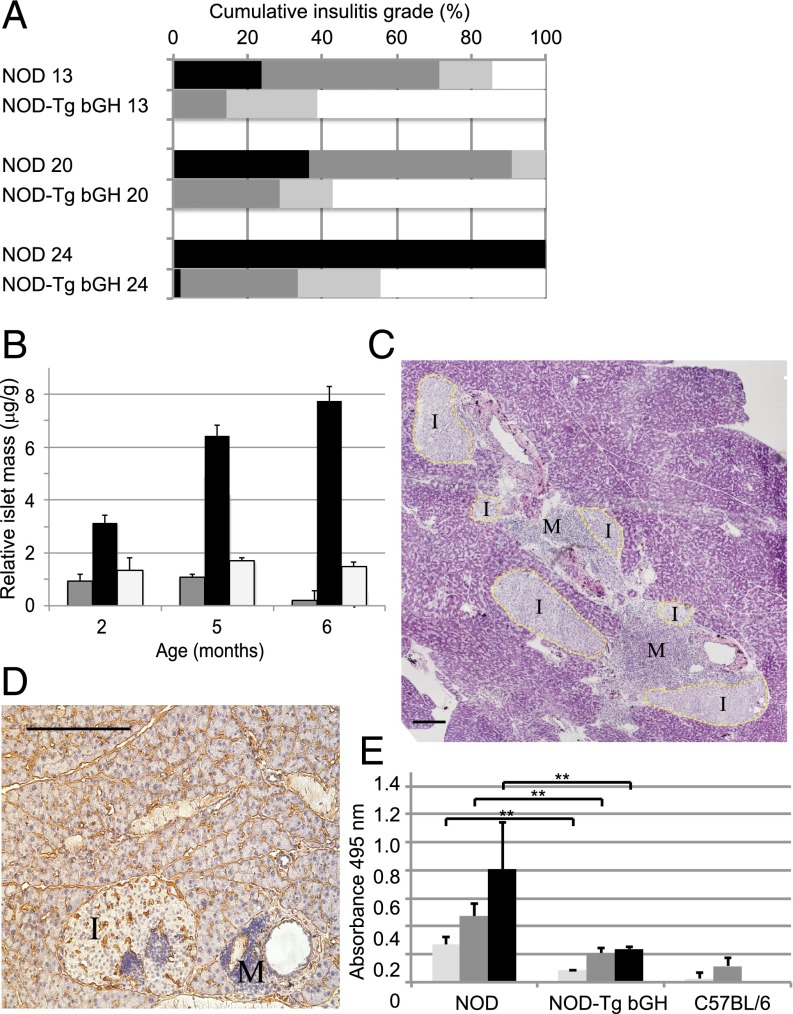
Early in type 1 diabetes progression, an inflammatory environment is established around the islets of Langerhans, usually interpreted as a result of anomalous macrophage activity during postnatal remodeling of the endocrine pancreas (23). Mononuclear cells are recruited into the pancreas and situate around the pancreatic islets shortly after weaning. The insular parenchyma is invaded progressively by immune cells, which destroy insulin-producing β-cells. On serial pancreas sections from NOD-Tg bGH, NOD littermates, and control C57BL/6 mice at different ages, we used H&E staining to evaluate islet size as well as insular and periinsular infiltration (Fig. 1 C–F). In NOD-Tg bGH mice, insular infiltration was delayed and most β-cells were conserved over time (Fig. 3A). The islet-cell mass thus diminished progressively in NOD mice, as predicted, whereas it increased continuously in NOD-Tg bGH mice (Fig. 3B). These data concur with the reported GHR-dependent islet hyperplasia (24) and the compensatory hyperinsulinemia mechanism associated with GH-dependent insulin resistance (25).
Fig. 3.
Type 1 diabetes symptoms are largely suppressed in NOD-Tg bGH mice. (A) Severity of insulitis and destructive lesions in NOD, NOD-Tg bGH, and C57BL/6 (control) mice. After H&E staining, ∼50–100 random islets per pancreas were evaluated. Three mice were analyzed for each genotype and age group (13, 20, and 24 wk). The degree of mononuclear cell infiltration was graded independently by two observers as follows: 0, normal (white); 1, mild periinsulitis (light gray); 2, severe periinsulitis (dark gray); or 3, insulitis (black; see Materials and Methods for details). (B) Islet mass estimated from percentage of endocrine area (relative to total pancreas surface) as a function of age for NOD (gray), NOD-Tg bGH (black), and C57BL/6 mice (white; n = 3 for each age group). (C and D) Late infiltration in islets from NOD-Tg bGH mice. Frozen pancreas sections of 7-mo-old NOD-Tg bGH mice. Islets (I) and infiltrates (M) are labeled. (Scale bar, 100 μm.) (C) Merged image of H&E staining showing giant coalescent islets. Original magnification, 6×. (D) Section stained with antilaminin antibody to identify the basal layer of pancreatic acini, blood vessels, and periinsular sheet of Schwann cells. Hematoxylin counterstain shows perivascular and islet infiltrates. Mononuclear infiltrates were surrounded by this laminin sheet and hence did not invade the islet parenchyma. Original magnification, 10×. (E) Evaluation of prodromal antiinsulin antibodies (NOD-Tg bGH, n = 5; NOD, n = 7; C57BL/6 control, n = 3) at 10 (light gray), 12 (dark gray), and 14 wk (black). ELISA for human insulin did not detect autoantibodies in NOD-Tg bGH mice. Background optical density was subtracted. Student t test, **P < 0.01.
Using immunohistochemistry and flow cytometry, we analyzed infiltrate composition in pancreata from 3- to 5-mo-old mice. Snap-frozen organs were sectioned and stained with anti-CD4, -CD8, -F4/80, -CD11c, and -B220 antibodies. Although individual variation was broad, the inflammation grade in NOD-Tg bGH mouse pancreas was lower than that of NOD mice (Fig. 3A). Infiltrate composition was nonetheless grossly similar, with a predominance of T cells, mainly CD4+ (Fig. S3). We observed no immune cell infiltration in NOD-Tg bGH islet parenchyma, even in older mice in which infiltration was massive, but always restricted to the islet periphery (Fig. 3C). This inflammation coincides with no apparent degradation of the periinsular laminin layer, which defines the basal lamina of the sheet of Schwann cells thought to be the first target of autoimmune attack (26) (Fig. 1 E and F). In some cases, we found intrainsular foci in mature mice (Fig. 3 C and D), although they must be considered perivascular, because they are surrounded by an intact laminin sheet.
Because type 1 diabetes is also characterized by development of a humoral response to islet antigens, we evaluated antiinsulin antibodies in prediabetic NOD-Tg bGH and control prediabetic NOD mice. Whereas NOD mice had high antiinsulin antibody titers, indicative that insulin is the primary antigen for type 1 diabetes in these mice (27), as it is in humans (28), NOD-Tg bGH mice had much lower titers (barely detectable even at 12 wk; Fig. 3E). Spleen B cells from NOD and NOD Tg-bGH mice were activated in vitro with anti-mouse IgM antibody (10 μg/mL, 180–360 min, 37 °C) alone or with exogenous GH (5 μg/mL); activation was similar, as demonstrated by flow cytometry using anti-CD69 and anti-CD86 antibodies (Fig. S4). These findings rule out B-cell activation defects in NOD Tg-bGH mice.
