Significance
About 12% of human genetic disorders involve premature stop codons (PTC) that may produce abnormal, short proteins. Some antibiotics and other compounds have been proposed to restore full-length proteins by reading through PTC. We studied skin cells from xeroderma pigmentosum (XP) patients with different PTC in a DNA repair gene. XP patients have a DNA repair defect and a 10,000-fold increased risk of sunlight-induced skin cancer. Using several readthrough compounds, we found increased levels of DNA repair protein, assembly of DNA repair proteins at the DNA damage site, and repair of UV damage in some XP cells. Even small amounts of increased DNA repair protein may provide potential therapy for XP patients and may reduce disease severity.
Keywords: readthrough compounds, UV radiation
Abstract
About 12% of human genetic disorders involve premature termination codons (PTCs). Aminoglycoside antibiotics have been proposed for restoring full-length proteins by readthrough of PTC. To assess the efficiency of readthrough, we selected homozygous and compound heterozygous skin fibroblasts from xeroderma pigmentosum (XP) patients with different PTCs in the XPC DNA repair gene. XP patients have a nucleotide excision repair defect and a 10,000-fold increased risk of UV-induced skin cancer. In six of eight PTC-containing XP-C cells, treatment with Geneticin and gentamicin resulted in (i) stabilized XPC–mRNA, which would have been degraded by nonsense-mediated decay; (ii) increased expression of XPC protein that localized to UV-damaged sites; (iii) recruitment of XPB and XPD proteins to UV DNA damage sites; and (iv) increased repair of 6–4 photoproducts and cyclobutane pyrimidine dimers. Expression of PTC in a transfected vector revealed that readthrough depends on the PTC sequence and its location within the gene. This sensitive DNA repair assay system demonstrates the complexity of response to PTC readthrough inducers. The efficiency of aminoglycoside-mediated readthrough depends on the type and copy number of PTC, the downstream 4+ nucleotide, and the location within the exon. Treatment with small-molecule nonaminoglycoside compounds (PTC124, BZ16, or RTC14) resulted in similarly increased XPC mRNA expression and photoproduct removal with less toxicity than with the aminoglycosides. Characterizing PTC structure and parameters governing effective PTC readthrough may provide a unique prophylactic therapy for skin cancer prevention in XP-C patients.
The rare autosomal recessive disorder xeroderma pigmentosum (XP) has a more than 10,000-fold increased skin cancer risk caused by a defective nucleotide excision repair (NER) of UV radiation–induced cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts (6–4PPs) (1). Cells from XP patients fall into seven complementation groups (XP-A through XP-G) and a variant form with a mutation in polymerase eta, resulting in defective translesion bypass (2). XP has a frequency of about one per million in the United States and Europe (1, 3). Following lesion recognition by DDB2 (XPE), the XPC protein—in association with Rad23B and centrin-2—senses DNA damage and recruits other NER proteins (4). XP-C cells show proficient transcription-coupled (TC)-NER but defective global genome repair (GGR) of damaged DNA, whereas cells from XP complementation groups A, B, D, F, and G are defective in both pathways (2). Premature termination codons (PTCs) have been identified in 24 (15%) of 159 XP-C patients (5–13) (Table S1). A PTC can reduce the level of mRNA and protein via nonsense-mediated mRNA decay (NMD), a mechanism that detects and degrades PTC-bearing transcripts. NMD prevents the expression of truncated proteins that might be nonfunctional or deleterious due to dominant-negative or gain-of-function (14).
About 12% of genetic disorders are caused by nonsense mutations that result in a primary PTC (15, 16). Secondary PTC would not be amenable to readthrough abrogation. Compounds that induce readthrough of PTC and induction of functional protein (15, 17, 18) bind to ribosomal RNA of the small ribosomal subunit, leading to conformational changes that reduce codon–anticodon pairing, thus increasing error-prone translation (19). In vitro studies and mouse models have demonstrated that certain aminoglycosides can read through PTCs and restore the expression and function of missing proteins in different genetic diseases with PTCs (17, 18, 20). Clinical trials using aminoglycosides in cystic fibrosis (CF) and Duchenne muscular dystrophy patients have demonstrated readthrough in vivo and partial correction of protein function (21). However, systemic aminoglycoside administration may be associated with severe side effects such as kidney damage and hearing loss, thus impeding their clinical application (22). Nonaminoglycoside compounds such as the synthetic oxadiazole derivative PTC124 may have less toxicity (23). Du et al. developed a high throughput screening assay and identified four new groups of nonaminoglycoside small-molecule readthrough compounds that induced readthrough of the ATM gene, leading to increased expression of functional ATM protein (24, 25).
Readthrough of XP PTC has not been previously reported. We developed a sensitive method for assessing efficiency of readthrough with a panel of four homozygous and four compound heterozygous skin fibroblasts from XP-C patients with all three types of PTC mutations (TGA, TAG, and TAA). We measured the level of the XPC protein, recruitment of NER proteins to sites of UV DNA damage, and the efficiency of repair of photoproducts. We tested aminoglycosides and small-molecular-weight nonaminoglycoside compounds with potential for blood–brain barrier penetration (25).
Results
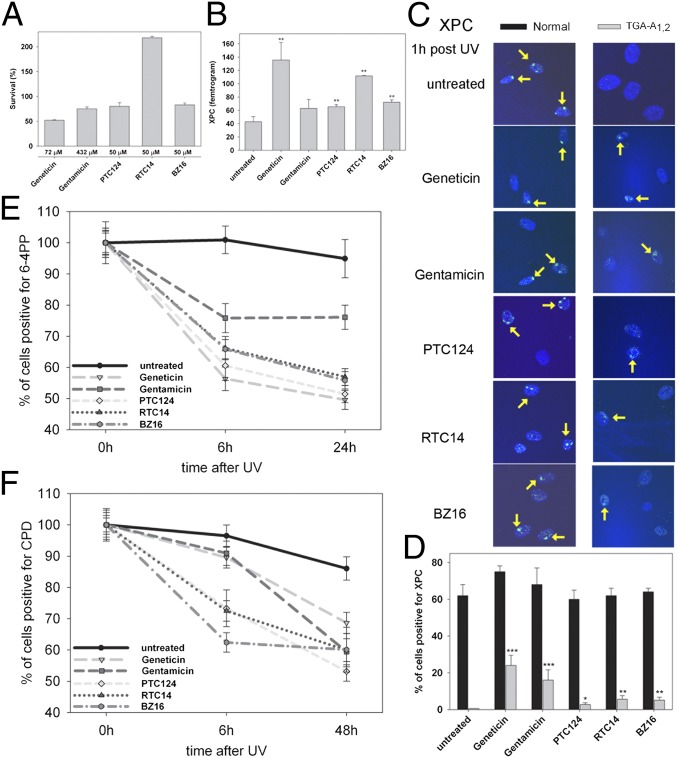
Geneticin Induces Readthrough of XPC mRNA.
