Significance
Steroid hormones coordinate multiple behaviors into a functional response (reproduction, stress). It is easy to assess such coordination when comparing actions in different target tissues but is more of a challenge when numerous functions are combined in the brain. Our study illustrates how steroids act on distinct cell groups to regulate separate components of a testosterone-dependent behavior, learned birdsong. Testosterone in the medial preoptic nucleus (POM) increased singing but not optimal song performance. Moreover, testosterone in the POM enhanced volumes of forebrain regions that control song, presumably reflecting the effect of activity-dependent plasticity. Thus, optimal performance of a complex, learned behavior requires testosterone at multiple loci, and activity and/or transsynaptic influences may stimulate forebrain neuroplasticity.
Keywords: activity-driven plasticity, singing motivation, preoptic area
Abstract
Steroid hormones regulate multiple but distinct aspects of social behaviors. Testosterone (T) has multiple effects on learned courtship song in that it regulates both the motivation to sing in a particular social context as well as the quality of song produced. The neural substrate(s) where T acts to regulate the motivation to sing as opposed to other aspects of song has not been definitively characterized. We show here that T implants in the medial preoptic nucleus (POM) of castrated male canaries (Serinus canaria) increase song rate but do not enhance acoustic features such as song stereotypy compared with birds receiving peripheral T that can act globally throughout the brain. Strikingly, T action in the POM increased song control nuclei volume, consistent with the hypothesis that singing activity induces neuroplasticity in the song control system independent of T acting in these nuclei. When presented with a female canary, POM-T birds copulated at a rate comparable to birds receiving systemic T but produced fewer calls and songs in her presence. Thus, POM is a key site where T acts to activate copulation and increase song rate, an appetitive sexual behavior in songbirds, but T action in other areas of the brain or periphery (e.g., HVC, dopaminergic cell groups, or the syrinx) is required to enhance the quality of song (i.e., stereotypy) as well as regulate context-specific vocalizations. These results have broad implications for research concerning how steroids act at multiple brain loci to regulate distinct sociosexual behaviors and the associated neuroplasticity.
Steroid hormones such as testosterone (T) can have multiple effects on physiology, morphology, and behavior (1–4). These pleiotropic effects of steroids allow coordinating suites of traits into an organized functional response (5, 6). In the case of the regulation of behavior, both motivational and performance aspects of behavior as well cognitive components are often activated by the same hormone (2, 7). The neural sites of steroid action coordinating these distinct aspects of an integrated behavioral response have not been well characterized. In this study, we investigated the site of hormone action in relation to the activation of different aspects of birdsong to illustrate how such an integrated regulation can occur.
Birdsong is a species-typical, stereotypic set of usually long, learned, complex vocalizations produced in reproductive contexts (8). A discrete network of interconnected brain nuclei orchestrates song learning and production (from here on called the song control system or SCS) (3, 9–11). Areas such as HVC (acronym is proper name) and the robust nucleus of the arcopallium (RA) regulate the production of song, whereas areas such as Area X and LMAN are involved in song learning. These forebrain nuclei can undergo remarkable plasticity in response to seasonally changing T (12, 13; see refs. 2 and 14 for review). There is also evidence that other factors such as singing activity (15–17; reviewed in refs. 18 and 19), social cues (20, 21), and photoperiod (22, 23) can contribute to the occurrence of seasonal neuroplasticity independently of T. The distinct functional roles of these nuclei and their robust neuroplasticity have made songbirds an excellent model taxon in which to study the neural bases of complex learned motor behavior with distinct components (24).
Throughout the songbird brain, there are multiple sites of steroid action (2, 25). For instance, androgen receptors are expressed in HVC, RA, lMAN, and throughout the hypothalamus and midbrain, and estrogen receptors are expressed in HVC as well as in the hypothalamus. This observation, in line with a plethora of correlational evidence, has alluded to the possibility that androgens such as T act in the SCS to activate song in songbirds (2, 9, 13, 26–28). However, multiple studies have indicated that the mechanism is not so simple, and steroids may act at multiple levels of the songbird brain to regulate specific aspects of song (5). For instance, blocking T actions in HVC reduces song quality but does not affect song rate (29). Also, implanting T in the HVC or RA of castrated white-crowned sparrows does not activate singing (30). Moreover, in the classic study by Nottebohm et al. (9), male canaries with lesioned HVCs produced “silent song,” during which they assumed all of the postural components associated with song production but did not produce audible vocalizations. Therefore, although songbirds represent an excellent model system in which to study the distinctive functional roles of steroid hormones, it has not been clearly identified where outside the SCS T acts to regulate the probability of singing or song rate.
The medial preoptic area is an area of the brain that is a key substrate for T to activate male-typical sexual behavior. For instance, studies in rodents and in Japanese quail (Coturnix japonica) show that T actions in this brain region are sufficient for activating male-typical sexual motivation and performance (31–34). Relevant to the current study, Riters and Ball (35), using bilateral electrolytic lesions of the medial preoptic nucleus (POM) of male European starlings (Sturnus vulgaris), demonstrated that this nucleus is required for increases in song rate that occur in response to the presentation of a female. Hence, the POM is a strong candidate neural substrate for T activating the motivational aspects of song in songbirds.
