Significance
Major depressive disorder is a significant contributor to the global burden of disease, affecting 350 million people according to an estimation of the World Health Organization. Today, no valid biomarkers of depression, which could predict the efficacy of a certain treatment in a certain group of patients, exist. Sleep deprivation is an effective and rapid-acting antidepressive treatment. However, the biomechanism of this effect is largely unknown. This study shows the effects of sleep deprivation on human brain functional connectivity alterations via the dorsal nexus, an area which is crucial in major depressive disorder. Here, we offer a neurobiological explanation for the known antidepressive action of sleep deprivation.
Abstract
In many patients with major depressive disorder, sleep deprivation, or wake therapy, induces an immediate but often transient antidepressant response. It is known from brain imaging studies that changes in anterior cingulate and dorsolateral prefrontal cortex activity correlate with a relief of depression symptoms. Recently, resting-state functional magnetic resonance imaging revealed that brain network connectivity via the dorsal nexus (DN), a cortical area in the dorsomedial prefrontal cortex, is dramatically increased in depressed patients. To investigate whether an alteration in DN connectivity could provide a biomarker of therapy response and to determine brain mechanisms of action underlying sleep deprivations antidepressant effects, we examined its influence on resting state default mode network and DN connectivity in healthy humans. Our findings show that sleep deprivation reduced functional connectivity between posterior cingulate cortex and bilateral anterior cingulate cortex (Brodmann area 32), and enhanced connectivity between DN and distinct areas in right dorsolateral prefrontal cortex (Brodmann area 10). These findings are consistent with resolution of dysfunctional brain network connectivity changes observed in depression and suggest changes in prefrontal connectivity with the DN as a brain mechanism of antidepressant therapy action.
Sleep deprivation has been used for decades as a rapid-acting and effective treatment in patients with major depressive disorder (MDD) (1, 2). Although clinically well established, the mechanisms of action are largely unknown.
Brain imaging studies have shown that sleep deprivation in depressed patients is associated with renormalized metabolic activity, mainly in limbic structures including anterior cingulate (ACC) as well as dorsolateral prefrontal cortex (DLPFC) (3–6), and that changes in limbic and DLPFC activity correlated with a relief of depression symptoms (7–9). Recent studies in patients with depression point to a critical importance of altered large-scale brain network connectivity during the resting state (10, 11). Among these networks, the default mode network (DMN), which mainly comprises cortical midline structures including precuneus and medial frontal cortex as well as the inferior parietal lobule (12–15), is most consistently characterized. In functional magnetic resonance imaging (fMRI) studies, the DMN shows the strongest blood oxygenation level–dependent (BOLD) activity during rest and decreased BOLD reactivity during goal-directed task performance. The DMN is anticorrelated with the cognitive control network (CCN), a corresponding task-positive network, which encompasses bilateral fronto-cingulo-parietal structures including lateral prefrontal and superior parietal areas (16). A third system with high relevance for depression—the affective network (AN)—is based in the subgenual and pregenual parts of the ACC [Brodman area (BA) 32] (17). The AN is active during both resting and task-related emotional processing, and forms strong functional and structural connections to other limbic areas such as hypothalamus, amygdala, entorhinal cortex, and nucleus accumbens (18, 19).
Increased connectivity of DMN, CCN, and AN with a distinct area in the bilateral dorsomedial prefrontal cortex (DMPFC) was recently found in patients with depression compared with healthy controls (20). This area within the DMPFC was termed dorsal nexus (DN) and was postulated to constitute a converging node of depressive “hot wiring,” which manifests itself in symptoms of emotional, cognitive, and vegetative dysregulation. This led to the hypothesis that a modification in connectivity via the DN would be a potential target for antidepressant treatments (20).
Recent studies in healthy subjects reported reduced functional connectivity within DMN and between DMN and CCN in the morning after total (21) and in the evening after partial sleep deprivation (22). However, brain network connectivity via the DN was not examined in these studies. Given the recently proposed role of the DN in mood regulation, here we specifically tested whether sleep deprivation as a well-known antidepressant treatment modality affects connectivity via the DN. Based on our previous findings on network changes by ketamine (23), we hypothesized that sleep deprivation leads to a reduction in connectivity via the DN.