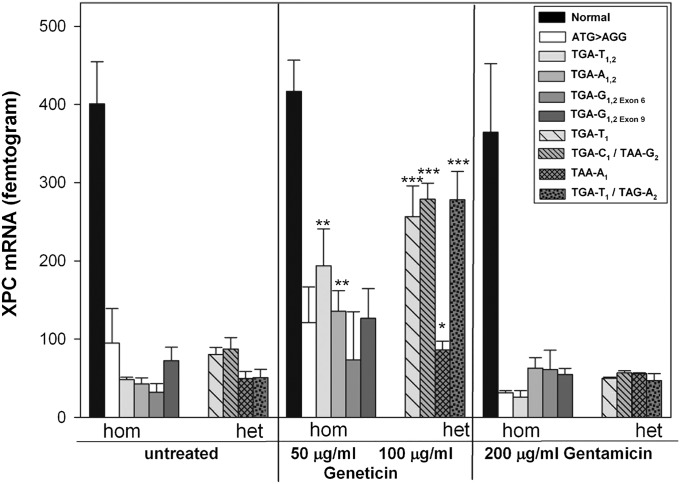
Quantitative real-time PCR was performed to measure the levels of XPC mRNA in primary XP-C cells with or without aminoglycoside treatment. All untreated XP-C cells showed markedly reduced levels of XPC mRNA (mean 62 femtogram, fg), representing 15% of normal (400 fg, Fig. 1), as found in other XP-C cells (6). To demonstrate that this reduced level is caused by NMD in the cells with PTC, we treated cells with cycloheximide, a known NMD inhibitor (26) and found a 4- to 14-fold increase in XPC mRNA (Fig. S1). Incubation with Geneticin (also known as G418) for 3 d led to a significant increase in XPC mRNA to levels 20–70% of normal in two homozygous cell lines TGA-T1,2 (194 fg) and TGA-A1,2 (136 fg), and in the four compound heterozygous cell lines TGA-T1 (257 fg), TGA-C1/TAA-G2 (279 fg), TAA-A1 (86 fg), and TGA-T1/TAG-A2 (278 fg) (Fig. 1). XPC mRNA was also increased after Geneticin treatment of lymphoblast cell lines, corresponding to the same fibroblast cell lines TGA-T1 and TGA-T1/TAG-A2 (Fig. S2). In contrast to Geneticin, 3 d incubation with gentamicin did not significantly increase XPC mRNA in any of the eight XP-C cell lines tested (Fig. 1).
Fig. 1.
Increased XPC mRNA with Geneticin but not gentamicin. XP-C cells containing PTC were incubated with Geneticin or gentamicin for 3 d and mRNA was measured. Data are mean ± SD of three experiments each in triplicate. *P < 0.05, **P ≤ 0.005, ***P ≤ 0.0005.
Aminoglycosides Induce XPC Protein Expression and Localization at Sites of UV-Induced DNA Damage.
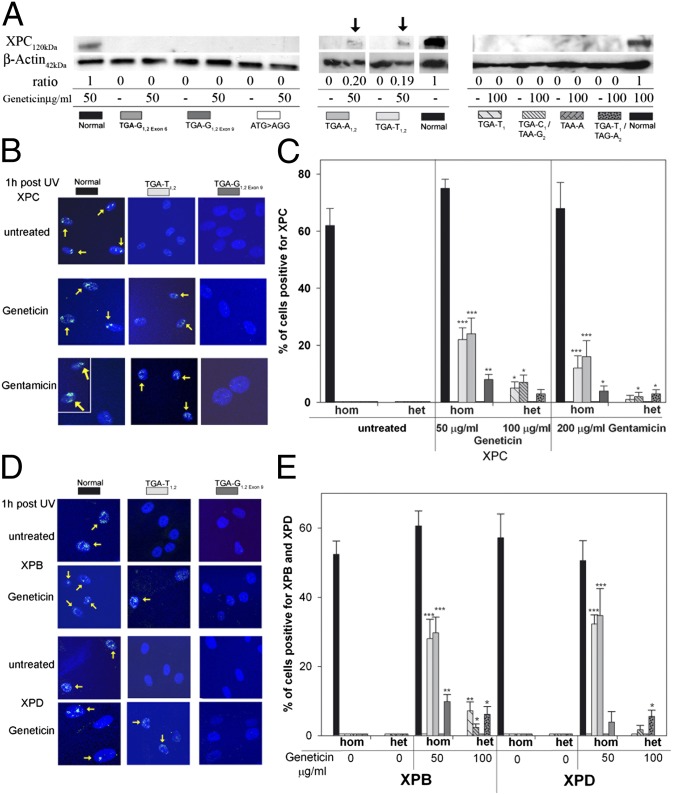
To determine whether the increased XPC mRNA is functional and translates into XPC protein, we performed immunoblot analysis. As in previous studies of cultured cells (6, 27) and intact skin (28), XPC protein was not detectable in cells from XP-C patients. Following Geneticin treatment for 4 d, XPC protein was detectable only in TGA-A1,2 and TGA-T1,2 cells (Fig. 2A). Insufficient sensitivity of immunoblotting for the detection of readthrough-induced protein has been reported previously (18). We therefore irradiated cells with UV through a 5-μm polycarbonate isopore membrane to generate localized areas of DNA damage (29, 30). Using fluorescent antibody labeling, 1 h after localized UV exposure, foci of XPC protein were visualized in 62–75% nuclei of untreated normal cells but were not detectable in untreated XP-C cells (Fig. 2 B and C and Fig. S3A). In contrast, in the homozygous cell lines TGA-A1,2 and TGA-T1,2, about 24% and 22% of cells, respectively, were XPC protein positive after incubation with Geneticin and about 16% and 12%, respectively, after gentamicin treatment. The homozygous cell line TGA-G1,2exon 9 showed only few XPC-positive cells after Geneticin (8%) and gentamicin treatment (4%). Similar low levels of positive cells with Geneticin were obtained in the compound heterozygote cell lines TGA-T1 (5%), TGA-C1/TAA-G2 (7%), and TGA-T1/TAG-A2 (3%). We tested an additional XP-C patient cell line we recently received (XP495BE) that is a compound heterozygote with the same TAG-A1 (Lys692X) PTC as the cell line XP54BE (Table S1). Like the XP54BE cells (Fig. 2C), this cell line had no detectable XPC protein localization in the absence of treatment, and 6% of the cells were positive for XPC following 100 μg/mL Geneticin treatment. Of the compound heterozygote cell lines treated with gentamicin, TGA-T1, TGA-C1/TAA-G2, and TGA-T1/TAG-A2 showed only a few XPC-positive cells (1%, 2%, and 3%, respectively). However, we found that XPC protein persisted 24 h and 48 h after UV irradiation in gentamicin-treated TGA-T1,2 and TGA-A1,2 cells in contrast to wild-type cells (Fig. S4). These results are consistent with a lower frequency but prolonged repair of UV-induced DNA damage in untreated XP-C cells compared with normal cells (31) and with experiments of Lai et al. (18) using PTC-carrying ataxia–telangiectasia cells.
