Significance
Mammalian Hox genes are important for limb development. Posterior abdominal B (AbdB) Hox groups (Hox9–Hox13) are required for establishment of the limb proximodistal axis. In addition, Hox9 genes control the onset of Hand2 expression in the posterior forelimb, and HoxA/D AbdB genes are responsible for the initiation and maintenances of Sonic Hedgehog (Shh). In this study, we generated Hox5 triple mutants, resulting in embryos with severe forelimb anterior patterning defects. We found that Hox5 proteins interact with promyelocytic leukemia zinc finger to restrict Shh expression in the forelimb bud. The hindlimb in Hox5 mutants develops normally, revealing distinct differences in anteroposterior field establishment in the forelimb and hindlimb and unanticipated roles for non-AbdB Hox genes, including HoxB and HoxC group genes, in limb development.
Keywords: limb development, organogenesis, anteroposterior limb patterning, gene interactions, mouse developmental genetics
Abstract
To date, only the five most posterior groups of Hox genes, Hox9–Hox13, have demonstrated loss-of-function roles in limb patterning. Individual paralog groups control proximodistal patterning of the limb skeletal elements. Hox9 genes also initiate the onset of Hand2 expression in the posterior forelimb compartment, and collectively, the posterior HoxA/D genes maintain posterior Sonic Hedgehog (Shh) expression. Here we show that an anterior Hox paralog group, Hox5, is required for forelimb anterior patterning. Deletion of all three Hox5 genes (Hoxa5, Hoxb5, and Hoxc5) leads to anterior forelimb defects resulting from derepression of Shh expression. The phenotype requires the loss of all three Hox5 genes, demonstrating the high level of redundancy in this Hox paralogous group. Further analyses reveal that Hox5 interacts with promyelocytic leukemia zinc finger biochemically and genetically to restrict Shh expression. These findings, along with previous reports showing that point mutations in the Shh limb enhancer lead to similar anterior limb defects, highlight the importance of Shh repression for proper patterning of the vertebrate limb.
Limb buds initially emerge as small bulges protruding from the embryonic lateral plate mesenchyme, and development proceeds along three axes: dorsoventral (DV), proximodistal (PD), and anteroposterior (AP) (1). Numerous factors involved in the establishment of these three axes have been defined; for example, DV patterning depends on the antagonism between Wnt7a from the dorsal ectoderm and bone morphogenetic protein genes (BMPs) and Engrailed1 (EN1) from the ventral ectoderm, growth along the PD axis is regulated mainly by fibroblast growth factor genes (Fgfs) secreted from the apical ectodermal ridge (AER), and establishment of the AP axis requires signaling from a region of the posterior limb bud termed the zone of polarizing activity (ZPA). Sonic Hedgehog (Shh) is the morphogen secreted from this region (2), and loss of Shh function results in the absence of posterior limb elements (3, 4). Previous research has identified a limb-specific enhancer located in the fifth intron of limb region 1 protein homolog gene appeoximately 1 Mb from the Shh coding sequence, designated the ZPA regulatory sequence (ZRS) (5). Deletion of this enhancer leads to defects similar to Shh loss-of-function mutants (6–8).
Hox genes also have been shown to play pivotal roles in limb PD patterning of the limb skeletal elements. The HoxA and HoxD genes from groups 9–13 impact forelimb development along the PD axis (9–14). Hoxa9/d9 and Hox10 paralogs specify stylopod patterning (humerus and femur) (10, 14, 15). Loss of function of Hoxa11 and Hoxd11 results in dramatic mispatterning of the zeugopod (radius/ulna and tibia/fibula) (9, 14). Loss of autopod elements (i.e., handplate and footplate) in Hoxa13/d13 mutants reveals important roles for this group in autopod patterning (11).
In addition, the HoxA/D9–13 paralogous group genes are collectively required for the activation and maintenance of Shh expression in limb AP patterning (12, 13). Although misexpression of more anterior Hox genes in mice reportedly affects limb patterning (16), no loss-of-function mutants of anterior, non–abdominal B (AbdB)-related genes have demonstrated defects in the patterning of limb skeletal elements. Moreover, no HoxB or HoxC group genes had been shown to play a role in forelimb development until a report by our group demonstrated that all four Hox9 paralogous genes (Hoxa9, Hoxb9, Hoxc9, and Hoxd9) are required in the early lateral plate mesoderm to define the posterior forelimb field by regulating the onset of Hand2 expression (15).
Numerous human syndromes and mouse mutants that affect AP limb patterning have been identified. Disruption of Shh expression accounts for some of these phenotypes. Some mutations in the Shh limb enhancer ZRS lead to loss of posterior digits reminiscent of loss of Shh function (3, 4, 8, 17). In addition, many point mutations in the ZRS identified in spontaneous mouse mutants (Hx and M100081) (18), human patients (PPD2, Cuban mutation, Werner mesomelic syndrome, and others) (5–7, 18–27), chickens (17), and cats (28) that lead to anteriorized and/or ectopic expression of Shh, indicating that the ZRS enhancer not only directs activation of Shh in the ZPA, but also is responsible for repression of Shh in the anterior limb.
Mutations in factors that have not been associated with Shh signaling also can lead to anterior limb defects. For example, patients with Holt–Oram syndrome (HOS) and Okihiro syndrome (OS), which are caused by mutations of TBX5 and the Spalt family zinc finger transcription factor SALL4, respectively, show anterior forelimb defects, including loss of a thumb or a triphalangeal digit and/or hypoplasia of the radius (29–36). Loss of function of promyelocytic leukemia zinc finger gene (Plzf) function in both human patients and mouse mutants also results in similar limb AP patterning defects (37–39). Interactions among the myriad of required factors in limb AP patterning and their relationships to Shh signaling and other signaling pathways remain incompletely understood.