Results
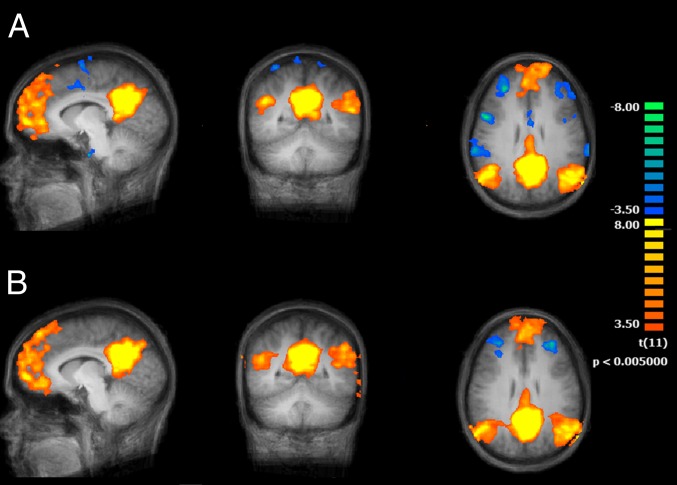
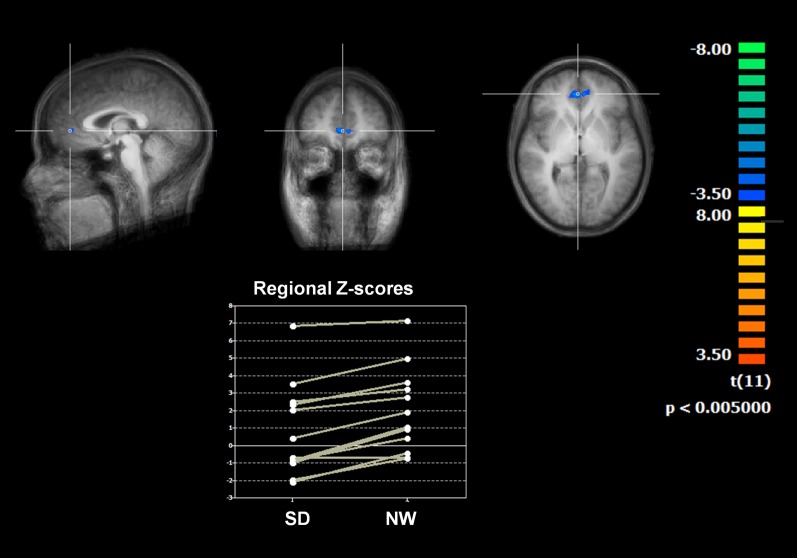
Both after normal sleep and sleep deprivation, posterior cingulate cortex (PCC) seed-based analysis revealed a clear depiction of the DMN, with positively correlated clusters in orbital cortex and DMPFC, inferior parietal lobule, and precuneus (all bilateral) and negatively correlated clusters in medial frontal and lateral prefrontal cortex (Fig. 1). The DMN was determined using a seed region identical to a previous work (center of this region: x = −8, y = −49, z = 28) (23). It is important to note that, consistent with previous studies (22, 24), sleep deprivation reduced functional connectivity of the PCC with the ACC (BA 32; coordinates: +5, +43, +3; z score = 3.7173, P = 0.000201; cluster size = 359 mm3) (Fig. 2). On the other hand, when we selected a seed region in the precuneus, the resulting functional connectivity analysis yielded no differential effects between sleep deprivation and normal conditions over the entire brain at the used statistical threshold.
Fig. 1.
Functional connectivity maps (n = 12) of the brain using the PCC as seed region and comparing two conditions, normal wakefulness (A) and sleep deprived (B) (main effects, P < 0.05 cluster corrected).
Fig. 2.
The contrast of the two conditions shows a reduced connectivity between the PCC seed and the bilateral ACC (BA 32; coordinates: +5, +43, +3; z score = 3.7173, significance = 0.000201; cluster size = 359 mm) after sleep deprivation (SD) compared with normal wakefulness (NW).
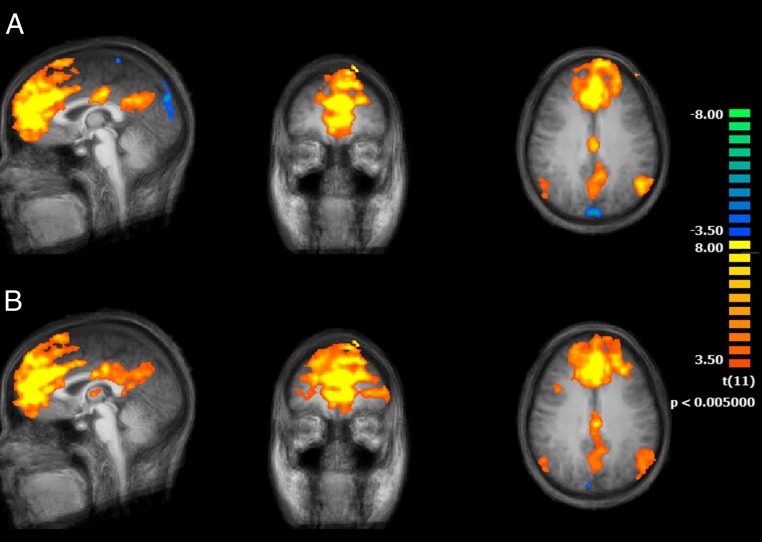
The DN seed-based analysis also allowed a delineation of the main DMN nodes. The overlap with the DMN, however, was only partial. The contribution of positively connected regions was more prominent in the anterior part of the brain, including putative regions of other networks such as CCN and AN, and less prominent in the posterior part of the brain (Fig. 3).