In addition to the maintenance of normal glycemia, the lack of antiinsulin antibodies and the absence of islet-infiltrating, putative antigen-specific CD4+ T cells in NOD-Tg bGH mice suggests that GH modulates the adaptive immune response in type 1 diabetes.
Circulating GH Levels Are Responsible for the Protective Phenotype.
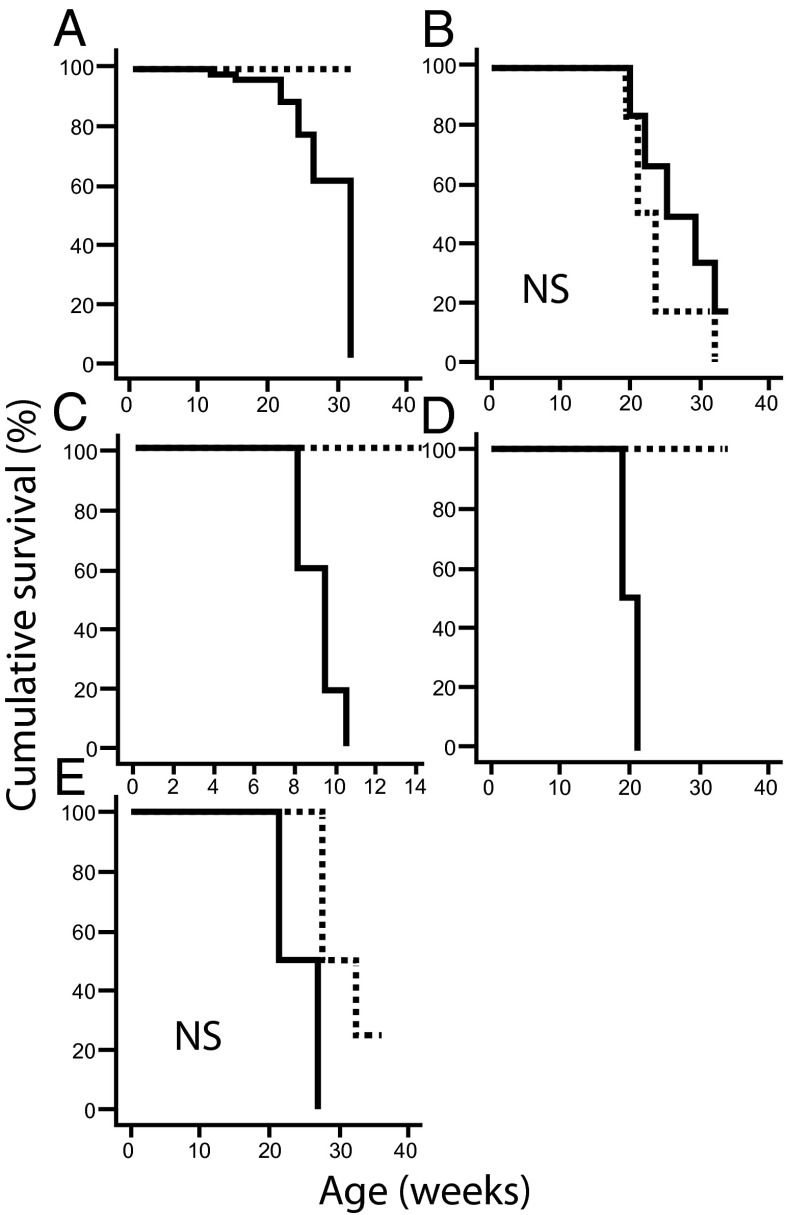
For detailed characterization of the role of the adaptive immune response in NOD-Tg bGH mice, we evaluated diabetogenic cell populations and suppressor cell activity. We transferred total splenocytes from NOD-Tg bGH or NOD mice into untreated or sublethally (7 Gy) irradiated NOD or NOD-Tg bGH mice. Splenocytes from NOD-Tg bGH mice did not protect untreated NOD mice from disease development (Fig. 4A), suggesting that NOD-Tg bGH regulatory cells had insufficient suppressive capacity in NOD mice. In addition, cells from the transgenic mice did not accelerate diabetes in sublethally irradiated NOD mice (Fig. 4B), indicating a lack of diabetogenic effector cells in the NOD-Tg bGH splenocyte population.
Fig. 4.
Kaplan–Meyer analysis for adoptive transfer protocols. (A) Seven-week-old NOD mice (continuous line, n = 4) were inoculated with 2 × 107 NOD-Tg bGH mouse splenocytes; inoculated NOD-Tg bGH mice were used as controls (dashed line). No delay in hyperglycemia was observed compared with the normal behavior of NOD mice. (B) Seven-week-old NOD mice (continuous line, n = 6) were sublethally irradiated and inoculated with 2 × 107 NOD-Tg bGH mouse splenocytes. No delay was observed in manifestation of overt diabetes compared with untreated NOD control mice (dashed line). (C) Six-week-old NOD-Tg bGH mice (dashed line, n = 5) were sublethally irradiated and inoculated with 2 × 107 diabetic NOD mouse splenocytes. Irradiated NOD littermates (continuous line, n = 4) were used as controls. Accelerated diabetes development was observed in NOD controls, whereas NOD-Tg bGH mice remained fully resistant. (D) The experiment in C was repeated using lethally irradiated NOD-Tg bGH mice (dashed line, n = 4). NOD mice (n = 2) were used as controls for accelerated diabetes development, and uninoculated mice (NOD, n = 2; NOD-Tg bGH, n = 2) as controls of lethality; all four untransferred mice died within 2 wk of irradiation. (E) NOD/SCID mouse sensitivity to diabetes development after splenocyte transfer (NOD, continuous line; NOD + NOD-Tg bGH, dashed line; 12 wk old, n = 4 for both groups). Log-rank test P < 0.05 was considered significant. NS, not significant.
Radiomimetic drugs trigger type 1 diabetes in NOD mice by targeting the CD4+CD25+FoxP3+ T-cell population and impairing their recovery in pancreas infiltrates (29). To determine the role of Treg cells in our model, we transferred splenocytes (2 × 107) from a pool of three overtly diabetic 6-mo-old NOD mice into 6-wk-old sublethally (7 Gy) irradiated NOD-Tg bGH mice. Diabetes did not develop in the recipients (Fig. 4C), whereas diabetes was accelerated in irradiated control NOD littermates. These data suggest a resistant suppressive mechanism in irradiated NOD-Tg bGH mice that blocked NOD effector cells.
Because Treg cells are reported to be relatively radioresistant (30), we transplanted diabetogenic splenocytes into lethally irradiated (12 Gy) NOD and NOD-Tg bGH recipients for a 3-wk radioprotection/accelerated diabetes assay. Recipient mice were rescued from lethality and, though NOD mice became hyperglycemic within 7–10 d, transgenic mice remained normoglycemic throughout the experiment (Fig. 4D). Untransferred mice of both genotypes, used as a lethality control, died during the first week. The results imply a suppressive mechanism for diabetogenic cells in NOD-Tg bGH mice, which are resistant even to a high dose of full-body irradiation. The relative radioresistance of Treg cells and monocytes might account for this suppression.