In this study, we demonstrate that Hox5 genes perform a novel function in limb AP patterning. Loss of function of Hox5 paralogous genes (Hoxa5, Hoxb5, and Hoxc5), an anterior set of Hox genes not belonging to the AbdB-related Hox group, results in defects in anterior forelimb patterning that closely resemble some point mutations in the ZRS in both mice and humans. Early pattering of the anterior and posterior limb compartments is not disrupted in these mutants; however, the limb defects in Hox5 mutants are associated with ectopic Shh expression in the anterior forelimb buds, and we provide molecular and genetic evidence indicating that Hox5 interacts with Plzf to restrict Shh expression and pattern the anterior forelimb.
Results
Inactivation of Hox5 Paralogous Group Genes Results in Anterior Forelimb Defects.
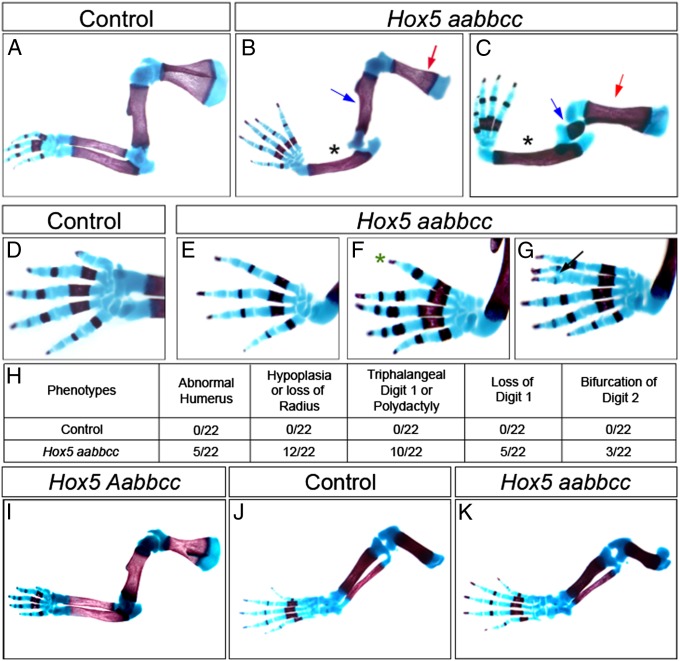
Single mutants for Hoxa5, Hoxb5, and Hoxc5 (the three mammalian Hox5 paralogous group genes) have been generated previously (40–42). Although loss of Hoxa5 function results in a smaller scapula (43), no limb patterning abnormalities have been reported for any of the three Hox5 single mutants despite the expression of these genes in the developing forelimb and hindlimb (42, 43) (Fig. S1). Compound mutants deficient for any combination of as many as five of the six Hox5 alleles did not exhibit limb defects (Fig. 1 A and I). Only when all six Hox5 alleles were mutated were defects in the anterior forelimb skeletal elements observed (Fig. 1 A–H). The humerus of Hox5 triple mutants was variably affected (Fig. 1 B and C), the radius was truncated or lost (Fig. 1 B and C), and digit 1 was often missing or transformed into a triphalangeal digit, with the distal portion of digit 2 occasionally bifurcated (Fig. 1 E–G). Hindlimb development was not affected in Hox5 mutant mice, even though Hox5 was expressed at early hindlimb bud stages (Fig. 1 J and K).
Fig. 1.
Loss of function of Hox5 paralogous genes results in anterior forelimb defects. (A–G) Skeletal analysis of control and Hox5 triple-mutant forelimbs at E18.5. The scapula is reduced in Hox5 triple mutants compared with controls, as observed for Hoxa5 single mutants (A–C, red arrows). The stylopod is reduced or truncated only in embryos with a phenotype in the radius (B and C, blue arrow). The radius of mutant forelimbs is missing or severely truncated (B and C, black asterisk). (D–G) The most anterior digit develops abnormally in Hox5 mutants compared with controls. Digit 1 is often missing (E) or triphalangeal (F and G, green asterisk). Less frequently, Hox5 mutants also have a bifurcated digit 2 (G, black arrow). Digit phenotypes do not correlate with the severity of stylopod/zeugopod defects. (H) Table summarizing forelimb phenotypes of Hox5 mutant forelimbs. (I) Compound mutants deficient for as many as five of the six Hox5 alleles do not exhibit limb defects. (J and K) Hindlimb development is not affected in Hox5 mutants.
Shh Is Ectopically Activated in Hox5 Mutant Forelimb Buds.
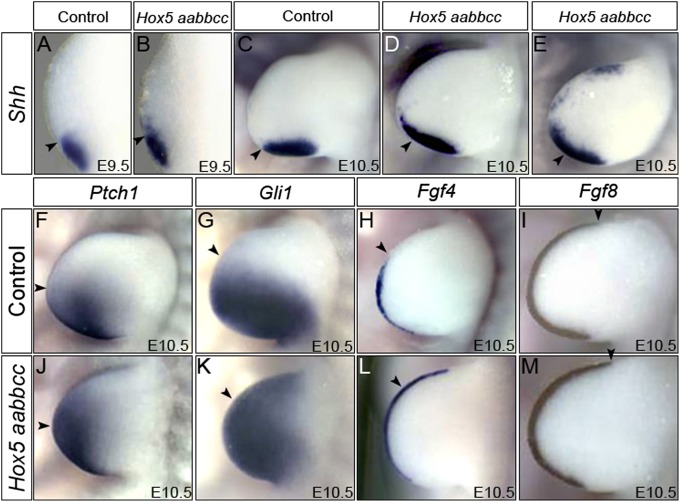
Given the clear disruption of AP limb patterning and the importance of Shh in this process, we examined Shh expression in Hox5 mutants. Shh expression expanded anteriorly in early forelimb buds of Hox5 mutants, and ectopic Shh expression was observed in anterior domains in some instances (Fig. 2 A–E). The expression of downstream factors Ptch1 and Gli1 was consistently anteriorized in Hox5 triple-mutant embryos (Fig. 2 F, G, J, and K). Fgf4 expression in the AER extended anteriorly compared with controls (Fig. 2 H and L), consistent with anteriorized Shh expression, whereas Fgf8 was expressed normally in the AER (Fig. 2 I and M).