Fig. 3.
Functional connectivity maps (n = 12) of the brain using the DN as seed region and comparing the two conditions, normal wakefulness (A) and sleep deprived (B) (main effects, P < 0.05 cluster corrected).
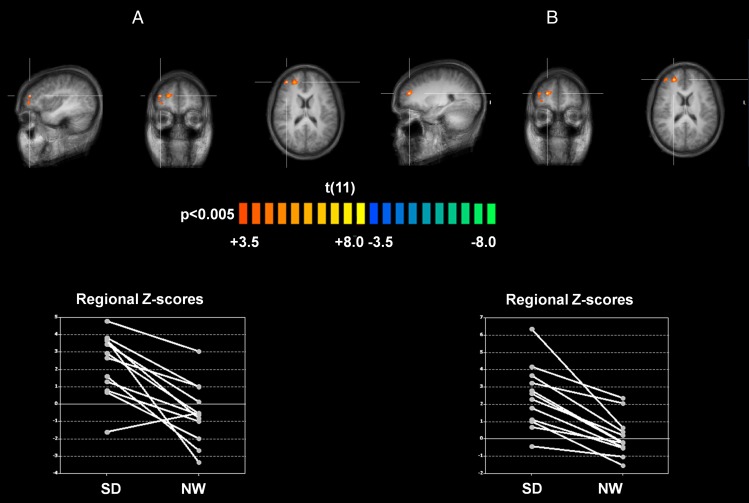
It is interesting that, in contrast to the known effects of sleep deprivation on DMN connectivity, DN connectivity was altered in unexpected manner. More specifically, compared with the normal sleep condition, DN connectivity after sleep deprivation was significantly increased in right anterior middle (coordinates: +38, +46, +18; z score = 3.5413, significance = 0.000498; cluster size = 324 mm3) and superior frontal gyri (coordinates: +23, +46, +18; z score = 4.202, significance = 0.000026; cluster size = 1,092 mm3), both located in DLPFC and pertaining to the dorsolateral part of BA 10 (border to BA 46; Fig. 4). These frontal regions appear consistently and positively correlated with DN only in the sleep deprivation condition, whereas in the normal wakefulness condition, half or more of the subjects exhibited negative scores.
Fig. 4.
The contrast of the two conditions shows an increased connectivity between the DN seed and two areas (A) on the right middle (BA 10, DLPFC, spot 1: coordinates: +38, +46, +18; z score = 3.5413, significance = 0.000498; cluster size = 324 mm) and (B) on the right superior frontal gyrus (BA 10, DLPFC; spot 2: coordinates: +23, +46, +18; z score = 4.202, significance = 0.000026; cluster size = 1,092 mm) after SD compared with NW.