Fig. 2.
Effect of Geneticin and gentamicin on XPC, XPB, and XPD proteins. (A) XP-C cells were incubated with geneticin for 4 d and immunoblot was performed. XPC protein was detectable via immunoblotting in Geneticin-treated TGA-T1,2 and TGA-A1,2 only (arrows). The ratio (%) of the intensity of the XPC band to b-actin band is indicated. In total, three different experiments were performed. Shown are three representative western blots. (B) Cells were incubated with Geneticin or gentamicin for 3 d, and an immunofluorescence assay 1 h after local UV irradiation was performed. Geneticin and gentamicin induce post-UV XPC protein localization in TGA-T1,2 (yellow arrows) but not in TGA-G1,2exon 6. For the gentamicin-treated normal cells (Lower Left), two representative areas of the same coverslip are shown. (C) Quantification of XPC protein detected via immunofluorescence at sites of UV damage 1 h after UV exposure. One hundred nuclei were scored. Bars indicate mean ± SD of the percent positive cells for XPC. *P < 0.05, **P < 0.005, ***P < 0.0005. (D) Cells were incubated with Geneticin for 3 d, and an immunofluorescence assay 1 h after local UV irradiation was performed. Geneticin induced post-UV XPB or XPD protein recruitment in TGA-T1,2 (yellow arrows) but not in TGA-G1,2exon 6. (E) Quantification of XPB and XPD proteins detected via immunfluorescence at sites of UV damage 1 h after UV exposure. Bars indicate mean ± SD of the percent positive cells for XPB and XPD, and 100 nuclei were scored. *P < 0.05, **P < 0.005, ***P < 0.0005.
Geneticin Readthrough Induces Functional XPC Protein and DNA Damage Removal.
Because XPC has a central role in DNA damage recognition and recruitment of other NER factors, we investigated if Geneticin treatment recruits XPB and XPD helicases to sites of DNA damage. In untreated XP-C cells, there was no localization of these helicases (32), whereas after Geneticin treatment, XPB protein was significantly recruited to nuclear foci in six of eight cell lines (Fig. 2 D and E and Fig. S3B). The proportion of XPD-positive cells was significant only in TGA-T1,2, TGA-A1,2, and TGA-T1/TAG-A2. Our results demonstrate that the Geneticin-induced XPC protein is functional because it recruits XPB and XPD helicases to sites of DNA damage.
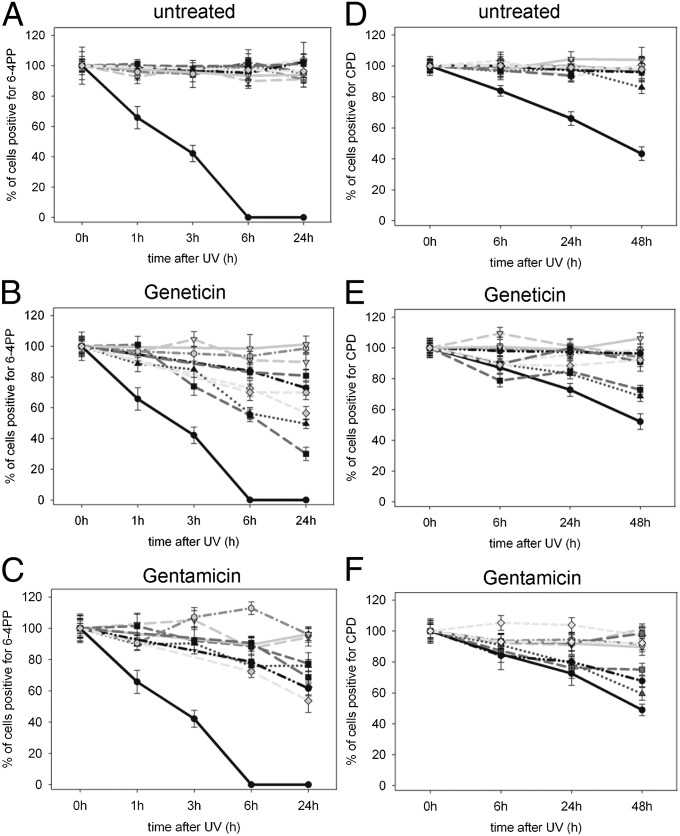
To investigate whether XPC, XPB, and XPD proteins are able to repair localized UV-induced DNA damage, we analyzed the removal of 6–4 pyrimidine pyrimidone photoproducts (6–4PPs) and CPDs. At 24 h after UV exposure, normal cells had no detectible 6–4PPs, whereas UV-exposed XP-C cells showed a high proportion of 6–4PPs, indicating little to no repair (Fig. 3A and Fig. S5A). Incubation with Geneticin or gentamicin (Fig. 3 B and C) resulted in fewer 6–4PP–positive nuclei in those XP-C cell lines that were positive for XPC, XPB, and XPD proteins, indicating a repair of these photoproducts. Counting the nuclei containing one or more 6–4PP immediately (0 h), and 1, 3, 6, and 24 h after local UV irradiation, revealed that among all cell lines TGA-T1,2 showed the highest repair rate of 6–4PPs when treated with Geneticin (70% 6–4PP removal at 24 h, P < 0.001). Furthermore, TGA-T1,2 cells showed a significantly higher repair rate than TGA-A1,2 cells (50% at 24 h, P = 0.001). Both cell lines also exhibited the highest proportion of XPC-, XPB-, and XPD-positive cells (Fig. 2E). The repair rate in Geneticin-treated compound heterozygous cells that were positive for XPC, XPB, or XPD was less compared with homozygous cells, which was in accordance with the fewer positive cells for XPC, XPB, and XPD proteins (Fig. 2E). TGA-T1 had the most pronounced 6–4PP removal rate (43% after 24 h, P = 0.04) among the compound heterozygous cells. Interestingly, this cell line (XP24BE) shares the same PTC at the same position in exon 4 (p.Arg155X) and the identical downstream nucleotide (T) with the homozygous cell line TGA-T1,2 (XP62DC), which showed the highest repair rate of 6–4PPs among the homozygous cell lines. This same p.Arg155X mutation is also present in the compound heterozygous cell line, XP54BE (Table S1), which showed lower 6–4PP repair. Geneticin induced a significantly greater removal of 6–4PPs compared with gentamicin in TGA-T1,2 (70% vs. 23% repair, P < 0.001) and TGA-A1,2 (50% vs. 24% repair, P < 0.001), whereas in TGA-G1,2exon 9, gentamicin was more effective (31% vs. 19% repair, P = 0.017).