Fig. 2.
Shh signaling is disrupted in Hox5 mutant forelimbs. (A–E) Shh expression is anteriorized in Hox5 mutant forelimbs. At E9.5, Hox5 mutant forelimbs display slightly anteriorized Shh expression compared with controls (A and B). Anteriorization of Shh expression is observed by E10.5 in Hox5 mutant forelimbs compared with controls (C–E), and ectopic Shh expression appears in anterior regions of some mutant forelimbs (E). (F–M) Expression of Ptch1 and Gli1 is consistently shifted anteriorly in Hox5 mutants at E10.5 compared with controls (F, J, G, and K). Fgf4 expression is also anteriorized in Hox5 mutant forelimbs compared with controls (H and L), whereas Fgf8 expression is unchanged (I and M). Black arrowheads in each panel mark the WT anterior boundary of expression for each probe.
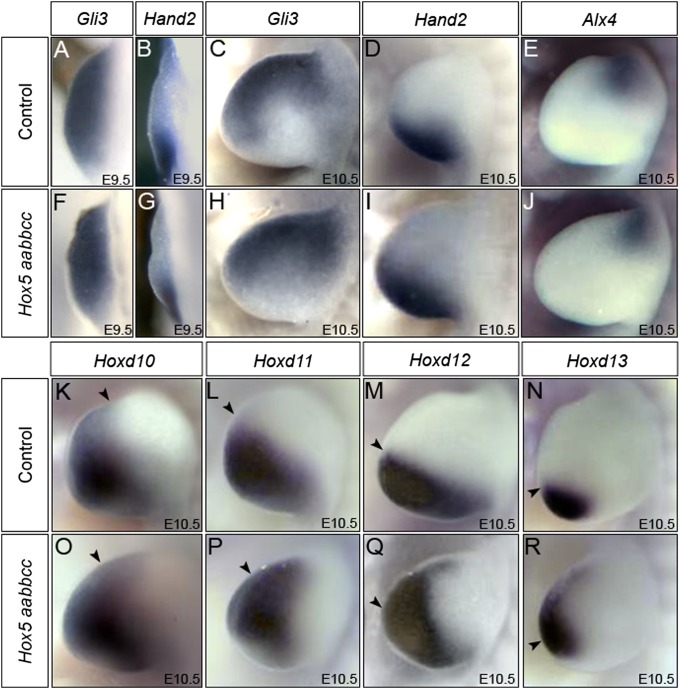
Because Shh expression is disrupted at early stages, we examined AP patterning regulators upstream of Shh signaling. In somite-matched Hox5 mutants and controls, there were no observable differences in the expression of Gli3 (Fig. 3 A, C, F, and H) or Hand2 (Fig. 3 B, D, G, and I) at any stage examined. Alx4, another early regulator of anterior limb patterning, was expressed normally in Hox5 mutants (Fig. 3 E and J).
Fig. 3.
Early limb patterning pathways are not disrupted, but posterior HoxD gene expression is anteriorized in Hox5 mutants. (A–J) Early AP patterning factors are not disrupted in Hox5 mutants. The expression of Gli3 is not altered at E9.5 (A and F) or E10.5 (C and H). Hand2 is expressed normally in Hox5 mutant forelimbs at E9.5 (B and G) and E10.5 (D and I). Alx4 expression in Hox5 mutants is also comparable to that in controls at E10.5 (E and J). (K–R) The expression limits of posterior HoxD genes are anteriorized in E10.5 Hox5 mutant forelimbs. Hoxd10 (K and O), Hoxd11 (L and P), Hoxd12 (M and Q), and Hoxd13 (N and R) are all expressed more anteriorly in Hox5 mutant forelimb buds (O–R) compared with controls (K–N). Black arrowheads mark the WT anterior boundary of expression for each probe.
Misexpression of HoxD genes results in preaxial polydactyly phenotypes bearing some resemblance to Hox5 mutants (12, 13, 16, 44), and ectopic or anteriorized Shh expression also leads to coincident anteriorization of Hoxd10-13 (45). Expression of Hoxd10-13 genes in Hox5 mutants was shifted anteriorly in Hox5 mutant forelimbs at embryonic day (E) 10.5 (Fig. 3 K–R), consistent with misregulation of Shh. It is important to note that HoxD genes are not linked to the HoxA, HoxB, or HoxC clusters, and thus these effects cannot be due to cis effects from the targeted mutations introduced into the Hox5 alleles.
Plzf Is a Potential Coregulator of Shh Repression.
Several additional regulators of anterior limb patterning have been identified in human disease syndromes as well as in mouse mutants. Forelimb defects similar to those seen in Hox5 mutants have been identified in patients with HOS caused by Tbx5 mutations (32–36), and Hox genes have been reported to be capable of driving Tbx5 expression (46), OS caused by Sall4 mutations (29, 30), Townes–Brocks syndrome caused by Sall1 mutations (31, 47) and Saethre–Chotzen syndrome caused by Twist1 mutations, which also show limb phenotypes in mutant mice (48–50). Mutations of both the human and mouse limb enhancer of Shh, ZRS (7, 18, 19, 24), and human multiple congenital anomaly/mental retardation syndromes caused by mutations of Plzf, as well as loss-of-function mutations in Plzf in mice (37, 39), lead to similar phenotypes. Based on these similarities, we investigated the expression of Tbx5, Sall1, Sall4, Twist1, and Plzf in our Hox5 mutants. We found no change in the expression of any of these genes in Hox5 mutant forelimbs (Figs. S2 A–L and S3 A–D).