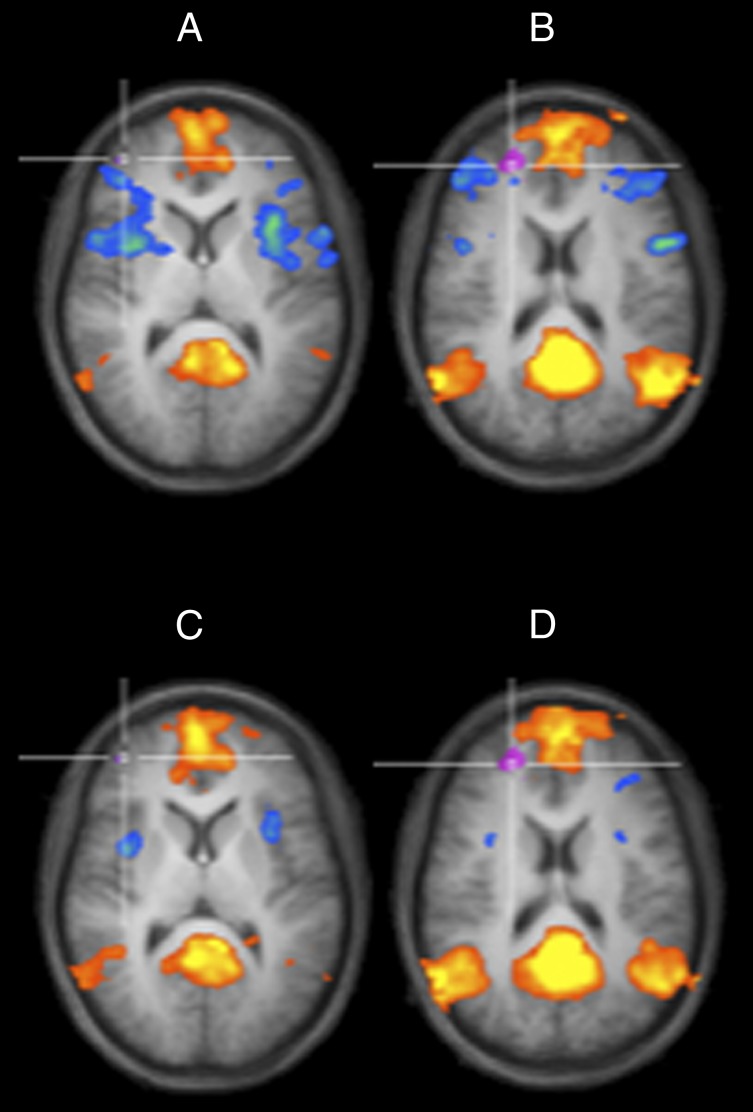
A comparison of the DMN connectivity changes and the DN connectivity changes due to sleep deprivation (Fig. 5) shows a reduction of the DMN–CCN anticorrelations from the normal wakefulness (Fig. 5 A and B) to the sleep deprivation (Fig. 5 C and D) condition. Although it does not reach statistical significance, which might be due to the small sample size, this tendency is in line with previous studies (21, 22). More important, this comparison clarifies that the main effect of sleep deprivation on the functional connectivity of the DN is not to reduce DMN anticorrelations in these regions, but rather to promote their positive correlation to the DN.
Fig. 5.
Comparison of the locations of the DMN-CCN spots of Fig. 1 after (A) normal wakefulness and (C) SD with the DN spots of Fig. 4 after (B) normal wakefulness and (D) SD. An axial cut was generated at the location of the DN spots, keeping the positive and negative correlations of the PCC seed overlaid on the same average anatomy to show in detail the location of these spots with respect to the CCN spots.
To control vigilance during fMRI, the EEG was recorded. In both conditions, subjects were awake for more than 95% of imaging time, and no differences in vigilance states were found between the conditions (P > 0.6). As expected, spectral power in delta and theta frequencies (<8 Hz) was slightly enhanced after prolonged wakefulness, yet did not differ significantly from the normal wakefulness condition (pall > 0.3; Table 1).
Table 1.
Results of EEG spectral analysis during fMRI scanning
| Frequency range (Hz) | Normal wakefulness (μV2) | Sleep deprivation (μV2) | Difference ± SEM | t value | P value |
| 0.5–4.5 | 4.64 ± 0.57 | 5.28 ± 0.65 | 0.65 ± 0.64 | 1.01 | 0.33 |
| 4.5–8.0 | 4.29 ± 0.82 | 4.59 ± 0.78 | 0.30 ± 0.60 | 0.49 | 0.64 |
| 8.0–11.0 | 2.01 ± 0.42 | 2.06 ± 0.40 | 0.05 ± 0.21 | 0.23 | 0.82 |
| 11.0–15.0 | 1.17 ± 0.21 | 1.26 ± 0.33 | 0.09 ± 0.24 | 0.38 | 0.71 |
| 15.0–25.0 | 0.96 ± 0.81 | 0.87 ± 0.56 | −0.09 ± 0.25 | −0.35 | 0.74 |
Means ± SEM (n = 12) represent EEG spectral power values in consecutive frequency bands (delta, theta, alpha, and beta) averaged over all recording electrodes during fMRI scanning in normal wakefulness and sleep deprivation conditions.
Discussion
Here we delineate a differential pattern of resting-state connectivity after sleep deprivation, which is a potent, rapidly acting antidepressant intervention with a largely unknown mode of action. More specifically, using resting-state fMRI in healthy subjects, we found that sleep deprivation reduced functional connectivity between the PCC and the bilateral ACC (BA 32), yet increased connectivity between the DN and two areas within the right DLPFC (BA 10). Although the previous observation was consistent with our a priori hypothesis, the latter observation was not expected. Visual inspection and quantification of the EEG during scanning confirmed that the changes in connectivity were not caused by the occurrence of spontaneous sleep after prolonged waking.