We tested whether transgenic splenocytes protect mice in an accelerated diabetes model. Two groups of 12-wk-old NOD/SCID (severe combined immunodeficiency) mice were inoculated i.v. with 2 × 107 splenocytes from a pool of two 5-mo-old diabetic NOD mice. One group also received 2 × 107 splenocytes from a pool of two 5-mo-old transgenic mice (Fig. 4E). All mice that received NOD splenocytes alone developed hyperglycemia by 4 wk posttransfer. The group inoculated with splenocytes from NOD + NOD-Tg bGH mice showed a slight, nonsignificant delay in hyperglycemia (log-rank test P = 0.094), suggesting loss of a hypothetical protective mechanism in the absence of circulating GH.
Suppressive Potential in NOD-Tg bGH Mice.
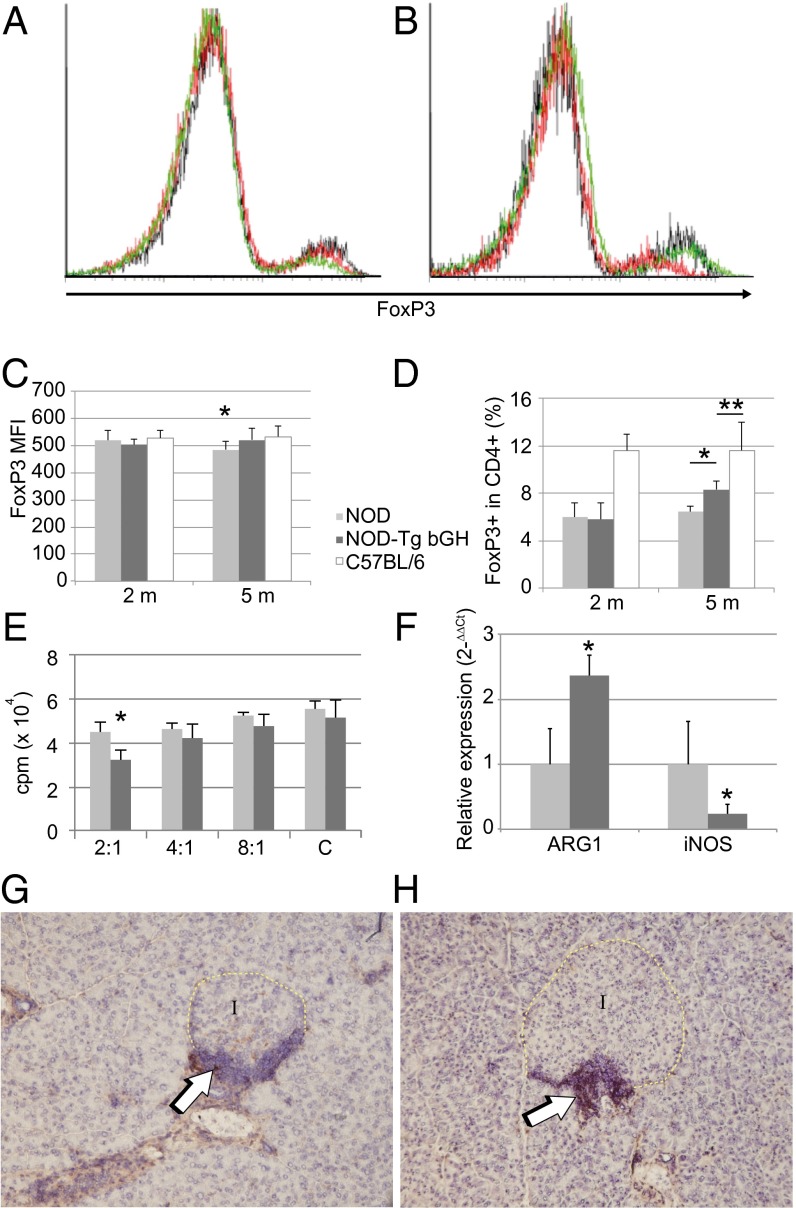
Although Treg cells are broadly implicated in type 1 diabetes development, their precise function during the prediabetic stage is not well understood (31–34). CD4+CD25+ Treg cells control disease progression through various potential mechanisms, inhibiting activation, proliferation, and/or migration of islet-specific T cells in lymph nodes and in pancreas (35). Because Treg cell suppressive potential is associated with FoxP3 levels (36), we used flow cytometry to determine FoxP3 expression on CD4+CD25+ peripheral blood lymphocytes from C57BL/6, NOD, and NOD-Tg bGH mice at 2 mo of age, before hyperglycemia was detected (Fig. 5A), and at 5 mo, when NOD mice were hyperglycemic (Fig. 5B). We found no differences in FoxP3 expression in any 2-mo-old mice (Fig. 5 A and C), whereas CD4+CD25+ lymphocytes from 5-mo-old NOD mice showed a clear reduction in FoxP3 levels compared with those from B6 mice; this down-regulation was not observed for NOD-Tg bGH CD4+CD25+ Treg cells (Fig. 5 B and C). The results suggest a GH effect on the maintenance of Treg cell activity.
Fig. 5.
Bias toward a regulatory phenotype in NOD-Tg bGH mice. Down-regulation of FoxP3 in NOD Treg cells. Blood samples from 2-mo-old (A) and 5-mo-old mice (B) of C57BL/6, NOD, and NOD-Tg bGH genotypes were labeled for CD4, CD25, and FoxP3; Treg cells were gated by light-scatter properties (forward scatter and side scatter), CD25 and CD4. One representative sample of nine is shown. C57BL/6, black line; NOD, red line; NOD-Tg bGH, green line. (C) FoxP3 expression in CD4+CD25+ cells assessed by flow cytometry. FoxP3 mean fluorescence intensity in blood samples from 2- and 5-mo-old mice. (D) Relative abundance of FoxP3+ cells in the blood CD4+ population, assessed by flow cytometry, at 2 and 5 mo. (C and D) NOD (light gray), NOD-Tg bGH (dark gray), and C57BL/6 (white) mice mean values + SD for four experiments (total n = 9 for each mouse group). Student t test, *P < 0.5; **P < 0.1. (E) Suppressive activity of CD4+CD25+ splenocytes from NOD (light gray) and NOD-Tg bGH (dark gray) mice at various ratios of CD4+CD25− effector cells (letter C indicates no suppressor cells added). [3H]thymidine incorporation after coculture is shown as a percentage of the value for cultured pure effector cells. (F) M1 and M2 macrophage marker expression. Quantitative RT-PCR was used to quantify relative levels of NOS2 and arginase-1 mRNA in pancreatic lymph nodes from 3-mo-old mice. Values (2−∆∆Ct) are relative to the mean level of each message in samples from NOD mice. (G and H) Immunohistochemistry showing arginase-1 expression within the periinsular infiltrate in young mice (8 wk). Anti–arginase-1 antibody was visualized with horseradish peroxidase and diaminobenzidine (arrows); hematoxylin was used as counterstain (G, NOD; H, NOD-Tg bGH). Original magnification, 20×.