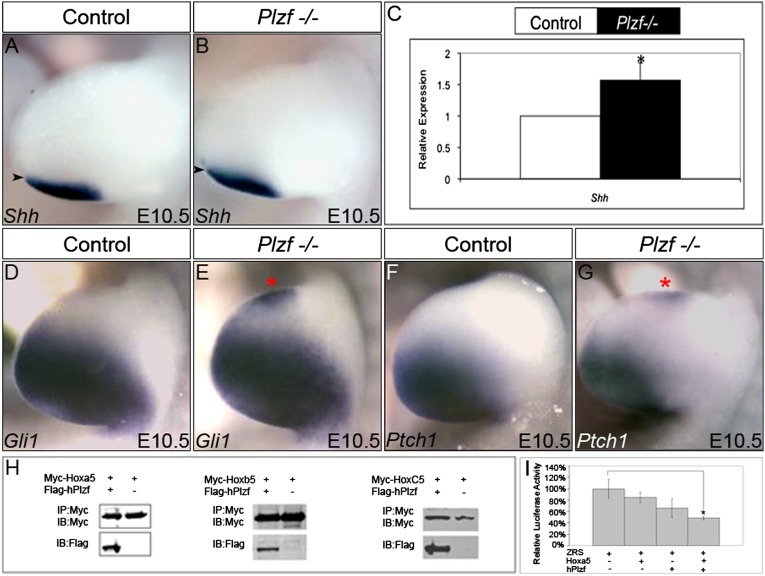
To test whether these factors might act in parallel in the same pathway as Hox5 and affect Shh expression, we examined Shh expression in Tbx5 heterozygotes with or without loss of Sall4 function, as well as in Plzf mutant embryos. Consistent with previous reports that Shh expression is not altered with loss of Tbx5 function (51), we found that Shh was not altered in Tbx5 or Sall4 heterozygous mutants or compound Tbx5/Sall4 heterozygous mutants (Fig. S3 I–L); however, Plzf mutants showed a small but reproducible anterior shift in Shh expression (Fig. 4 A and B). In addition, Shh transcripts were increased by ∼50% in Plzf mutant limbs (Fig. 4C). To confirm changes in Shh expression, we examined the expression of Ptch1 and Gli1, factors immediately downstream of Shh. In Plzf mutants, Ptch1 and Gli1 were consistently anteriorized and ectopically expressed (Fig. 4 D–G), demonstrating that Shh expression is affected downstream of Plzf in the developing limb.
Fig. 4.
Hox5 and Plzf cooperatively mediate repression of Shh expression via ZRS. (A–G) The Shh signaling pathway is disrupted in Plzf mutant forelimbs similar to that in Hox5 mutant forelimbs. The expression of Shh is anteriorized in Plzf mutant forelimb buds compared with controls (A and B; black arrowheads mark the WT anterior boundary), and qRT-PCR analysis demonstrates an increase in Shh levels in Plzf mutants (C; asterisk indicates significant differences from controls; P < 0.05). Ectopic, anteriorized Gli1 and Ptch1 expression is observed in all Plzf mutant forelimbs at E10.5, confirming anteriorized Shh activity (D–G; red asterisks mark ectopic anterior expression). (H) Coimmunoprecipitation assays from cell lysates cotransfected with Myc-tagged Hox5 proteins and Flag-tagged Plzf protein. Immunoprecipitation with anti-Myc (Hox) antibodies and immunoblotting (IB) for anti-Flag (Plzf) results in coimmunoprecipitation (IP) of Myc-tagged Hoxa5, Hoxb5, and Hoxc5 with Plzf. (I) ZRS-driven luciferase reporter activity trends downward when cotransfected with Hox5 protein expression constructs or with the Plzf protein expression construct; however, cotransfections of the ZRS reporter with both Hox5 and Plzf proteins result in statistically significant down-regulation of expression from this reporter.
Hox5 Proteins Interact with Plzf and Can Regulate Shh Expression Through the ZRS in Vitro.
Plzf mouse mutants have been found to have similar forelimb phenotypes as Hox5 mutant mice, although with low penetrance (37). The mice used in the present study demonstrated a similar phenotype (Fig. 5 A and B), but with 100% penetrance in the forelimb (52). Heterozygous embryos had no limb phenotype (Fig. 5C). Having already demonstrated no changes in Plzf expression in our Hox5 mutants, we investigated the possibility that Hox5 acts downstream of Plzf in forelimb AP patterning, but found normal Hox5 expression levels in Plzf mutant forelimbs (Fig. S3 E–G).
Fig. 5.