Fig. 3.
Effect of Geneticin and gentamicin in removal of 6–4PPs and CPDs. XP-C cells were incubated with Geneticin or gentamicin for 3 d, and an immunofluorescence assay for detection of 6–4PPs and CPDs after local UV irradiation was performed. (A–C) Quantification of 6–4PP removal 0, 1, 3, 6, and 24 h after UV in (A) untreated, (B) Geneticin-treated, and (C) gentamicin-treated cells. (D–F) Quantification of CPD removal 0, 6, 24, and 48 h after UV irradiation in (D) untreated, (E) Geneticin-treated, and (F) gentamicin-treated cells. One hundred nuclei were scored. Bars indicate mean ± SD of the percent positive cells for 6–4PPs and CPDs. Legend:  Normal;
Normal;  ATG > AGG;
ATG > AGG;  TGA-T1,2;
TGA-T1,2;  TGA-T1;
TGA-T1;  TGA-A1,2;
TGA-A1,2;  TGA-G1,2 Exon 6;
TGA-G1,2 Exon 6;  TGA-C1/TAA-G2;
TGA-C1/TAA-G2;  TAA-A1; TGA-G1,2 Exon 9;
TAA-A1; TGA-G1,2 Exon 9;  TGA-T1/TAG-A2.
TGA-T1/TAG-A2.
The ratio of UV-induced CPDs to 6–4PPs is 3:1 and the 6–4PP create a larger DNA distortion and they are usually repaired faster than CPDs (33). After 48 h, although 47–50% of normal cells and 100% of untreated XP-C cells are CPD positive (Fig. 3D and Fig. S5B), Geneticin and gentamicin treatment (Fig. 3 E and F) induced repair of CPD only in the homozygous cell lines TGA-T1,2 (73% and 75% CPD-positive cells, respectively) and TGA-A1,2 (69% and 59% CPD-positive cells, respectively). Of interest, repair of CPD in TGA-C1/TAA-G2 cells was detected after gentamicin (72% CPD-positive cells) but not Geneticin treatment. These data suggest that aminoglycosides are less effective in the removal of CPDs than in removal of 6–4PPs, and this difference is more pronounced in the compound heterozygous XP-C cell lines.
To measure repair of uniform damage in the entire cell culture, using an ELISA we analyzed the removal of 6–4PP from DNA of cultures irradiated directly without polycarbonate isopore filter. At 6 h after UV exposure, normal cells had about 17% of the 6–4PPs remaining, whereas two different XP-C cell lines (TGA-T1,2 and TGA-T1/TAG-A2) had 38–45% 6–4PPs remaining, which is consistent with their DNA repair defect. Treatment with 100 μg/mL Geneticin significantly reduced the amount of 6–4PPs remaining to 13% in both XP-C cell lines (Fig. S6). Thus, both the localized UV treatment method (Fig. 3B) and the diffuse UV treatment demonstrated increased removal of 6–4PPs following Geneticin treatment.
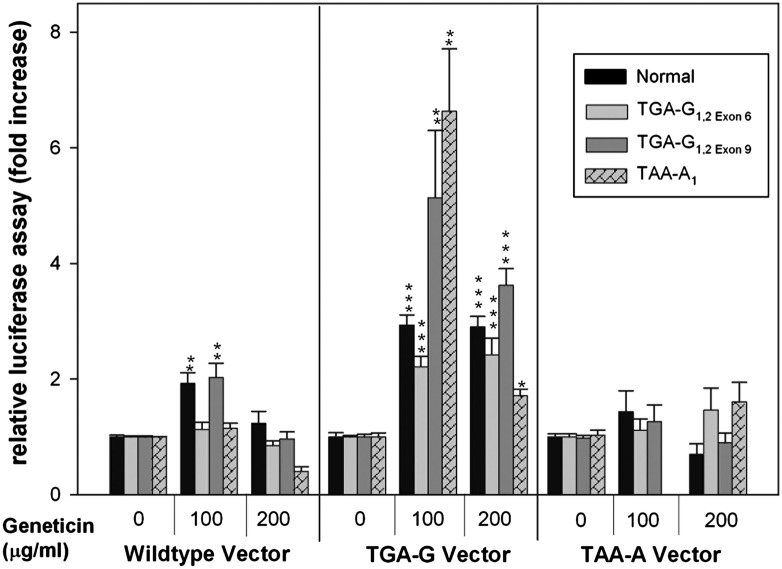
Geneticin Readthrough of pTGA-G but Not of pTAA-A.
The XP-C cell lines with TGA-G1,2exon 6 and TAA-A2 PTC showed no readthrough response (Figs. 2 and 3). To determine whether the location or context of the PTC within the gene was important for readthrough, we created these same TGA-G and TAA-A PTC in luciferase expression vectors using site-directed mutagenesis and transfected them into normal cells and into the XP-C cells TGA-G1,2exon 6, TGA-G1,2exon 9, and TAA-A2. Compared with wild-type vector, we found less than 1% luciferase activity of the PTC-containing vectors after transfection into normal or XP-C cells, indicating the strong termination efficiency of these PTCs (Fig. 4). Geneticin treatment resulted in two- to sevenfold increase of luciferase activity of TGA-G mutated vector in normal cells and in XP-C cells with TGA-G1,2exon 6, TGA-G1,2exon 9, or TAA-A2 PTC. Given that TGA-G1,2exon 6 showed no readthrough response in the other assays compared with TGA-G1,2exon 9, these results suggest that the readthrough efficiency of TGA-G depends on the specific location in XPC. In contrast, none of the transfected cells showed readthrough of the TAA-A mutated vector with Geneticin treatment, indicating that this sequence does not respond to treatment with this aminoglycoside. This finding is consistent with previous reports concerning poor efficiency of TAA readthrough (17, 34).