There is growing evidence that brain connectivity within the DMN and between the DMN and other brain networks is altered in patients with MDD compared with healthy volunteers, primarily reflecting dysfunctional self-referential processing such as rumination, negative anticipation, and excessive feelings of guilt and shame (11). We explored the functional connectivity of the PCC as a core seed of the DMN, after normal wakefulness and sleep deprivation in healthy subjects. In both conditions, we found a clear depiction of the DMN (13), including the PCC, orbital and dorsal parts of the medial prefrontal cortex (MPFC), inferior parietal lobule, and precuneus (all bilateral; Fig. 1). The comparison between normal sleep and sleep deprivation revealed a significantly reduced connectivity of the PCC to the bilateral ACC (BA 32) after sleep deprivation (Fig. 2).
The ACC is seen as a key structure for emotional processing and depressive psychopathology and is part of the AN (25). Numerous studies using event-related and resting-state fMRI designs point to alterations in ACC activity in depressed patients (26–29). The first resting-state fMRI study with MDD patients revealed a hyperconnectivity between the subgenual ACC and the DMN, confirming previous PET studies, which found resting state overactivity in ACC in these patients (30). Hyperconnectivity in the MPFC and ventral ACC was correlated with rumination in another fMRI study (31). Here, we found that sleep deprivation reduced connectivity on the ACC–PCC axis. This is of particular interest because resting-state DMN dominance is associated with increased maladaptive, depressive rumination, and reduced adaptive, reflective rumination (32). Depressed patients typically fail to down-regulate DMN activity during emotional stimulation (33, 34). Our finding of reduced intrinsic DMN connectivity after sleep deprivation resembles a pattern of normalization with regard to the depressive state. Keeping in mind the multiple functional connections of the ACC, a connectivity reduction to the DMN might be viewed as a potential therapeutic effect in patients who suffer from excessive ACC network contribution during rest, as it is observed during rumination.
Our study focused on the DN as a specific seed region, which plays a crucial role in the pathophysiology of depression, and was recently discovered as a node mediating dramatic functional hyperconnectivity between DMN, CCN, and AN in patients with MDD (20). This hot wiring via the DN was proposed to underlie core depressive symptoms such as rumination and hyperarousal, as well as affective and vegetative dysregulation. A reduction of DN connectivity may, thus, represent a neurobiological target for antidepressant treatment strategies and a potential biomarker of antidepressant response. Two recent studies tested this so-called DN hypothesis using psychopharmacological challenges in healthy subjects. The first revealed reduced functional connectivity between DN and hippocampus after 7 d of citalopram administration (35). The second showed reduced DN connectivity with the PCC and the pregenual ACC 24 h after ketamine infusions (23). These studies support the hypothesis that reducing DN connectivity to subcortical structures and the DMN network may represent a biomechanism of antidepressant action.
In the present study, we observed similar DN connectivity to the key structures of the DMN and the AN after normal sleep and sleep deprivation. We found in both conditions significant DN connectivity to the bilateral PCC, bilateral precuneus, and bilateral parietal lobules (Fig. 3), indicating that the DN is highly correlated with intrinsic DMN connectivity in healthy subjects. Compared with normal sleep, sleep deprivation induced an expansion of the DN connectivity pattern with the DMPFC toward more dorsolateral regions in the deprivation condition. This effect led to the delineation of two sleep deprivation–related areas of significant hyperconnectivity in the right anterior middle and superior frontal gyrus, both of which are located in DLPFC (BA 10/BA 46; Fig. 4). These areas showed negative scores in the normal wakefulness condition in half or more of the subjects, suggesting the possibility that these regions could be anticorrelated with the DN in their initial status. From a metabolic perspective this means that sleep deprivation may actually weaken the anticorrelation, rather than increasing the correlation of these regions to the DN. However, as a differential effect, we observe a change in the functional connectivity distribution where these regions are initially not part of a positive or negative DN network, which in turn seems to recruit them specifically in the sleep deprivation–altered condition.
The comparison of the functional connectivity changes of the DMN and DN networks after normal wakefulness and sleep deprivation (Fig. 5) shows that the negative correlations supporting the DMN–CCN anticorrelation result weakened in the sleep deprivation condition in line with previous studies. Furthermore, the DN region functionally connects to a frontally emphasized DMN due to its overlap with the DMPFC node of the PCC-based DMN pattern. After sleep deprivation, new regions in the superior and lateral frontal cortex participate to this network, although these regions are not part of the CCN.
Our results are consistent with several PET studies highlighting the importance of two distinct brain structures for the pathophysiology and treatment of depression, namely the ACC and the DLPFC.