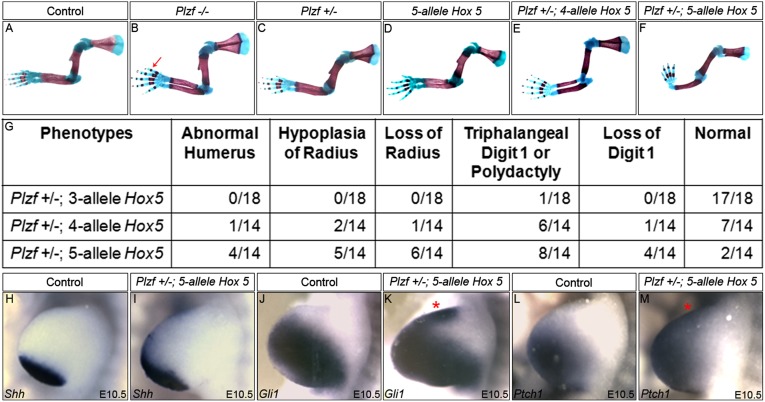
Hox5 and Plzf genetically interact to pattern the anterior limb and Shh expression in vivo. (A–G) Skeletal preparations from E18.5 Plzf, 5-allele Hox5, and compound Hox5;Plzf mutants. Plzf heterozygotes and embryos with up to five mutant Hox5 alleles (Hox5Aabbcc) are indistinguishable from controls (C and D), whereas Plzf mutants exhibit preaxial defects with 100% penetrance in our background (B; arrow denotes triphalangeal digit 1). Compound mutants heterozygous for Plzf plus three mutant Hox5 alleles rarely display a phenotype (G), but Plzf heterozygotes with four or five mutant Hox5 alleles display increases in the penetrance of anterior limb defects (E–G), demonstrating strong genetic interaction between Hox5 and Plzf. (H–M) The Shh pathway is derepressed in Hox5;Plzf compound mutants. Shh (H and I), Gli1 (J and K), and Ptch1 (L and M) are ectopically expressed in Plzf +/−;Hox5 5-allele compound mutants compared with controls. Controls include WT, Plzf heterozygous, and compound Hox5 mutants with five or fewer mutant alleles; red asterisks denote anteriorized expression.
To test whether Hox5 and Plzf proteins are capable of interacting to regulate downstream limb target genes, we examined potential physical interactions between these proteins in vitro. In cells cotransfected with epitope-tagged Hoxa5, Hoxb5, or Hoxc5 protein in combination with tagged Plzf, we found coprecipitation of all three Hox5 proteins with Plzf protein (Fig. 4H and Fig. S3H), consistent with the possibility that these proteins interact to regulate downstream targets.
If Hox5 and Plzf function together to repress Shh expression anteriorly and affect AP patterning of the forelimb, then we would expect these genes to interact in vivo. Hox5 mutants demonstrate no forelimb defects unless all six alleles of the three Hox5 genes are mutated (compare Fig. 1 A–G and Fig. 5D). Plzf mutants exhibited forelimb phenotypes only with loss of both alleles, whereas heterozygous animals had no phenotype (Fig. 5 A–C). Compound mutants heterozygous for Plzf combined with either one or two mutant Hox5 alleles did not exhibit any forelimb defects. Embryos heterozygous for Plzf plus three Hox5 mutant alleles resulted in preaxial limb skeletal defects in only 1 of 18 forelimbs (Fig. 5G). Compound mutants heterozygous for Plzf plus four Hox5 mutant alleles showed more severe forelimb defects with higher penetrance (Fig. 5 E and G). Forelimb defects were further exacerbated in compound mutants heterozygous for Plzf plus five Hox5 mutant alleles, with 12 of 14 forelimbs demonstrating anterior forelimb defects (Fig. 5 F and G). These findings indicate that strong genetic interactions occur between Hox5 and Plzf in vivo, supporting the hypothesis that these proteins cooperatively regulate forelimb AP patterning events.
If Hox5 and Plzf coordinately regulate Shh expression, then the phenotypes observed in the compound mutants should result in changes in Shh pathway expression. Obvious anteriorization of Shh expression was observed in Plzf/Hox5 compound mutants (Fig. 5 H and I), similar to that seen in both Hox5 triple mutants and Plzf mutants, but not in Plzf heterozygotes or in compound Hox5 mutants carrying up to five mutant alleles. Gli1 and Ptch1 expression also was anteriorized in the forelimb of the compound Hox5/Plzf mutants (Fig. 5 J–M), but not in control WT embryos, Plzf heterozygotes, or compound Hox5 mutants harboring up to five mutant alleles. These genetic data further support the assertion that Hox5 and Plzf cooperatively repress anterior Shh expression during forelimb development to influence forelimb AP patterning.
To provide evidence supporting possible direct regulation at the ZRS, we examined the ability of transfected Hox5 proteins and Plzf to regulate reporter expression through the ZRS enhancer. Transfection of any of the three Hox5 proteins or Plzf alone with the ZRS reporter did not result in any statistically significant change in expression, although the levels trended downward; however, transfection of any of the Hox5 proteins and Plzf together resulted in a statistically significant down-regulation of baseline expression, consistent with direct regulation of Shh expression through the ZRS enhancer (Fig. 4I). We made multiple attempts to perform ChIP of both Plzf and Hox5 at the ZRS promoter in vivo. Despite the successful use of the antibodies at other enhancers, the very high level of background (control antibody) binding at the ZRS and the potentially small numbers of responding cells in the limb bud precluded conclusive evidence of binding of these proteins in vivo (Fig. S4 and SI Materials and Methods).
Discussion
In this study, we show a unique and unexpected role for Hox5 genes in limb AP patterning. A myriad of genetic studies have defined important roles for HoxA and HoxD group 9–13 genes in forelimb development (9–14). Recently, we reported that Hox9 genes from HoxB and HoxC complex, along with Hoxa9 and Hoxd9, are also required to define the posterior forelimb compartment (15). However, previous loss-of-function studies have provided no evidence that anterior, non-AbdB Hox genes participate in patterning limb skeletal elements. Here we report limb phenotypes resulting from loss of Hox5 paralogous gene function, further demonstrating pivotal roles for HoxB and HoxC complex genes in forelimb AP patterning, with Hoxa5, Hoxb5, and Hoxc5 controlling anterior forelimb patterning.