Fig. 4.
Increased readthrough of TGA-G but not TAA-A luciferase expression vectors with Geneticin. XP-C and normal cells incubated with or without Geneticin for 2 d were transfected with wild-type or mutated luciferase expression vectors containing indicated PTCs. Relative luciferase activity at 48 h after transfection is expressed as percent activity of mutated plasmid in Geneticin-treated cells compared with untreated cells. Bars indicate mean ± SD of the relative luciferase activity of three different experiments each in triplicate. The expression levels of the wild-type plasmid varied between 300,000 and 700,000 relative light units. *P < 0.05, **P < 0.005, ***P < 0.0005.
Nonaminoglycoside Readthrough of TGA-A1,2.
Because both aminoglycosides showed a high readthrough in the homozygous cell line TGA-A1,2 (Figs. 2 and 3), we tested the nonaminoglycoside compounds PTC124, BZ16 (a derivative of RTC13), and RTC14 in this cell line. We found that their effective doses were less toxic compared with Geneticin and gentamicin (Fig. 5A). XPC mRNA was significantly increased after treatment with PTC124 (65 fg), BZ16 (72 fg), and RTC14 (111 fg) compared with untreated cells (43 fg; Fig. 5B). Furthermore, all three compounds induced XPC protein localization following localized UV damage, but in a lower proportion compared with Geneticin and gentamicin (Fig. 5 C and D). This is in accord with Du et al. (24), showing RTC14 to be less effective than gentamicin and Geneticin in production of ATM protein. However, there was a significant increase in the repair of 6–4PPs and CPDs (Fig. 5 E and F) with PTC124, BZ16, and RTC14, indicating that they stimulated functional NER to a similar extent as aminoglycosides.
Fig. 5.
Increased readthrough of TGA-G1,2 with nonaminoglycosides. XP-C cells with TGA-G1,2 were incubated with nonaminoglycosides and aminoglycosides for 3 d. (A) MTT survival assay. The effective doses of PTC124, BZ16, and RTC14 used were less toxic in TGA-A1,2 compared with Geneticin and gentamicin. (B) Measurement of XPC mRNA. Treatment with PTC124, BZ16, and RTC14 and Geneticin resulted in significantly increased XPC mRNA. **P < 0.005. (C) Immunofluorescence assay in normal and XP-C TGA-G1,2 cells 1 h after local UV irradiation. Geneticin, gentamicin, PTC124, BZ16, and RTC14 induce post-UV XPC protein localization. (D) Quantification of XPC protein at sites of UV damage 1 h post UV (from C). Bars indicate mean ± SD of the percent positive cells for XPC. One hundred nuclei were scored for each bar. *P < 0.05, **P < 0.005, ***P < 0.0005. (E and F) Immunofluorescence assay for detection of 6–4PPs (E) and CPDs (F) 6 and 24 or 48 h after local UV irradiation. Bars indicate mean ± SD of the percent positive cells for 6-4PP and CPDs; 100 nuclei were scored.
Discussion
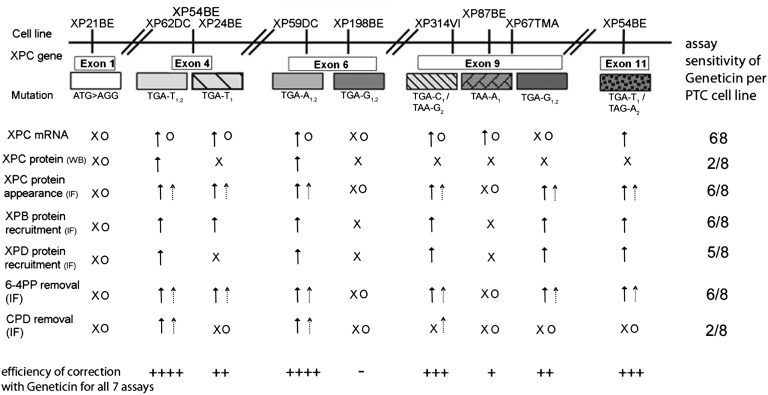
Previous studies of readthrough used in vitro assay systems that measured the response to isolated PTCs that were not located in cells from affected patients (15). Our cell-based assay system uses skin cells from XP patients and is probably more likely to be representative of physiological effects in human skin. We used a panel of DNA-repair–deficient XP-C cells carrying a variety of homozygous and compound heterozygous PTCs in different exons of XPC. We used seven different assays to assess the various steps of the post-UV NER pathway (2) (Fig. 6) and found that the immunofluorescence assay was extremely sensitive for detecting the appearance of localized XPC protein at the single cell level (six of the eight cell lines were positive in up to 24% of the nuclei). XPC protein function was demonstrated by XPB protein recruitment to localized UV damage and removal of 6–4PPs in the same six cell lines. However, we do not know whether the correct amino acid was incorporated opposite the PTC. In prokaryotes, Nilsson and Rydén-Aulin (35) reported that glutamine was inserted at UAG or UAA PTC, whereas UGA miscoded to tryptophan.
Fig. 6.
Summary of assays and XP-C PTC readthrough results. Summary of XP-C cell lines tested (top row), the type of PTC mutation in the XPC gene, the assays used to assess various steps of the post-UV NER pathway (first column: IF, immunofluorescence; WB, Western blot), the response to Geneticin and gentamicin in each assay, and summary assay sensitivity of the eight PTC cell lines for Geneticin (last column). The efficiency of correction with Geneticin for all seven assays (bottom row) is indicated by ++++ positive in seven assays, +++ positive in five assays, ++ positive in four assays, + positive in one assay, and – none.  , Geneticin response;
, Geneticin response;  , gentamicin response; x, no response to Geneticin; O, no response to gentamicin.
, gentamicin response; x, no response to Geneticin; O, no response to gentamicin.