Baseline brain metabolism, as measured with PET, is consistently increased in MDD patients in limbic structures such as the MPFC and ACC, and is consistently reduced in the DLPFC (36–41). In depressed patients, selective serotonin reuptake inhibitor (SSRI) treatment resulted in increased metabolic activity in the middle frontal gyrus (42) and the prefrontal cortex (43). Furthermore, a PET study showed that increased ACC activity before antidepressant sleep deprivation was correlated with reduction in depression symptoms (4). It is intriguing that the positive treatment response following sleep deprivation correlated with decreased activity in the ACC (BA 32) and increased metabolism in areas including the right DLPFC (BA 46). This relationship of depression symptom relief with a metabolic decrease in inferior and orbital frontal areas and an increase in the DLPFC was recently partly confirmed with a higher resolution MRI scanner (6). In addition, patients with bipolar depression who responded to sleep deprivation had decreased activity in the ACC (BA 32, 24) and an increased activity in the DLPFC (BA 10, 46) in reaction to negative visual stimuli during an fMRI task (comparing pretreatment to posttreatment) (7). Increased prefrontal responsivity to cognitive demands after sleep deprivation was also observed in healthy subjects (44). Wu et al. and Benedetti et al. interpreted the increase of DLPFC activity—which correlated with symptom improvement—as a sleep deprivation–related reactivation of top–down control on negative emotional processing (6, 7). This interpretation is supported by reports of DLPFC reactivity in relation to voluntary suppression of sadness (45) and decrease of DLPFC metabolism in PET after induction of transient sadness in healthy subjects (46). Regarding brain network connectivity, the DLPFC is a canonical structure of the CCN, representing conscious control of executive functions and mental representations (16).
In conclusion, the current pattern of altered resting-state connectivity induced by sleep deprivation—dissociation of ACC from DMN and recruitment of CCN to DN—may indicate a shift from affective to cognitive network contributions to the DMN, which could be beneficial in depressed patients who suffer from excessive ACC and/or impaired DLPFC function. Therefore, our data warrant an extension of the DN hypothesis and the integration of differential changes in brain network connectivity into the framework of mechanisms underlying antidepressant effects of sleep deprivation. Further research is needed to identify potential therapeutic benefits of matching altered functional connectivity patterns in depression with corresponding patterns of action of antidepressant treatments.
Materials and Methods
Study Subjects.
Healthy female subjects (n = 12, mean age, 23.42 ± 3.12 [SD]) without any psychiatric, neurological, or medical illness were self-referred from online study advertisements. The study was approved by the University of Zurich institutional review board, and subjects gave written informed consent before screening. All subjects underwent a psychiatric interview and medical examination. Exclusion criteria were a history of psychiatric/neurological diseases, sleep–wake cycle abnormalities, drug abuse, concurrent medication, cardiovascular disease, anemia, or thyroid disease, MR exclusion criteria, and pregnancy. The week before the experiments, subjects were obliged to follow a regular sleep–wake pattern with bedtimes between 10:00 PM and 8:00 AM. Wrist actigraphy was assessed for the sleep deprivation night and the following day.
Sleep Deprivation and Experimental Protocol.
Subjects underwent two fMRI measurements—once well-rested after normal sleep and once after sleep deprivation—at 6:00 PM or 8:30 PM (randomized, two subjects scanned after each other, scanning time kept constant). During sleep deprivation, participants slept from 3:06 AM (± 1:36 h [SD]) until 6:48 AM (± 2:48 h [SD]), with a sleep duration of 3 h 42 min (± 1:40 h [SD]), and then stayed awake until the measurement the next evening. Such an early sleep deprivation protocol may elicit an antidepressant response in distinct cases, particularly in younger women (47). The experiments were performed with an interval of two days. Participants had to abstain from caffeine and alcohol on the experimental days. Assessments were performed at the neuroimaging center of the Psychiatric Hospital of the University of Zurich and the Child and Adolescence Psychiatry, sleep deprivation was conducted at home.
EEG Recording During fMRI Scanning.
Presence of wakefulness and sleep during scanning was confirmed by standard polysomnographic recordings using a 32-channel MR-compatible EEG montage (Brain Products). Impedances were kept below 20 kΩ. In addition to online control for sleep, the polysomnographic records were visually scored offline by two independent raters according to standard criteria (48).
To quantify the EEG during the 8-min resting-state intervals, records were subjected to power spectral analysis. Data of all channels were filtered between 0.3 and 35 Hz with a slope of 48 dB/oct and rereferenced to averaged mastoids. Artifacts were excluded. Power spectra between 0 and 25 Hz (fast Fourier transform routine; 10% Hanning window; 0.2-s overlap) of artifact-free, 4-s EEG epochs sampled with 1,024 Hz were calculated with Brain Vision Analyzer. Individual mean power across all channels was determined for the following EEG bands: 0.5–4.5 Hz (delta), 4.5–8.0 Hz (theta), 8.0–11.0 Hz (alpha), 11.0–15 Hz (beta 1), and 15.0–25.0 Hz (beta 2).