The limb defects in our Hox5 mutants are restricted to forelimbs, with no defects in hindlimb development observed. The limb defects in quadruple Hox9 mutants are also restricted to the forelimbs (15). Taken together, our findings highlight significant differences in how anterior and posterior limb compartments are established in forelimbs and hindlimbs. This is surprising, considering the downstream factors currently known to play critical roles in AP patterning (i.e., Hand2, Gli3, and Shh) function similarly in both forelimbs and hindlimbs. Our findings from both loss of Hox9 paralog function (15) and the present study suggest that early axial Hox expression in the lateral plate mesoderm controls the establishment of the anterior (Hox5) and posterior (Hox9) compartments of the forelimb. None of the numerous combinations of posterior Hox loss-of-function mutants reported to date (9–14) are known to lead to hindlimb AP patterning defects analogous to those reported for loss of Hox9 or Hox5 paralogs. Itou et al. (53) recently demonstrated that LIM-homeodomain factor Islet1 is a critical regulator of Hand2 expression and the posterior compartment in hindlimbs. How the hindlimb anterior compartment is established remains to be discovered.
It is also interesting to note that although Hox5 paralogs and Hox9 paralogs control anterior and posterior patterning, respectively, Hox9 paralogs are responsible for initiation of Hand2, whereas no disruption of early anterior/posterior compartment formation is observed in Hox5 mutants (ref. 15 and this paper). In Hox5 mutant limbs, the initial Hand2/Gli3 pattern is normal, but downstream expression of Shh is affected, consistent with Hox5 regulating Shh expression more directly. This finding is also consistent with previous reports demonstrating that numerous point mutations in the ZRS of mouse, humans, chickens, and cats result in ectopic Shh activity in anterior domains of the limb bud and result in phenotypes similar to those that we detected in Hox5 mutant mice, including defects in the stylopod, anterior zeugopod, and digits (5–7, 17–28).
The findings reported here also reveal a role for Plzf in regulating Shh expression in limb AP patterning. We detected anteriorized Shh expression in Plzf mutants, in contrast to a previous report (37). The discrepancy may be related to the use of different Plzf mutant alleles; we observed 100% penetrance of forelimb defects in the mutants used in the present study, significantly higher than in the previously reported mutant allele (37). Our finding that Shh is regulated downstream of Plzf in limb AP patterning is further supported by changes in Ptch1 and Gli1 expression in addition to Shh expression.
Our genetic and molecular analyses of Hox5 function in forelimb development support a model in which Hox5 proteins, interacting with Plzf, act as repressors of Shh expression. Among the many potential binding sites in the ZRS are several putative Hox- binding sites and at least one putative Plzf-binding site. The putative Plzf site is mutated in the “Cuban mutation,” one of the human mutations with limb phenotypes similar to those in the mice reported here, including radial aplasia (7). There is also a report of three independent probands with anterior forelimb phenotypes (mostly triphalangeal thumbs) that harbor a mutation in one of the three putative Hox-binding sites (19).
The activity of Hox5 and Plzf likely combine with numerous other factors that bind to this enhancer to both activate and repress Shh expression. Several factors, including Hand2 and HoxD, have been shown to activate Shh expression via the ZRS limb enhancer element (54, 55). Etv4/Etv5 and Twist 1 have been shown to cooperate to restrict Shh expression to the posterior limb bud (50, 56). The ZRS is more than 700 bp long, and thus it is likely that a myriad of factors converge at this critical regulatory hub to direct proper expression of Shh in the developing vertebrate limb. A complete understanding of the factors that bind to these sites and how they interact remains to be delineated in future studies.
Materials and Methods
Mice and Whole-Mount in Situ Hybridization.
All mouse mutant strains used in this study have been reported previously (41, 52). Control mice included both WT embryos and low-allele littermates from Hox and Hox/Plzf crosses. The results were identical, and thus we use the term “control” throughout for clarity. Mutant mouse strains, early skeletal preparations, and standard whole-mount in situ hybridization were as described previously (14, 41, 52). All in situ probes were prepared as described previously (15, 57, 58). All experiments were performed following protocols approved by the University of Michigan’s Institutional Committee on the Use and Care of Animals.
Cell Culture, Transfections, Luciferase Assays, and Coimmunoprecipitation Assays.
HEK293 or HEK293T cells were used and plated as described previously (59). Cell transfections were performed by CaPO4 precipitation. Coimmunoprecipitation assays were performed as described previously (59). Hox5 and Plzf protein-coding sequences were amplified from their cDNAs using the primers listed in SI Materials and Methods, then subcloned into pCS2+MT or p3XFlag-CMV vectors (Sigma-Aldrich). Details of plasmid generation and reporter assays have been reported previously (59). The highly conserved ZRS core region was amplified using the primers listed in SI Materials and Methods and then subcloned into a pGL3 promoter vector (Promega). The Student t test was used to determine statistical significance. All experiments were repeated at least three times in independent experiments.
RNA Isolation and Quantitative RT-PCR.
RNA was isolated from mouse limbs with the Qiagen RNeasy Micro Kit. Quantitative RT-PCR (qRT-PCR) was carried out using Roche FastStart SYBR Green Master Mix. Primer sequences have been described previously (51). Relative expression values were calculated as 2−ΔΔCt, and values of controls were normalized to 1. GAPDH served as an internal control for normalization in all qRT-PCR experiments, and the Student t test was used to determine statistical significance (P < 0.05). All experiments were repeated at least three times.
Supplementary Material
Acknowledgments
We thank Drs. Benoit Bruneau, Xin Sun, and Licia Selleri for the in situ hybridization probes. This work was supported by National Institutes of Health Grants AR057018 and AR061402 (to D.M.W.) and National Center for Research Resources Grant UL1RR024986 (to S.M.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315075110/-/DCSupplemental.