In 2-mo-old mice, the percentage of CD4+CD25+FoxP3+ cells was higher in C57BL/6 than in NOD-Tg bGH mice or NOD littermates, with no difference between the last two groups. The percentage of CD4+CD25+FoxP3+ cells was higher in 5-mo-old NOD-Tg bGH mice than in NOD littermates, although in both cases it was lower than that in C57BL/6 mice (Fig. 5D), which suggests higher suppressive T-cell activity in NOD-Tg bGH than in NOD mice.
We used an in vitro suppression assay to test for a correlation between reduced FoxP3 expression on CD4+CD25+ lymphocytes and a reduction in their suppressive capacity. Coculture of CD4+CD25− NOD T cells with varying proportions of CD4+CD25+ T cells from 5-mo-old NOD or NOD-Tg bGH mice led to slight but significantly higher suppressive activity by NOD-Tg bGH-derived cells (Fig. 5E).
These results indicate that GH participates in maintenance of the suppressive potency of Treg cells as well as of the relatively high FoxP3 levels in CD4+CD25+ cells. This effect might be potentiated in vivo, because NOD-Tg bGH cells are continuously exposed to high levels of circulating GH. Treg cells in vivo might also be activated locally by antigen-presenting cells (37) specifically charged with islet antigens, which were absent in our in vitro experiments.
GH Effect on Macrophage Polarization.
Macrophages have a key role in pancreas remodeling (23) and are also present in pancreas infiltrate in NOD and NOD-Tg bGH mice. There are two main macrophage types: M1 or inflammatory macrophages, characterized by high NOS2 (inducible nitric oxide synthase) expression, and M2 or anti-inflammatory macrophages characterized by arginase-1 expression. We used quantitative real-time PCR to quantify M1/M2 macrophage markers in pancreatic lymph nodes from 3-mo-old NOD and NOD-Tg bGH mice. We found lower NOS2 and higher arginase-1 RNA levels in nodes from NOD-Tg bGH than from NOD mice (Fig. 5F). These data were confirmed by immunohistochemical identification of arginase-1–producing cells in NOD and NOD-Tg bGH mouse pancreas (Fig. 5 G and H). The results indicate a GH effect on macrophage polarization toward the M2 phenotype in NOD-Tg bGH mice.
GH Modulates Th17 Cell Plasticity.
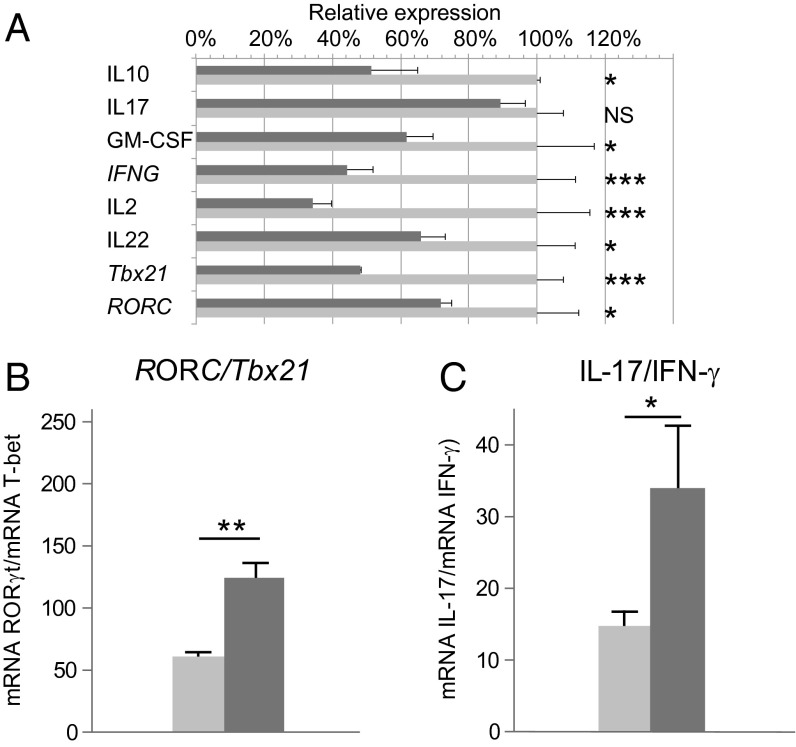
Diabetes development is reported to be associated with acquisition of a Th1-like phenotype by Th17 cells (38), which then express T-bet and secrete IFN-γ. We tested the GH effect on Th17/Th1 plasticity using RT-PCR to determine IL-17, IFN-γ, IL-2, IL-22, and GM-CSF mRNA levels in total pancreas of age-matched NOD-Tg bGH and prediabetic NOD mice, as well as of ROR-γT and T-bet transcription factors as specific markers of Th17 and Th1 polarization, respectively (Fig. 6A). To avoid interference due to different numbers of infiltrating cells in the pancreas, we compared the ratio for messages characteristic of Th17 and Th1 cells, which is more indicative of the relative abundance of nonpathogenic Th17 cells than are individual Th17 or Th1 values (39). The results showed a higher RORC/Tbx21 (Fig. 6B) and IL-17/IFN-γ (Fig. 6C) ratios in NOD-Tg bGH mouse pancreas. In addition, we detected higher IFN-γ, IL-2, IL-22, and GM-CSF mRNA levels in NOD mouse pancreas (Fig. 6A). These data indicate a significant reduction of pathogenic Th17 cells infiltration and their plasticity to Th1 in NOD-Tg bGH mouse pancreas.
Fig. 6.
Cytokine expression in the pancreas of NOD prediabetic and NOD-Tg bGH diabetes-resistant mice. RNA from 13-wk-old mouse whole pancreas was quantified by quantitative RT-PCR. Student t test (n = 6); *P < 0.05; **P < 0.01; ***P < 0.001. (A) Relative expression (2−∆∆Ct) of characteristic Th1 and Th17 transcription factors and cytokines in NOD and NOD-Tg bGH mice pancreas, relative to mean values in NOD. The ratios between mRNA expression of RORC/Tbx21 (B) and IL-17/IFN-γ (C) in NOD (light gray) and NOD-Tg bGH (dark gray) mice are shown.
Discussion
Type 1 diabetes is a multifactorial disease caused by the concurrence of genetic and environmental factors that include infectious agents, diet, and illness (40). Endocrinopathies characterized by chronic overproduction of hormones whose action opposes that of insulin, such as epinephrine, glucagon, cortisol, or GH, generally cause diabetes by triggering insulin resistance (41); nonetheless, very little is known of their potential to influence autoimmune diabetes. Here we observed that a transgenic mouse strain on the NOD background that expresses bGH under the control of the rat PEPCK promoter (NOD-Tg bGH) did not develop type 1 diabetes. The pancreatic inflammatory phenotype characteristic of the NOD background was severely reduced in the presence of GH. These mice also showed almost complete suppression of the adaptive immune response. We detected no prodromal antiislet antibody production or diabetogenic cell expansion; hence, there was no islet destruction in these mice.