Functional MRI Data Acquisition.
Measurements were performed on a Philips Achieva 3.0T TX 3-tesla whole-body magnetic resonance unit equipped with an eight-channel head coil array. The subjects were told to lie still in the scanner with their eyes closed during the acquisition of resting-state data. The functional images were collected in 8-min runs (196 volumes) using a sensitivity-encoded single-shot echo-planar sequence (TE = 20 ms; field of view = 22 cm; acquisition matrix = 88 × 85, interpolated to 96 × 96, voxel size = 2.50 × 2.50 × 2.50 mm3, reconstructed to 2.29 × 2.29 × 2.5 mm3, and sensitivity-encoded acceleration factor R = 2.5) sensitive to BOLD contrast (T2* weighting). Using a midsagittal scout image, 42 contiguous axial slices were placed along the anterior–posterior commissure plane covering the entire brain with a repetition time of 2,500 ms. A 3D T1-weighted anatomical scan was obtained for structural reference.
Postprocessing and fMRI Data Analysis.
Standard image data preparation and preprocessing, as well as statistical analysis and visualization were performed with the software BrainVoyager QX (Brain Innovation BV). Functional data preprocessing included a correction for slice scan timing acquisition, a 3D rigid body motion correction, a spatial smoothing (Gaussian kernel of 4 mm full width half maximum), a temporal high-pass filter with cutoff set to two cycles per time course, and a temporal low-pass filter (Gaussian kernel of 3 s). Structural and functional data were coregistered and spatially normalized to the Talairach standard space using a 12-parameter affine transformation. In the course of this procedure, the functional images were resampled to an isometric 3-mm grid covering the entire Talairach box. Nuisance signals (global signal, white matter, and cerebrospinal fluid signals) were regressed out from each data set together with motion translation and rotation estimates after segmenting the entire brain, the white matter, and ventricles from the normalized T1 volume.
A seed-based analysis (49, 50) was performed to study the functional connectivity from the PCC and DN to the entire brain similar to previous studies (20, 23). A control seed region was defined by tracing a 6-mm radius sphere centered at (x = −8, y = −60, z = 21). This region was anatomically located in the precuneus (51), and fell slightly more posteriorly and more inferiorly, albeit not overlapping, to the PCC seed. To compute functional connectivity maps corresponding to a selected seed region of interest (ROI), the mean regional time course was extracted from all ROI voxels and correlated against all voxels of the brain. Two ROIs were studied, the definition of which was based on our previous work (23), and separate correlation maps were produced for each subject, condition, and ROI. The correlation maps were applied the Fisher’s r-to-z transform z = 0.5 Ln [(1 + r)/(1 − r)] before entering a second-level random-effects statistical analysis where the main and differential effects of the two studied conditions were summarized as t-statistic maps. This analysis was carried out by treating the individual subject map values as random observations at each voxel, thereby one- and two-sample t tests were performed at each voxel to map the whole-brain distribution of the seed-based functional connectivity for the single condition and the difference between the two conditions. The statistical maps were thresholded at P = 0.05 (corrected for multiple comparisons) and overlaid on the average normalized T1 volume of all subjects. To correct for multiple comparisons in the voxel-based analysis, regional effects resulting from the voxel-based comparative tests were only accepted for compact cluster surviving the joint application of a voxel- and cluster-level statistical threshold chosen with a nonparametric randomization approach. Namely, an initial voxel-level threshold was set to P = 0.005 (uncorrected) and a minimum cluster size was estimated after 1,000 Monte Carlo simulations that protected against false positive clusters up to 5% (52).