Aminoglycosides and nonaminoglycoside small-molecule readthrough compounds were able to read through different types of PTC of XPC. Six of eight XP-C cell lines tested responded to aminoglycoside treatment (Fig. 6). This response depends on the type, copy number, and gene location of the PTC, the downstream 4+ base, and the readthrough compound used (16). Four of the XP-C cell lines had homozygous PTCs and two others were compound heterozygotes with two different PTCs. It appears that the efficiency of readthrough was in the order TGA > TAG > TAA (Fig. S7), in agreement with earlier studies in different species and assay systems (17, 21, 34). In support of this conclusion, we found Geneticin induced readthrough of a TGA PTC in a luciferase vector, whereas readthrough of TAA was not detected, indicating that the critical factor for TAA readthrough is the codon sequence (Fig. 4). As the two homozygous TGA-G1,2 cells responded differently (Fig. S7), the location of the PTC in the XPC gene seems to be crucial, as readthrough was detected in the vector (Fig. 4). Stop codons that are located more than 50 nt upstream of an exon–exon junction are generally recognized as premature, resulting in NMD (14). Among the XP-C cell lines tested, TGA-G1,2exon 6 is the only cell line whose PTC is located 38 nt upstream of exon 7 and whose mRNA would escape NMD. However, we measured substantial NMD (Fig. S1). Because Geneticin and gentamicin show no such readthrough with TGA-G1,2exon 6, other factors may affect its readthrough. We found a different readthrough response to Geneticin depending on the copy number of PTC present (Fig. 6 and Fig. S7): The homozygous cell line XP62DC (Arg155X) showed readthrough in all seven assays, XP54BE (Arg155X and Arg415X) had readthrough in five assays, and XP24BE (Arg155X) showed readthrough in four assays.
The efficiency of readthrough of PTC has been reported to be related to the downstream 3′ base (referred to as 4+ wobble). Out of 12 different sequences (3 PTCs × 4 bases at next 3′ position), eight types of PTC have been identified in XP-C cells, and we tested seven of them (Table S1 and Fig. S7). Among the homozygous XP-C cells, those with TGA-A1,2 and TGA-T1,2 responded in all seven repair assays, whereas XP-C cells with TGA-G1,2 responded in none (XP198BE) or four (XP67TMA) (Fig. 6 and Fig. S7). This supports the conclusion that readthrough of 3′ A or T is more efficient than that of G. However, that this hierarchy depends on the aminoglycoside, the gene, and the assay is shown in other studies [C > G>A > U (17), C > U>G > A (21), C > A, G > U (34)].
We found intriguing differences in photoproduct repair (Figs. 3 and 6 and Fig. S5). Although CPDs are about threefold more prevalent (36), 6–4PPs create greater DNA distortion and are repaired faster (33). In GGR, the XPC-Human Homolog of RAD23B protein complex acts together with the XPA–Replication Protein A complex to sense CPDs and 6–4PPs. They may have different affinities for each type of UV damage and might need DDB2 for recruitment to CPD, whereas XPC efficiently recognizes 6–4PPs (4, 37). Nevertheless, Emmert et al. (38) reported that a truncated XPC protein leads to repair of CPDs but not of 6–4PPs. Our data indicate that the ability to repair both types of DNA damage may depend on the amount of available XPC protein. The high XPC readthrough levels in TGA-T1,2 and TGA-A1,2 cells lead to repair of both 6–4PPs and CPDs, whereas the lesser amount of induced XPC protein in the XP-C compound heterozygous cells is sufficient to detect and repair 6–4PPs only. These findings suggest that the pathophysiology of the disease may play a role in readthrough efficacy.
Even low levels of XPC protein (3%) led to the recruitment of XPB (6%) and XPD (6%) in Geneticin-treated TGA-T1/TAG-A2 cells. This finding is in agreement with a previous report showing that a reduced level of XPC protein is sufficient to induce localization of other NER factors (32) and to reduce the frequency of skin cancers in XP-C patients (39). Strikingly, although TGA-T1 showed low levels of XPC protein (5%), we could measure a high 6–4PP removal rate. It is possible that readthrough in different exons of XPC renders dissimilar XPC protein structures due to incorporation of amino acids other than the normal ones, which affect repair efficiency. As little as 1% of normal protein function after readthrough may be sufficient to restore a near-normal or clinically less severe phenotype (40).
Our study serves as a “proof of principle” that readthrough of XPC PTC can be achieved with certain compounds despite their clinical toxicity. Topical application might be useful for the prevention of sunlight-induced skin cancers in XP-C patients and would eliminate much of the toxicity resulting from parenteral delivery (41). Shiozuka et al. (42) demonstrated that topically applied 0.1% gentamicin cream to shaved mdx dystrophin mouse skin induces readthrough of UGA. Similarly, one patient with Hailey–Hailey disease with a PTC in the ATP2C1 gene responded better to topical 0.1% gentamicin than to a boric acid control (43). The expressed XPC protein after treatment with less toxic small molecule nonaminoglycosides BZ16 and RTC14 might be as efficient in inducing DNA repair as aminoglycoside-induced XPC expression. Studies with CF patients showed that PTC124 effectively induced synthesis of full-length Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein and improved CFTR activity (44). Du et al. (24) reported that RTC13 and RTC14 are as efficient in readthrough of the ATM gene as Geneticin and gentamicin. Thus, these nonaminoglycosides might be unique therapeutic candidates for XP-C patients. Because they are small molecules (25) they may pass through the blood brain barrier, enabling treatment of neurodegenerative forms of XP (1, 2) and other diseases such as ataxia-telangectasia and Hurler syndrome.
The XP-C cell assay system is a promising model for evaluating readthrough of PTC and for testing new drugs. The assay permits the ability to identify which XP-C patients are likely to respond to a specific agent, the hallmark of personalized, or precision, medicine. The selection of study patients and agents on the basis of their in vitro efficacy will facilitate demonstrating efficacy and minimizing toxicity in clinical trials.
Materials and Methods
Cells Lines, Culture Conditions, and Drug Treatment.
Normal primary human skin fibroblasts (AG13154), from the Human Genetic Mutant Cell Repository, and XP-C fibroblasts containing PTC (Table S1) were cultured as described (30). The aminoglycosides Geneticin (G418 sulfate) and gentamicin sulfate (Enzo Life Sciences) were dissolved in sterile deionized water (5 mg/mL stock) and diluted in media as indicated. To examine the cytotoxicity of drugs post-UV, cell survival (Fig. S8) using CellTiter96 Non-Radioactive Cell Proliferation Assay (Promega) was assessed. BZ16, RTC14, and PTC124 synthesized by Michael Jung at the University of California, Los Angeles were dissolved in DMSO (50 mM stock) and diluted in media as indicated.
Quantitative Real-Time PCR.
Total RNA extracted from cells incubated for 3 d with compounds was used to quantitate the XPC mRNA as described (9). For measurement of nonsense-mediated decay, cells were incubated for 5 h in 200 μg/mL Cycloheximide (Sigma), and XPC mRNA was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of the sense and antisense primers used for GAPDH amplification were 5′-TGCACCACCAACTGCTTAGC-3′ and 5′-GGCATGGACTGTGGTCATGAG-0.3′, respectively.
Immunoblotting.