There is considerable evidence for distinct GH effects on pancreatic β-cells (16), some direct and others through its main mediator, IGF1 (42). Exogenous GH in rat islet cultures thus stimulates DNA synthesis and insulin production (43); IGF1 and GH signaling have mitogenic effects on INS-1 cells (44). We detected a large β-cell mass in NOD-Tg bGH mice that correlated with the higher proliferation rate and lower apoptotic β-cell numbers detected in the pancreas of these mice, which could explain in part the lack of hyperglycemia in these mice. By activating the JAK/STAT pathway, GH can also stimulate β-cell survival. The JAK/STAT-activated suppressors of cytokine signaling block damage triggered by cytokines such as IFN-γ or TNF-α (45), and expression of a constitutively active form of STAT5b has a protective effect on β-cells in a model of streptozotocin-induced diabetes (46).
NOD-Tg bGH mice showed periinsulitis, although we found no sign of degradation of the periinsular laminin layer, and no antiinsulin antibodies in serum. The results confirm a GH effect on the immune system in addition to its effect on β-cells.
Although differences in immune cell activity are associated with high levels of circulating GH (47), we detected no major differences in any of the circulating cell populations in either mouse type; B220+, CD3+, CD4+, CD8+, CD11b+, or Gr1+ cell numbers were similar, as were the lymph node T-cell activation markers (CD25, CD69, CD44, and CD62L). This observation concurs with the similar response in a DTH assay of NOD and NOD-Tg bGH mice to immunization with allogeneic splenocytes, ruling out general defects in the T-cell response and/or antigen presentation. It is nonetheless postulated that APC, antigen presenting cell, defects are responsible for the lack of adequate regulatory potential in NOD mice (48). It could be argued that the halt observed at the periinsulitis checkpoint is due to a reduced Th2 response and Ig production in NOD-Tg bGH mice. Overexpression of bGH in C57BL/6 mice alters the humoral response to egg albumin by reducing Th2 cytokine production (1). We did not observe such Th2 defects on the NOD background, as assessed by measurement of circulating cytokine levels. In any case, antiislet antibodies are not directly linked to β-cell destruction, and the role of β-cells appears to be restricted to their antigen-presenting activity (49). β-cells from NOD and NOD-Tg bGH mice showed no defects in their in vitro activation by anti-IgM antibodies. We previously observed that after stimulation with conventional antigens, the antigen response is reduced in Tg bGH mice, with a IgG1 to IgG2 isotype shift (1). These findings implicate GH in altering T-cell function.
Transferred NOD-Tg bGH splenocytes did not protect NOD mice from diabetes, and NOD splenocytes did not promote diabetes when transferred into NOD-Tg bGH mice, suggesting that control of T-cell responses in NOD-Tg bGH mice is dependent on circulating GH levels.
At 5 mo of age, with a well-developed inflammatory environment, CD4+CD25+ Treg cell numbers are maintained at stable low levels in NOD mice, whereas they rise in NOD-Tg bGH mice. In NOD-Tg bGH mice, we did not observe the down-regulation of FoxP3 expression found in hyperglycemic NOD mice. Because FoxP3 expression is directly linked to the regulatory action of Treg cells (50), it is thus possible that GH regulates FoxP3 activation via STAT5b or STAT3. A single point mutation in STAT5b, which encodes a transcription factor involved in GH signaling (51), limits FoxP3 expression by Treg cells (11). Sustained activation of STAT3 (another transcription factor involved in signaling through the GHR) is needed to maintain FoxP3 expression by Treg cells (52). Through STAT5B or STAT3 activation, GH might thus increase FoxP3 levels, regulating Treg cell activity.
In the adoptive transfer experiments, sublethal and even lethal irradiation did not alter the protective effect of GH expression in NOD-Tg bGH mice. GH promotes radioprotection in a variety of cell types, and radiation sensitivity differs in some immune system niches. For example, GH and IGF1 enhance hematopoietic stem cell radioresistance and proliferation (53). These resistance mechanisms nonetheless appear to be insufficient for the rapid expansion of suppressive cells that would be needed to control disease in an accelerated diabetes model. It is more likely that GH increases the radioresistance of the Treg cell population; lethally irradiated wild-type hosts transferred with scurfy bone marrow cells did not develop autoimmune disease, due to suppression of sf-derived T cells by radioresistant host FoxP3+ Treg cells (30).
We tested the in vitro suppressive capacity of Treg cells from NOD and NOD-Tg bGH mice and found slight but significant differences. These differences might be considered insufficient to explain complete resistance to diabetes development; nonetheless, T-cell differentiation and activity are dependent on antigen-presenting cell type and the microenvironment in which presentation occurs, and Treg cells might be induced only locally by M2 macrophages (37). Macrophages have two distinct phenotypes (54): inflammatory (M1) macrophages participate in antigen recognition and secretion of inflammatory cytokines, and noninflammatory (M2) macrophages are involved in tissue repair and remodeling (55). M1 polarization is induced by IFN-γ and characterized by high NOS2 expression and by secretion of proinflammatory cytokines IL-1 and IL-12, whereas M2 are induced by IL-4 and IL-13 and are characterized by high arginase-1 expression and by IL-10 secretion. Our data for pancreatic lymph nodes and pancreata from prediabetic NOD mice showed the presence of M1 macrophages (arginase-1lowNOS2high), whereas age-matched NOD-Tg bGH mouse macrophages had an M2 phenotype (arginase-1highNOS2low). This difference might be the result of the high circulating levels of IL-17 (56), and could also influence increased local Treg cell activity.
Autoimmune diabetes is a well-characterized Th1 pathology. We detected high IFN-γ and IL-2 mRNA levels in NOD mouse pancreas, which were lower in NOD-Tg bGH pancreas. Both mouse models also had high IL-17 mRNA levels, suggesting Th17 cell involvement in type 1 diabetes. Th17 cells are found in some autoimmune diseases such as experimental autoimmune encephalomyelitis (57) and rheumatoid arthritis (58); NOD and NOD-Tg bGH mouse pancreas also showed high ROR-γT mRNA levels.
Recent evidence suggests that depending on the microenvironment, Th17 cells can alter their differentiation program to induce protective or proinflammatory responses (59, 60). We found a significant reduction in mRNA levels of IFN-γ, IL-2, and GM-CSF, characteristic mediators for Th17 pathogenic cells (59), in NOD-Tg bGH pancreas compared with that of NOD mice. Although, increasing evidences points to the role of Th17 cells in NOD mice, it seems that the conversion of this cell subset into Th1 is more important (22, 38). How the inflammatory microenvironment modulates this Th17 differentiation is not completely understood, although our results suggest that GH maintains a nonpathogenic profile of Th17 cells and reduces their Th1 potential in the pancreas.