Acknowledgments
We acknowledge the support of the Clinical Research Priority Programs Sleep and Health and Molecular Imaging granted by University of Zurich, as well as Dr. B. B. Quednow for his critical comments. The work was supported by Swiss National Science Foundation Grants 320030-135414 and PP00P1_133685.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Dallaspezia S, Benedetti F. Chronobiological therapy for mood disorders. Expert Rev Neurother. 2011;11(7):961–970. doi: 10.1586/ern.11.61. [DOI] [PubMed] [Google Scholar]
- 2.Pflug B, Tölle R. Disturbance of the 24-hour rhythm in endogenous depression and the treatment of endogenous depression by sleep deprivation. Int Pharmacopsychiatry. 1971;6(3):187–196. doi: 10.1159/000468269. [DOI] [PubMed] [Google Scholar]
- 3.Gillin JC, Buchsbaum M, Wu J, Clark C, Bunney W., Jr Sleep deprivation as a model experimental antidepressant treatment: Findings from functional brain imaging. Depress Anxiety. 2001;14(1):37–49. doi: 10.1002/da.1045. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156(8):1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 5.Wu JC, et al. Effect of sleep deprivation on brain metabolism of depressed patients. Am J Psychiatry. 1992;149(4):538–543. doi: 10.1176/ajp.149.4.538. [DOI] [PubMed] [Google Scholar]
- 6.Wu JC, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1-3):181–186. doi: 10.1016/j.jad.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, et al. Neural and genetic correlates of antidepressant response to sleep deprivation: A functional magnetic resonance imaging study of moral valence decision in bipolar depression. Arch Gen Psychiatry. 2007;64(2):179–187. doi: 10.1001/archpsyc.64.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Clark CP, et al. Improved anatomic delineation of the antidepressant response to partial sleep deprivation in medial frontal cortex using perfusion-weighted functional MRI. Psychiatry Res. 2006;146(3):213–222. doi: 10.1016/j.pscychresns.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CP, Frank LR, Brown GG. Sleep deprivation, EEG, and functional MRI in depression: Preliminary results. Neuropsychopharmacology. 2001;25(5) Suppl:S79–S84. doi: 10.1016/S0893-133X(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 10.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090, discussion 1097–1099. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142(1-3):6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Broyd SJ, et al. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 14.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 15.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niendam TA, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu C, et al. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage. 2011;54(4):2571–2581. doi: 10.1016/j.neuroimage.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 19.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 20.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107(24):11020–110. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59(2):1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Sämann PG, et al. Increased sleep pressure reduces resting state functional connectivity. MAGMA. 2010;23(5-6):375–389. doi: 10.1007/s10334-010-0213-z. [DOI] [PubMed] [Google Scholar]
- 23.Scheidegger M, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: Implications for antidepressant drug action. PLoS ONE. 2012;7(9):e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gujar N, Yoo SS, Hu P, Walker MP. The unrested resting brain: Sleep deprivation alters activity within the default-mode network. J Cogn Neurosci. 2010;22(8):1637–1648. doi: 10.1162/jocn.2009.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kühn S, Vanderhasselt MA, De Raedt R, Gallinat J. Why ruminators won’t stop: The structural and resting state correlates of rumination and its relation to depression. J Affect Disord. 2012;141(2-3):352–360. doi: 10.1016/j.jad.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Sheline YI, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greicius MD, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton JP, et al. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimm S, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4):932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 34.Grimm S, et al. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World J Biol Psychiatry. 2011;12(8):627–637. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- 35.McCabe C, et al. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry. 2011;16(6):592–594. doi: 10.1038/mp.2010.138. [DOI] [PubMed] [Google Scholar]
- 36.Baxter LR, Jr, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 37.Bench CJ, et al. The anatomy of melancholia—Focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22(3):607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 38.Cohen RM, et al. Evidence for common alterations in cerebral glucose metabolism in major affective disorders and schizophrenia. Neuropsychopharmacology. 1989;2(4):241–254. doi: 10.1016/0893-133x(89)90028-6. [DOI] [PubMed] [Google Scholar]
- 39.Mayberg HS. Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci. 1994;6(4):428–442. doi: 10.1176/jnp.6.4.428. [DOI] [PubMed] [Google Scholar]
- 40.Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 41.Mayberg HS, Lewis PJ, Regenold W, Wagner HN., Jr Paralimbic hypoperfusion in unipolar depression. J Nucl Med. 1994;35(6):929–934. [PubMed] [Google Scholar]
- 42.Buchsbaum MS, et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41(1):15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy SH, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158(6):899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 44.Drummond SP, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403(6770):655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 45.Lévesque J, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53(6):502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 46.Liotti M, et al. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol Psychiatry. 2000;48(1):30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 47.Parry BL, et al. Can critically timed sleep deprivation be useful in pregnancy and postpartum depressions? J Affect Disord. 2000;60(3):201–212. doi: 10.1016/s0165-0327(99)00179-2. [DOI] [PubMed] [Google Scholar]
- 48.Iber C, Ancoli-Israel S, Chessonn A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st Ed. IL: AASM, Westchester; 2007. [Google Scholar]
- 49.Joel SE, Caffo BS, van Zijl PC, Pekar JJ. On the relationship between seed-based and ICA-based measures of functional connectivity. Magn Reson Med. 2011;66(3):644–657. doi: 10.1002/mrm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 52.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]