References
- 1.Towers M, Tickle C. Generation of pattern and form in the developing limb. Int J Dev Biol. 2009;53(5-6):805–812. doi: 10.1387/ijdb.072499mt. [DOI] [PubMed] [Google Scholar]
- 2.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 3.Chiang C, et al. Manifestation of the limb prepattern: Limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236(2):421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 4.Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech Dev. 2001;100(1):45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 5.Lettice LA, et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12(14):1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- 6.Wieczorek D, Köster B, Gillessen-Kaesbach G. Absence of thumbs, A/hypoplasia of radius, hypoplasia of ulnae, retarded bone age, short stature, microcephaly, hypoplastic genitalia, and mental retardation. Am J Med Genet. 2002;108(3):209–213. doi: 10.1002/ajmg.10271. [DOI] [PubMed] [Google Scholar]
- 7.Zguricas J, et al. Clinical and genetic studies on 12 preaxial polydactyly families and refinement of the localisation of the gene responsible to a 1.9 cM region on chromosome 7q36. J Med Genet. 1999;36(1):32–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T. Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development. 2005;132(4):797–803. doi: 10.1242/dev.01613. [DOI] [PubMed] [Google Scholar]
- 9.Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375(6534):791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- 10.Fromental-Ramain C, et al. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development. 1996;122(2):461–472. doi: 10.1242/dev.122.2.461. [DOI] [PubMed] [Google Scholar]
- 11.Fromental-Ramain C, et al. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122(10):2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 12.Kmita M, et al. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435(7045):1113–1116. doi: 10.1038/nature03648. [DOI] [PubMed] [Google Scholar]
- 13.Tarchini B, Duboule D, Kmita M. Regulatory constraints in the evolution of the tetrapod limb anterior-posterior polarity. Nature. 2006;443(7114):985–988. doi: 10.1038/nature05247. [DOI] [PubMed] [Google Scholar]
- 14.Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301(5631):363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 15.Xu B, Wellik DM. Axial Hox9 activity establishes the posterior field in the developing forelimb. Proc Natl Acad Sci USA. 2011;108(12):4888–4891. doi: 10.1073/pnas.1018161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knezevic V, et al. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124(22):4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- 17.Maas SA, Suzuki T, Fallon JF. Identification of spontaneous mutations within the long-range limb-specific Sonic hedgehog enhancer (ZRS) that alter Sonic hedgehog expression in the chicken limb mutants oligozeugodactyly and silkie breed. Dev Dyn. 2011;240(5):1212–1222. doi: 10.1002/dvdy.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagai T, et al. Phylogenetic conservation of a limb-specific, cis-acting regulator of Sonic hedgehog (Shh) Mamm Genome. 2004;15(1):23–34. doi: 10.1007/s00335-033-2317-5. [DOI] [PubMed] [Google Scholar]
- 19.Furniss D, et al. A variant in the sonic hedgehog regulatory sequence (ZRS) is associated with triphalangeal thumb and deregulates expression in the developing limb. Hum Mol Genet. 2008;17(16):2417–2423. doi: 10.1093/hmg/ddn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albuisson J, et al. Identification of two novel mutations in Shh long-range regulator associated with familial pre-axial polydactyly. Clin Genet. 2011;79(4):371–377. doi: 10.1111/j.1399-0004.2010.01465.x. [DOI] [PubMed] [Google Scholar]
- 21.Farooq M, et al. Preaxial polydactyly/triphalangeal thumb is associated with changed transcription factor-binding affinity in a family with a novel point mutation in the long-range cis-regulatory element ZRS. Eur J Hum Genet. 2010;18(6):733–736. doi: 10.1038/ejhg.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurnett CA, et al. Two novel point mutations in the long-range SHH enhancer in three families with triphalangeal thumb and preaxial polydactyly. Am J Med Genet A. 2007;143(1):27–32. doi: 10.1002/ajmg.a.31563. [DOI] [PubMed] [Google Scholar]
- 23.Semerci CN, et al. Homozygous feature of isolated triphalangeal thumb-preaxial polydactyly linked to 7q36: No phenotypic difference between homozygotes and heterozygotes. Clin Genet. 2009;76(1):85–90. doi: 10.1111/j.1399-0004.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- 24.Wieczorek D, et al. A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS), while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum Mutat. 2010;31(1):81–89. doi: 10.1002/humu.21142. [DOI] [PubMed] [Google Scholar]
- 25.Wu L, et al. A ZRS duplication causes syndactyly type IV with tibial hypoplasia. Am J Med Genet A. 2009;149A(4):816–818. doi: 10.1002/ajmg.a.32740. [DOI] [PubMed] [Google Scholar]
- 26.Laurell T, et al. A novel 13 base pair insertion in the sonic hedgehog ZRS limb enhancer (ZRS/LMBR1) causes preaxial polydactyly with triphalangeal thumb. Hum Mutat. 2012;33(7):1063–1066. doi: 10.1002/humu.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Qattan MM, Al Abdulkareem I, Al Haidan Y, Al Balwi M. A novel mutation in the SHH long-range regulator (ZRS) is associated with preaxial polydactyly, triphalangeal thumb, and severe radial ray deficiency. Am J Med Genet A. 2012;158A(10):2610–2615. doi: 10.1002/ajmg.a.35584. [DOI] [PubMed] [Google Scholar]
- 28.Lettice LA, Hill AE, Devenney PS, Hill RE. Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. Hum Mol Genet. 2008;17(7):978–985. doi: 10.1093/hmg/ddm370. [DOI] [PubMed] [Google Scholar]