We cannot rule out that GH might also contribute to transgenic islet resistance to immune attack via STAT3 activation. STAT3 activation in Treg cells, associated with type 1 diabetes resistance in NOD mice (61), controls the macrophage IL-10–mediated anti-inflammatory response (62) and is an important survival factor in β-cells (63, 64). Our data show that GH-mediated interference in type 1 diabetes development involves an increase in β-cell mass, protection of the periinsular laminin layer, and a direct effect on immune cells—mainly macrophages, Th17, and possibly Treg cells. These results demonstrate the importance of endocrine control of immune functions, and indicate that therapies based on GH analogs and/or their signaling cascades should be considered for treatment of autoimmune diabetes.
Materials and Methods
Mice.
Mice transgenic for bGH under the control of the phosphoenolpyruvate carboxykinase promoter on a C57BL/6J × C3H/J hybrid background (65) were crossed on the NOD background until NOD polymorphic alleles were stabilized (F0; NOD-Tg PEPCK-bGH/Ccnb), as assessed by the length of single sequence repeats (66). The transgenic strain was maintained by continuous backcrosses on NOD females. Experimental results were obtained from F5 onward, always using strict littermates. Mice were fed a standard laboratory rodent diet (Global Diet 2918, Harlan Iberica; 18.5% protein, 5.5% oils and fat) and tap water ad libitum. The mice were monitored twice a week with Accutrend kits (Roche Diagnostics) for development of hyperglycemia, and declared diabetic when glucose was >200 mg/dL in two consecutive measurements. Overtly diabetic mice were killed. Mice were handled according to national and European Union guidelines, and experiments were approved by the Comité Ético de Experimentación Animal, Centro Nacional de Biotecnología.
Immunohistochemistry.
Pancreata were embedded in optimal cutting temperature freezing medium (Sakura) and snap-frozen in cooled isopentane. Sections (7 μm) were cut, air-dried, and fixed in cold acetone. When stored at −80 °C, sections were postfixed in ethanol/acetone (1:3) before staining. Primary antibodies were guinea pig anti-porcine insulin antibody (Dako), anti–arginase-1 (BD Biosciences), rabbit anti-laminin (Sigma), and rabbit anti-Ki67 (Novocastra antibodies); immunodetected with the tyramide signal amplification indirect staining kit (Perkin-Elmer); and visualized with diaminobenzidine. Hematoxylin was used for counterstaining.
β-Cell Mass Determination and Infiltration Level Count.
Pancreata from mice of different ages (three per group) were extracted, weighed, formaldehyde-fixed, and paraffin-embedded. For quantification, one 5-μm section was analyzed every 100 μm. Sections were H&E stained and photographed at 10× magnification. Composite images were generated with the photomerge function of Adobe Photoshop CS5. On merged images, a grid was superimposed and β-cell mass calculated from the ratio of intersections in endocrine vs. total pancreas and pancreas weight (67). Separation between grid lines was 50 μm. The same slides were used to determine infiltration level in islets. Insulitis was scored by the following criteria: insulitis (grade 3), infiltration in the islet parenchyma; severe periinsulitis (grade 2), three or more rows of mononuclear cell infiltrate surrounding the islet; mild periinsulitis (grade 1), less than three rows of periinsular infiltrating cells, and no insulitis (grade 0), absence of cell infiltration. Apparent intraislet area was sometimes counted as periinsular when the insular parenchyma was not invaded, as determined by the integrity of Schwann cell basal lamina.
Delayed Type Hypersensitivity.
C57BL/6J splenocytes were isolated by mechanical disaggregation and erythrocyte lysis with NH4Cl, washed once with PBS + 0.1% BSA and twice with PBS. The 6-wk-old mice were sensitized by i.v. injection of 2 × 105 C57BL/6J splenocytes, and challenged on day 6 in the right footpad with 1.5 × 107 cells in 50 μL PBS. Control left footpads received 50 μL PBS. Footpad thickness was measured with a vernier caliper (Mitutoyo Japan) at 24, 48, and 72 h after challenge. Results of footpad swelling were calculated as the difference between challenged vs. prechallenged footpad, expressed as a percentage. Student t test P values >0.05 were considered nonsignificant.
Adoptive Transfer.
Donor spleens were processed as above. The indicated number of splenocytes was injected i.v. into the tail of recipient mice. When indicated, host mice were previously irradiated lethally (12 Gy) or sublethally (7 Gy) with a single full-body dose from a 137Cs source. SPSS Statistics software was used for statistical analyses.
Cytokine Measurement.
Circulating cytokines were quantified in serum with a Bio-Plex kit and analyzer (BioRad). Serum samples were stored at −80 °C until use. Antiinsulin and total antibodies in serum were measured by standard ELISA at 495 nm. Plates were coated with 100 μL per well with human insulin (20 μg/mL; Novo-Nordisk) or goat anti-mouse Ig kappa chain (50 μg/mL) in PBS. Plates were incubated (overnight, 4 °C), washed with PBS, 0.1% Tween 20, and blocked with 1% BSA, 0.05% Tween-20 in PBS [1 h, room temperature (RT)]. Serial serum dilutions were added to washed plates and incubated (2 h, RT); after washing, peroxidase-conjugated anti-Ig (1:2,000; Dako) was added. Plates were incubated (1 h, RT), washed, and 100 μL orthophenylenediamine substrate solution (Sigma) was added. The reaction was terminated after 20 min and measured.
Cell Purification and Flow Cytometry.
To prepare single-cell suspensions, spleens and lymph nodes were harvested and minced on a 40-μm nylon mesh in RPMI medium 1640 (Lonza) supplemented with 10% FBS, 2 mM l-glutamine, and 50 μg/mL penicillin/streptomycin. For APC preparations, spleens were predigested with collagenase A and DNase I. CD11c+, CD4+CD25+, and CD4+CD25− cell populations were enriched by CD11c Microbeads Mouse (Miltenyi Biotech), Dynabeads Flow Comp Mouse CD4+CD25+ Treg Cells Kit (Invitrogen), and an AutoMACS Cell Sorter (Miltenyi Biotech). When required, murine B cells were purified using mouse pan-T Dynabeads (Invitrogen) and T cells with mouse T-cell negative isolation kit (Dynal). Purity of all cell preparations was routinely >95%. Blood samples were lysed with VersaLyse (Beckman Coulter).
Single-cell suspensions of lymphoid organs or blood leukocytes were prepared and blocked with anti-CD16/32 (BD Pharmingen) to impede Fc-mediated nonspecific antibody binding. Samples were stained with antibody conjugates by a standard procedure, using FITC anti-CD25, FITC anti-CD11b, and SPRD anti-Gr1 (Pharmingen); SPRD anti-CD4 (eBiosciences); FITC anti-CD3, FITC anti-CD8, PE anti-CD44, FITC anti-CD69, FITC anti-CD45, and APC anti-B220 (Beckman Coulter); PE anti-CD86 (BioLegend); and PE anti-CD62L (Southern). FoxP3 expression was determined after permeabilization and intracellular staining with a PE-labeled antibody (FoxP3 staining set; eBiosciences). When necessary, naïve B cells (92–95% pure) were first activated with 10 μg/mL goat anti-mouse IgM Ab (Jackson ImmunoResearch; 3 or 6 h, 37 °C), alone or with exogenous human GH (5 μg/mL, Genotonorm; Pfizer). Stained samples were analyzed on a flow cytometer (Cytomics FC 500; Beckman Coulter). FACS data were analyzed with FlowJo and CytoSpec software.