After 4 d of incubation with Geneticin or gentamicin, whole-cell lysates were prepared as described in ref. 38. Immunoblotting was performed using WesternBreeze Chemiluminescent Western Blot Immunodetection Kit (Invitrogen). The XPC antibody (Abcam) was used in a 1: 1,000 dilution; the β-actin antibody (Sigma) was used in a 1:5,000 dilution. The autoradiographic band intensity of XPC was measured with a laser densitometer and normalized to the intensity of the β-actin band using Image J.
Local UV Irradiation and Immunofluorescence.
Cells were grown on microscope cover glass (Fisher Scientific) and incubated for 3 d in media containing compounds. Cells were covered with a polycarbonate isopore membrane (pore size, 5 μm; diameter, 25 mm; Millipore). Local UV irradiation (254 nm, 100 J/m2) was performed as described (29). Following UV irradiation, cells were incubated in media with or without compounds for different times (0, 1, 3, 6, 24, and 48 h). Immunofluorescent labeling and imaging was performed as described (30) with the following changes: cells were incubated with antibodies against XPB, XPC, and XPD (Santa Cruz) overnight, and for the detection of CPD and 6–4PP cellular DNA was denatured for 20 min using 2N HCl before blocking and overnight incubation with TDM-2 and 64M-2 mouse monoclonal antibodies (Cosmo Bio).
ELISA.
Cells were incubated for 3 d in media containing 100 μg/mL Geneticin. Cells were irradiated with 10 J/m2 UVC and incubated in media with or without Geneticin for 0 h and 6 h. ELISA was performed using the OxiSelect UV-Incuded DNA Damage ELISA Kit (6-4PP Quantification; Cell Biolabs, Inc) with the following changes: FBS for the first blocking step and 6–4M-2 antibody (Cosmo Bio) was used.
Luciferase Assay.
The pGL3 luciferase vector (Promega) was subject to site-directed mutagenesis to create TGA (R261X) using Bioinnovatise’s Site-directed Mutagenesis Service (Bioinnovatise). For TAA, the QuikChange Site-Directed Mutagenesis Kit (Stratagene) was used as per vendor’s protocol and appropriate primers (forward, CGATCCCTTCAGGATTACTAAATTCAAAGTGCGTTGC; reverse, GCAACGCACTTTGAATTTAGTAATCCTGAAGGGATCG) to create TAA (K281X) in pGL3. Transfection of 70,000 fibroblasts with 1 μg plasmid was performed using AG Transgen Transfection Reagent and the vendor’s protocol (American Gene Technologies International Inc.). Luciferase activity was measured after 48 h in cell lysates using Promega’s Luciferase Assay System (Promega). Relative luciferase activity is expressed as percentage activity of Geneticin-treated plasmids compared with untreated plasmids.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH) (to C.K., J.J.D., S.G.K., and K.H.K.) and NIH R01 Grant NS05528 (to R.A.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312088110/-/DCSupplemental.
References
- 1.Bradford PT, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: Long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiGiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol. 2012;132(3 Pt 2):785–796. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleijer WJ, et al. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7(5):744–750. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Clement FC, et al. Dynamic two-stage mechanism of versatile DNA damage recognition by xeroderma pigmentosum group C protein. Mutat Res. 2010;685(1-2):21–28. doi: 10.1016/j.mrfmmm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Gozukara EM, et al. A stop codon in xeroderma pigmentosum group C families in Turkey and Italy: Molecular genetic evidence for a common ancestor. J Invest Dermatol. 2001;117(2):197–204. doi: 10.1046/j.1523-1747.2001.01424.x. [DOI] [PubMed] [Google Scholar]
- 6.Khan SG, et al. Reduced XPC DNA repair gene mRNA levels in clinically normal parents of xeroderma pigmentosum patients. Carcinogenesis. 2006;27(1):84–94. doi: 10.1093/carcin/bgi204. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Bales ES, Peterson CA, Legerski RJ. Characterization of molecular defects in xeroderma pigmentosum group C. Nat Genet. 1993;5(4):413–417. doi: 10.1038/ng1293-413. [DOI] [PubMed] [Google Scholar]
- 8.Khan SG, et al. Xeroderma pigmentosum group C splice mutation associated with autism and hypoglycinemia. J Invest Dermatol. 1998;111(5):791–796. doi: 10.1046/j.1523-1747.1998.00391.x. [DOI] [PubMed] [Google Scholar]
- 9.Khan SG, et al. The human XPC DNA repair gene: Arrangement, splice site information content and influence of a single nucleotide polymorphism in a splice acceptor site on alternative splicing and function. Nucleic Acids Res. 2002;30(16):3624–3631. doi: 10.1093/nar/gkf469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobelli S, et al. Xeroderma pigmentosum group C in a French Caucasian patient with multiple melanoma and unusual long-term survival. Br J Dermatol. 2008;159(4):968–973. doi: 10.1111/j.1365-2133.2008.08791.x. [DOI] [PubMed] [Google Scholar]
- 11.Soufir N, et al. A prevalent mutation with founder effect in xeroderma pigmentosum group C from north Africa. J Invest Dermatol. 2010;130(6):1537–1542. doi: 10.1038/jid.2009.409. [DOI] [PubMed] [Google Scholar]
- 12.Lam CW, et al. DNA-based diagnosis of xeroderma pigmentosum group C by whole-genome scan using single-nucleotide polymorphism microarray. J Invest Dermatol. 2005;124(1):87–91. doi: 10.1111/j.0022-202X.2004.23563.x. [DOI] [PubMed] [Google Scholar]
- 13.Schäfer A, et al. Molecular genetic analysis of 16 XP-C patients from Germany: Environmental factors predominately contribute to phenotype variations. Exp Dermatol. 2013;22(1):24–29. doi: 10.1111/exd.12052. [DOI] [PubMed] [Google Scholar]
- 14.Maquat LE. Nonsense-mediated mRNA decay in mammals. J Cell Sci. 2005;118(Pt 9):1773–1776. doi: 10.1242/jcs.01701. [DOI] [PubMed] [Google Scholar]
- 15.Lee HL, Dougherty JP. Pharmaceutical therapies to recode nonsense mutations in inherited diseases. Pharmacol Ther. 2012;136(2):227–266. doi: 10.1016/j.pharmthera.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Gatti RA. SMRT compounds correct nonsense mutations in primary immunodeficiency and other genetic models. Ann N Y Acad Sci. 2012;1250:33–40. doi: 10.1111/j.1749-6632.2012.06467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard MT, et al. Readthrough of dystrophin stop codon mutations induced by aminoglycosides. Ann Neurol. 2004;55(3):422–426. doi: 10.1002/ana.20052. [DOI] [PubMed] [Google Scholar]