- 29.Al-Baradie R, et al. Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am J Hum Genet. 2002;71(5):1195–1199. doi: 10.1086/343821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohlhase J, et al. Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet. 2002;11(23):2979–2987. doi: 10.1093/hmg/11.23.2979. [DOI] [PubMed] [Google Scholar]
- 31.Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18(1):81–83. doi: 10.1038/ng0198-81. [DOI] [PubMed] [Google Scholar]
- 32.Basson CT, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15(1):30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 33.Basson CT, et al. Different TBX5 interactions in heart and limb defined by Holt-Oram syndrome mutations. Proc Natl Acad Sci USA. 1999;96(6):2919–2924. doi: 10.1073/pnas.96.6.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brassington AM, et al. Expressivity of Holt-Oram syndrome is not predicted by TBX5 genotype. Am J Hum Genet. 2003;73(1):74–85. doi: 10.1086/376436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan C, Liu M, Wang Q. Functional analysis of TBX5 missense mutations associated with Holt-Oram syndrome. J Biol Chem. 2003;278(10):8780–8785. doi: 10.1074/jbc.M208120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li QY, et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15(1):21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- 37.Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal patterning. Nat Genet. 2000;25(2):166–172. doi: 10.1038/76014. [DOI] [PubMed] [Google Scholar]
- 38.Barna M, et al. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3(4):499–510. doi: 10.1016/s1534-5807(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 39.Fischer S, et al. Biallelic loss of function of the promyelocytic leukaemia zinc finger (PLZF) gene causes severe skeletal defects and genital hypoplasia. J Med Genet. 2008;45(11):731–737. doi: 10.1136/jmg.2008.059451. [DOI] [PubMed] [Google Scholar]
- 40.Jeannotte L, Lemieux M, Charron J, Poirier F, Robertson EJ. Specification of axial identity in the mouse: Role of the Hoxa-5 (Hox1.3) gene. Genes Dev. 1993;7(11):2085–2096. doi: 10.1101/gad.7.11.2085. [DOI] [PubMed] [Google Scholar]
- 41.McIntyre DC, et al. Hox patterning of the vertebrate rib cage. Development. 2007;134(16):2981–2989. doi: 10.1242/dev.007567. [DOI] [PubMed] [Google Scholar]
- 42.Rancourt DE, Tsuzuki T, Capecchi MR. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 1995;9(1):108–122. doi: 10.1101/gad.9.1.108. [DOI] [PubMed] [Google Scholar]
- 43.Aubin J, Déry U, Lemieux M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development. 2002;129(17):4075–4087. doi: 10.1242/dev.129.17.4075. [DOI] [PubMed] [Google Scholar]
- 44.Zakany J, Zacchetti G, Duboule D. Interactions between HOXD and Gli3 genes control the limb apical ectodermal ridge via Fgf10. Dev Biol. 2007;306(2):883–893. doi: 10.1016/j.ydbio.2007.03.517. [DOI] [PubMed] [Google Scholar]
- 45.Wada N, Kawakami Y, Nohno T. Sonic hedgehog signaling during digit pattern duplication after application of recombinant protein and expressing cells. Dev Growth Differ. 1999;41(5):567–574. doi: 10.1046/j.1440-169x.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 46.Minguillon C, et al. Hox genes regulate the onset of Tbx5 expression in the forelimb. Development. 2012;139(17):3180–3188. doi: 10.1242/dev.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohlhase J, et al. Molecular analysis of SALL1 mutations in Townes-Brocks syndrome. Am J Hum Genet. 1999;64(2):435–445. doi: 10.1086/302238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourgeois P, et al. The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum Mol Genet. 1998;7(6):945–957. doi: 10.1093/hmg/7.6.945. [DOI] [PubMed] [Google Scholar]
- 49.Krawchuk D, et al. Twist1 activity thresholds define multiple functions in limb development. Dev Biol. 2010;347(1):133–146. doi: 10.1016/j.ydbio.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, et al. Preaxial polydactyly: interactions among ETV, TWIST1 and HAND2 control anterior-posterior patterning of the limb. Development. 2010;137(20):3417–3426. doi: 10.1242/dev.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasson P, Del Buono J, Logan MP. Tbx5 is dispensable for forelimb outgrowth. Development. 2007;134(1):85–92. doi: 10.1242/dev.02622. [DOI] [PubMed] [Google Scholar]
- 52.Buaas FW, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36(6):647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 53.Itou J, et al. Islet1 regulates establishment of the posterior hindlimb field upstream of the Hand2-Shh morphoregulatory gene network in mouse embryos. Development. 2012;139(9):1620–1629. doi: 10.1242/dev.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capellini TD, et al. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133(11):2263–2273. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- 55.Galli A, et al. Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS Genet. 2010;6(4):e1000901. doi: 10.1371/journal.pgen.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z, Verheyden JM, Hassell JA, Sun X. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev Cell. 2009;16(4):607–613. doi: 10.1016/j.devcel.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verheyden JM, Lewandoski M, Deng C, Harfe BD, Sun X. Conditional inactivation of Fgfr1 in mouse defines its role in limb bud establishment, outgrowth and digit patterning. Development. 2005;132(19):4235–4245. doi: 10.1242/dev.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avantaggiato V, et al. Developmental analysis of murine Promyelocyte Leukemia Zinc Finger (PLZF) gene expression: Implications for the neuromeric model of the forebrain organization. J Neurosci. 1995;15(7 Pt 1):4927–4942. doi: 10.1523/JNEUROSCI.15-07-04927.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong KQ, Yallowitz AR, Sun H, Dressler GR, Wellik DM. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol Cell Biol. 2007;27(21):7661–7668. doi: 10.1128/MCB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.