In Vitro Suppression Assay.
CD4+CD25− NOD splenocytes (5 × 104) were cocultured with variable ratios of CD4+CD25+ cells (2:1, 4:1, and 8:1) and with NOD spleen CD11c+ cells (5 × 104; previously γ-irradiated, 15 Gy) in the presence of anti-CD3 (1 μg/mL). Cultures were prepared in triplicate in U-bottom 96-well plates (Nunclon Surface) with RPMI-1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 1 mM pyruvate, and 50 μM β-mercaptoethanol (72 h, 37 °C), and cells were pulsed (16 h) with 1 μCi [3H]thymidine (Perkin-Elmer) per well. The 3H incorporation was measured by liquid scintillation spectrometry using CytoScint mixture (MP Biomedical) and a 1450 MicroBeta counter (Perkin-Elmer).
Semiquantitative Real Time PCR.
cDNA sequences were obtained from the GenBank database. PCR primers were designed from the cDNA sequences using Primer-BLAST (68) (Table S2). RNA (5 μg) was used for reverse transcription. cDNA was obtained by SuperScript II reverse transcriptase (Invitrogen). Then cDNA was amplified by PCR analysis, using Power SYBR Green PCR Master Mix (Applied Biosystems), 0.3 μM of primers, and three serial dilutions of RT products. Triplicate samples were quantified using the ABI Prism HT7900 sequence detection system (Applied Biosystems). For relative quantification, we used the equation 2−ΔΔCt. We normalized each sample with β-actin (ΔCt), and ΔΔCt represents the difference between the Ct from each gene expression of NOD at 13 wk and each datum.
Supplementary Material
Acknowledgments
We are grateful for the gift of Genotonorm (Pfizer España). We thank R. Barroso and G. Cascio for technical support, L. Gómez for animal handling, C. Bastos for secretarial assistance, and C. Mark for editorial assistance. Support for this work was provided by Comunidad de Madrid Contract S2011/BMD-2502 (to R.V.); a La Caixa Fellowship (to D.K.); Spanish Ministry of Science and Innovation Grant SAF 2011-27370; European Union FP7-integrated project Masterswitch 223404; Fondo de Investigación Sanitaria, Instituto de Salud Carlos III RD12/009/009 and RD12/009/002; and the Comunidad de Madrid S2010/BMD-2350.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314985110/-/DCSupplemental.
References
- 1.Gonzalo JA, et al. Enterotoxin septic shock protection and deficient T helper 2 cytokine production in growth hormone transgenic mice. J Immunol. 1996;157(8):3298–3304. [PubMed] [Google Scholar]
- 2.Cross RJ, Bryson JS, Roszman TL. Immunologic disparity in the hypopituitary dwarf mouse. J Immunol. 1992;148(5):1347–1352. [PubMed] [Google Scholar]
- 3.Murphy WJ, Durum SK, Anver MR, Longo DL. Immunologic and hematologic effects of neuroendocrine hormones. Studies on DW/J dwarf mice. J Immunol. 1992;148(12):3799–3805. [PubMed] [Google Scholar]
- 4.Dardenne M, Mello-Coelho V, Gagnerault MC, Postel-Vinay MC. Growth hormone receptors and immunocompetent cells. Ann N Y Acad Sci. 1998;840:510–517. doi: 10.1111/j.1749-6632.1998.tb09589.x. [DOI] [PubMed] [Google Scholar]
- 5.Postel-Vinay MC, de Mello Coelho V, Gagnerault MC, Dardenne M. Growth hormone stimulates the proliferation of activated mouse T lymphocytes. Endocrinology. 1997;138(5):1816–1820. doi: 10.1210/endo.138.5.5108. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida A, Ishioka C, Kimata H, Mikawa H. Recombinant human growth hormone stimulates B cell immunoglobulin synthesis and proliferation in serum-free medium. Acta Endocrinol (Copenh) 1992;126(6):524–529. doi: 10.1530/acta.0.1260524. [DOI] [PubMed] [Google Scholar]
- 7.Merchav S, Tatarsky I, Hochberg Z. Enhancement of human granulopoiesis in vitro by biosynthetic insulin-like growth factor I/somatomedin C and human growth hormone. J Clin Invest. 1988;81(3):791–797. doi: 10.1172/JCI113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takagi K, Suzuki F, Barrow RE, Wolf SE, Herndon DN. Recombinant human growth hormone modulates Th1 and Th2 cytokine response in burned mice. Ann Surg. 1998;228(1):106–111. doi: 10.1097/00000658-199807000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiFedele LM, et al. Tumor necrosis factor alpha blockade restores growth hormone signaling in murine colitis. Gastroenterology. 2005;128(5):1278–1291. doi: 10.1053/j.gastro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Han X, et al. Tumour necrosis factor alpha blockade induces an anti-inflammatory growth hormone signalling pathway in experimental colitis. Gut. 2007;56(1):73–81. doi: 10.1136/gut.2006.094490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murawski MR, Litherland SA, Clare-Salzler MJ, Davoodi-Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: Implications for the NOD STAT5B mutation in diabetes pathogenesis. Ann N Y Acad Sci. 2006;1079:198–204. doi: 10.1196/annals.1375.031. [DOI] [PubMed] [Google Scholar]
- 12.King C, Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS ONE. 2011;6(2):e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard KM. Gender differences in autoimmunity associated with exposure to environmental factors. J Autoimmun. 2012;38(2-3):J177–J186. doi: 10.1016/j.jaut.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driver JP, Serreze DV, Chen Y-G. Mouse models for the study of autoimmune type 1 diabetes: A NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33(1):67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 15.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen JH, Svensson C, Galsgaard ED, Møldrup A, Billestrup N. Beta cell proliferation and growth factors. J Mol Med (Berl) 1999;77(1):62–66. doi: 10.1007/s001090050302. [DOI] [PubMed] [Google Scholar]
- 17.McGrane MM, et al. Developmental regulation and tissue-specific expression of a chimaeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic animals. J Reprod Fertil Suppl. 1990;41:17–23. [PubMed] [Google Scholar]
- 18.Olsson B, et al. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146(2):920–930. doi: 10.1210/en.2004-1232. [DOI] [PubMed] [Google Scholar]
- 19.Vasavada RC, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem. 2000;275(20):15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 20.Smaniotto S, et al. Growth hormone modulates thymocyte development in vivo through a combined action of laminin and CXC chemokine ligand 12. Endocrinology. 2005;146(7):3005–3017. doi: 10.1210/en.2004-0709. [DOI] [PubMed] [Google Scholar]
- 21.Lin MS, et al. A multivalent vaccine for type 1 diabetes skews T cell subsets to Th2 phenotype in NOD mice. Immunol Res. 2011;50(2-3):213–220. doi: 10.1007/s12026-011-8215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charré S, et al. Abnormalities in dendritic cell and macrophage accumulation in the pancreas of nonobese diabetic (NOD) mice during the early neonatal period. Histol Histopathol. 2002;17(2):393–401. doi: 10.14670/HH-17.393. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, et al. Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121(6):2422–2426. doi: 10.1172/JCI45027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valera A, et al. Glucose metabolism in transgenic mice containing a chimeric P-enolpyruvate carboxykinase/bovine growth hormone gene. FASEB J. 1993;7(9):791–800. doi: 10.1096/fasebj.7.9.8330686. [DOI] [PubMed] [Google Scholar]