- 18.Lai CH, et al. Correction of ATM gene function by aminoglycoside-induced read-through of premature termination codons. Proc Natl Acad Sci USA. 2004;101(44):15676–15681. doi: 10.1073/pnas.0405155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hainrichson M, Nudelman I, Baasov T. Designer aminoglycosides: The race to develop improved antibiotics and compounds for the treatment of human genetic diseases. Org Biomol Chem. 2008;6(2):227–239. doi: 10.1039/b712690p. [DOI] [PubMed] [Google Scholar]
- 20.Wilschanski M, et al. A pilot study of the effect of gentamicin on nasal potential difference measurements in cystic fibrosis patients carrying stop mutations. Am J Respir Crit Care Med. 2000;161(3 Pt 1):860–865. doi: 10.1164/ajrccm.161.3.9904116. [DOI] [PubMed] [Google Scholar]
- 21.Politano L, et al. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003;22(1):15–21. [PubMed] [Google Scholar]
- 22.Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev. 2003;16(3):430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirawat S, et al. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47(4):430–444. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- 24.Du L, et al. Nonaminoglycoside compounds induce readthrough of nonsense mutations. J Exp Med. 2009;206(10):2285–2297. doi: 10.1084/jem.20081940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du L, et al. A new series of novel small molecular weight compounds induce readthrough of all three types of nonsense mutations in the ATM gene. Mol Ther. 2013;21(9):1653–1660. doi: 10.1038/mt.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellais S, Le Goff C, Dagoneau N, Munnich A, Cormier-Daire V. In vitro readthrough of termination codons by gentamycin in the Stüve-Wiedemann Syndrome. Eur J Hum Genet. 2010;18(1):130–132. doi: 10.1038/ejhg.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavanne F, et al. Mutations in the XPC gene in families with xeroderma pigmentosum and consequences at the cell, protein, and transcript levels. Cancer Res. 2000;60(7):1974–1982. [PubMed] [Google Scholar]
- 28.de Feraudy S, et al. Diagnosing xeroderma pigmentosum group C by immunohistochemistry. Am J Dermatopathol. 2010;32(2):109–117. doi: 10.1097/DAD.0b013e3181af0a5e. [DOI] [PubMed] [Google Scholar]
- 29.Imoto K, et al. The total amount of DNA damage determines ultraviolet-radiation-induced cytotoxicity after uniformor localized irradiation of human cells. J Invest Dermatol. 2002;119(5):1177–1182. doi: 10.1046/j.1523-1747.2002.19514.x. [DOI] [PubMed] [Google Scholar]
- 30.Oh KS, Imoto K, Boyle J, Khan SG, Kraemer KH. Influence of XPB helicase on recruitment and redistribution of nucleotide excision repair proteins at sites of UV-induced DNA damage. DNA Repair (Amst) 2007;6(9):1359–1370. doi: 10.1016/j.dnarep.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins JH, Kraemer KH. Prolonged ultraviolet-induced thymidine incorporation into xeroderma pigmentosum lymphocytes: Studies on its duration, amount, localization and relationship to hydroxyurea. Biochim Biophys Acta. 1972;277(1):7–14. doi: 10.1016/0005-2787(72)90345-0. [DOI] [PubMed] [Google Scholar]
- 32.Khan SG, et al. XPC branch-point sequence mutations disrupt U2 snRNP binding, resulting in abnormal pre-mRNA splicing in xeroderma pigmentosum patients. Hum Mutat. 2010;31(2):167–175. doi: 10.1002/humu.21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi N, et al. Quantitation and visualization of ultraviolet-induced DNA damage using specific antibodies: Application to pigment cell biology. Pigment Cell Res. 2001;14(2):94–102. doi: 10.1034/j.1600-0749.2001.140204.x. [DOI] [PubMed] [Google Scholar]
- 34.Manuvakhova M, Keeling K, Bedwell DM. Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA. 2000;6(7):1044–1055. doi: 10.1017/s1355838200000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson M, Rydén-Aulin M. Glutamine is incorporated at the nonsense codons UAG and UAA in a suppressor-free Escherichia coli strain. Biochim Biophys Acta. 2003;1627(1):1–6. doi: 10.1016/s0167-4781(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 36.Sinha RP, Häder DP. UV-induced DNA damage and repair: A review. Photochem Photobiol Sci. 2002;1(4):225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 37.Moser J, et al. The UV-damaged DNA binding protein mediates efficient targeting of the nucleotide excision repair complex to UV-induced photo lesions. DNA Repair (Amst) 2005;4(5):571–582. doi: 10.1016/j.dnarep.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Emmert S, Kobayashi N, Khan SG, Kraemer KH. The xeroderma pigmentosum group C gene leads to selective repair of cyclobutane pyrimidine dimers rather than 6-4 photoproducts. Proc Natl Acad Sci USA. 2000;97(5):2151–2156. doi: 10.1073/pnas.040559697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan SG, et al. Two essential splice lariat branchpoint sequences in one intron in a xeroderma pigmentosum DNA repair gene: Mutations result in reduced XPC mRNA levels that correlate with cancer risk. Hum Mol Genet. 2004;13(3):343–352. doi: 10.1093/hmg/ddh026. [DOI] [PubMed] [Google Scholar]
- 40.Zingman LV, Park S, Olson TM, Alekseev AE, Terzic A. Aminoglycoside-induced translational read-through in disease: Overcoming nonsense mutations by pharmacogenetic therapy. Clin Pharmacol Ther. 2007;81(1):99–103. doi: 10.1038/sj.clpt.6100012. [DOI] [PubMed] [Google Scholar]
- 41.Fischel-Ghodsian N. Genetic factors in aminoglycoside toxicity. Pharmacogenomics. 2005;6(1):27–36. doi: 10.1517/14622416.6.1.27. [DOI] [PubMed] [Google Scholar]
- 42.Shiozuka M, et al. Transdermal delivery of a readthrough-inducing drug: A new approach of gentamicin administration for the treatment of nonsense mutation-mediated disorders. J Biochem. 2010;147(4):463–470. doi: 10.1093/jb/mvp185. [DOI] [PubMed] [Google Scholar]
- 43.Kellermayer R, Szigeti R, Keeling KM, Bedekovics T, Bedwell DM. Aminoglycosides as potential pharmacogenetic agents in the treatment of Hailey-Hailey disease. J Invest Dermatol. 2006;126(1):229–231. doi: 10.1038/sj.jid.5700031. [DOI] [PubMed] [Google Scholar]
- 44.Wilschanski M, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J. 2011;38(1):59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.