- 26.Winer S, et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat Med. 2003;9(2):198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent SC, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435(7039):224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 29.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu N, Hori S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc Natl Acad Sci USA. 2007;104(21):8959–8964. doi: 10.1073/pnas.0702004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Alise AM, et al. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc Natl Acad Sci USA. 2008;105(50):19857–19862. doi: 10.1073/pnas.0810713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171(8):4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 33.Kim D-H, et al. Inhibition of autoimmune diabetes by TLR2 tolerance. J Immunol. 2011;187(10):5211–5220. doi: 10.4049/jimmunol.1001388. [DOI] [PubMed] [Google Scholar]
- 34.Tarbell KV, Yamazaki S, Steinman RM. The interactions of dendritic cells with antigen-specific, regulatory T cells that suppress autoimmunity. Semin Immunol. 2006;18(2):93–102. doi: 10.1016/j.smim.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 35.You S, et al. Immunoregulatory pathways controlling progression of autoimmunity in NOD mice. Ann N Y Acad Sci. 2008;1150:300–310. doi: 10.1196/annals.1447.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manirarora JN, Kosiewicz MM, Parnell SA, Alard P. APC activation restores functional CD4(+)CD25(+) regulatory T cells in NOD mice that can prevent diabetes development. PLoS ONE. 2008;3(11):e3739. doi: 10.1371/journal.pone.0003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savage NDL, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181(3):2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 38.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119(3):565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 40.Peng H, Hagopian W. Environmental factors in the development of Type 1 diabetes. Rev Endocr Metab Disord. 2006;7(3):149–162. doi: 10.1007/s11154-006-9024-y. [DOI] [PubMed] [Google Scholar]
- 41.Yuen KC, Chong LE, Riddle MC. Influence of glucocorticoids and growth hormone on insulin sensitivity in humans. Diabet Med. 2013;30(6):651–663. doi: 10.1111/dme.12184. [DOI] [PubMed] [Google Scholar]
- 42.Hill DJ, Petrik J, Arany E, McDonald TJ, Delovitch TL. Insulin-like growth factors prevent cytokine-mediated cell death in isolated islets of Langerhans from pre-diabetic non-obese diabetic mice. J Endocrinol. 1999;161(1):153–165. doi: 10.1677/joe.0.1610153. [DOI] [PubMed] [Google Scholar]
- 43.Whittaker PG, Taylor KW. Direct effects of rat growth hormone in rat islets of langerhans in tissues culture. Diabetologia. 1980;18(4):323–328. doi: 10.1007/BF00251014. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes CJ. IGF-I and GH post-receptor signaling mechanisms for pancreatic beta-cell replication. J Mol Endocrinol. 2000;24(3):303–311. doi: 10.1677/jme.0.0240303. [DOI] [PubMed] [Google Scholar]
- 45.Flodström-Tullberg M, et al. Target cell expression of suppressor of cytokine signaling-1 prevents diabetes in the NOD mouse. Diabetes. 2003;52(11):2696–2700. doi: 10.2337/diabetes.52.11.2696. [DOI] [PubMed] [Google Scholar]
- 46.Jackerott M, et al. STAT5 activity in pancreatic beta-cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes. 2006;55(10):2705–2712. doi: 10.2337/db06-0244. [DOI] [PubMed] [Google Scholar]
- 47.Clark R. The somatogenic hormones and insulin-like growth factor-1: Stimulators of lymphopoiesis and immune function. Endocr Rev. 1997;18(2):157–179. doi: 10.1210/edrv.18.2.0296. [DOI] [PubMed] [Google Scholar]
- 48.Alard P, et al. Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes. 2006;55(7):2098–2105. doi: 10.2337/db05-0810. [DOI] [PubMed] [Google Scholar]
- 49.Serreze DV, et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161(8):3912–3918. [PubMed] [Google Scholar]
- 50.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 51.Gebert CA, Park SH, Waxman DJ. Regulation of signal transducer and activator of transcription (STAT) 5b activation by the temporal pattern of growth hormone stimulation. Mol Endocrinol. 1997;11(4):400–414. doi: 10.1210/mend.11.4.9904. [DOI] [PubMed] [Google Scholar]
- 52.Pallandre J-R, et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: Implications in graft-versus-host disease and antitumor immunity. J Immunol. 2007;179(11):7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 53.Chen BJ, et al. Growth hormone mitigates against lethal irradiation and enhances hematologic and immune recovery in mice and nonhuman primates. PLoS ONE. 2010;5(6):e11056. doi: 10.1371/journal.pone.0011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 55.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 56.Zizzo G, Cohen PL. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. J Immunol. 2013;190(10):5237–5246. doi: 10.4049/jimmunol.1203017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 58.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghoreschi K, Laurence A, Yang XP, Hirahara K, O’Shea JJ. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32(9):395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 Cells in Autoimmunity and Immunodeficiency: Protective or pathogenic? Front Immunol. 2012;3:129. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang R, et al. The role of STAT3 in antigen-IgG inducing regulatory CD4(+)Foxp3(+)T cells. Cell Immunol. 2007;246(2):103–109. doi: 10.1016/j.cellimm.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Gaba A, et al. Cutting edge: IL-10-mediated tristetraprolin induction is part of a feedback loop that controls macrophage STAT3 activation and cytokine production. J Immunol. 2012;189(5):2089–2093. doi: 10.4049/jimmunol.1201126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoang PT, et al. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59(3):343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mori H, et al. Suppression of SOCS3 expression in the pancreatic beta-cell leads to resistance to type 1 diabetes. Biochem Biophys Res Commun. 2007;359(4):952–958. doi: 10.1016/j.bbrc.2007.05.198. [DOI] [PubMed] [Google Scholar]
- 65.Cecim M, Kerr J, Bartke A. Effects of bovine growth hormone (bGH) transgene expression or bGH treatment on reproductive functions in female mice. Biol Reprod. 1995;52(5):1144–1148. doi: 10.1095/biolreprod52.5.1144. [DOI] [PubMed] [Google Scholar]
- 66.Dietrich WF, et al. A comprehensive genetic map of the mouse genome. Nature. 1996;380(6570):149–152. doi: 10.1038/380149a0. [DOI] [PubMed] [Google Scholar]
- 67.Montanya E, Téllez N. Pancreatic remodeling: Beta-cell apoptosis, proliferation and neogenesis, and the measurement of beta-cell mass and of individual beta-cell size. Methods Mol Biol. 2009;560:137–158. doi: 10.1007/978-1-59745-448-3_11. [DOI] [PubMed] [Google Scholar]
- 68.Ye J, et al. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.