In the present study, we demonstrate that the POM of songbirds is a critical site for the activation of sexual motivation and singing by T, but not for enhancing specific aspects of song stereotypy as well as the attentional components that coordinate singing with the suite of sexual behaviors. Specifically, we found that T in the POM increases song rate but not the latency and quality aspects (e.g., loudness and stereotypy) of song associated in birds with the systemic action of circulating T. Moreover, T in the POM was sufficient to activate copulation but not calls and pericopulatory songs in the presence of a female. Finally, T in the POM increased the volumes of SCS nuclei in parallel with the increased singing activity, thus providing strong additional evidence of activity-induced brain plasticity in these structures. These results suggest that T acts at multiple levels of the brain to regulate distinct components of complex sociosexual behaviors and acts at multiple, distinct neural substrates to coordinate the motivational, cognitive, and attentional aspects of sexual behavior.
Results
Serum T Concentrations and Brain Implant Location.
The two groups of birds that did not receive peripheral T implants had similarly low mean values for serum concentrations of T (Fig. 1A) and were statistically indistinguishable from one another (P > 0.9), whereas birds with peripheral T implants (PER-T) had substantially higher concentrations of circulating T. There was also no difference in plasma T concentration (P = 0.9) among POM-NO T birds between subjects with empty implants and subjects with T implants outside POM.
Fig. 1.
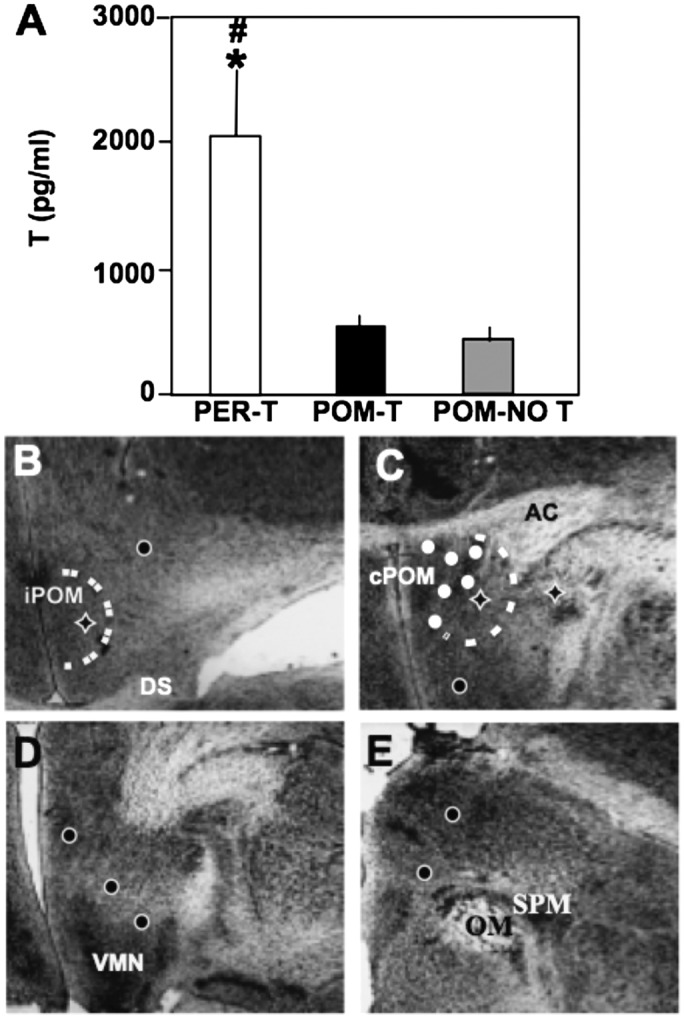
Concentrations of T and T implant sites. (A) Concentrations of T in the three treatment groups, PER-T, POM-T, and POM-NO T. *P < 0.05 vs. POM-NO T; #P < 0.05 vs. POM-T. (B–E) Implant sites in intermediate POM (iPOM) (B), caudal POM (cPOM) (C), near ventromedial nucleus of the hypothalamus (VMN) (D), and in dorsal thalamus near tractus occipitomesencephalicus (OM) and nucleus spiriformis medialis (SPM) (E). The white dashed lines demarcate the POM. The white circle filled in black indicate T implants that did not contact the POM; the white ones indicate implants that contacted the POM; the diamonds indicate empty implants. AC, anterior commissure; DS, supraoptic decussation.
The POM-T and POM-NO T groups also possessed POM volumes contralateral to the implant site approximately one-half the size of PER-T birds (Materials and Methods; POM-T: 2.66 × 105 ± 1.75 × 104; POM-NO T: 2.29 × 105 ± 1.24 × 104; PER-T: 5.20 × 105 ± 7.09 × 104 µm3; all means ± SEM). Volumes in the PER-T group were thus significantly larger than in the other two groups (P < 0.001 in both cases), but these groups were not significantly different (P > 0.56).
Six of 13 T-filled cannulae contacted the POM (Fig. 1C). T-filled cannulae that missed the POM (n = 7; Fig. 1 B–D) did not induce singing or other sexual behaviors. The latter group of birds was included in the group called POM-NO T to increase statistical power (n = 10; Materials and Methods), reflecting that neither group received T contacting their POM and did not show an activation of song or sexual behaviors. Importantly, among POM-NO T birds, there was also no difference in contralateral POM volume between birds implanted with an empty or a T-filled cannula (2.02 × 105 ± 5.26 × 103 µm3 and 2.46 × 105 ± 1.29 × 104 µm3, respectively; P > 0.17).
T in the POM Increases Song Rate but Not Song Quality.
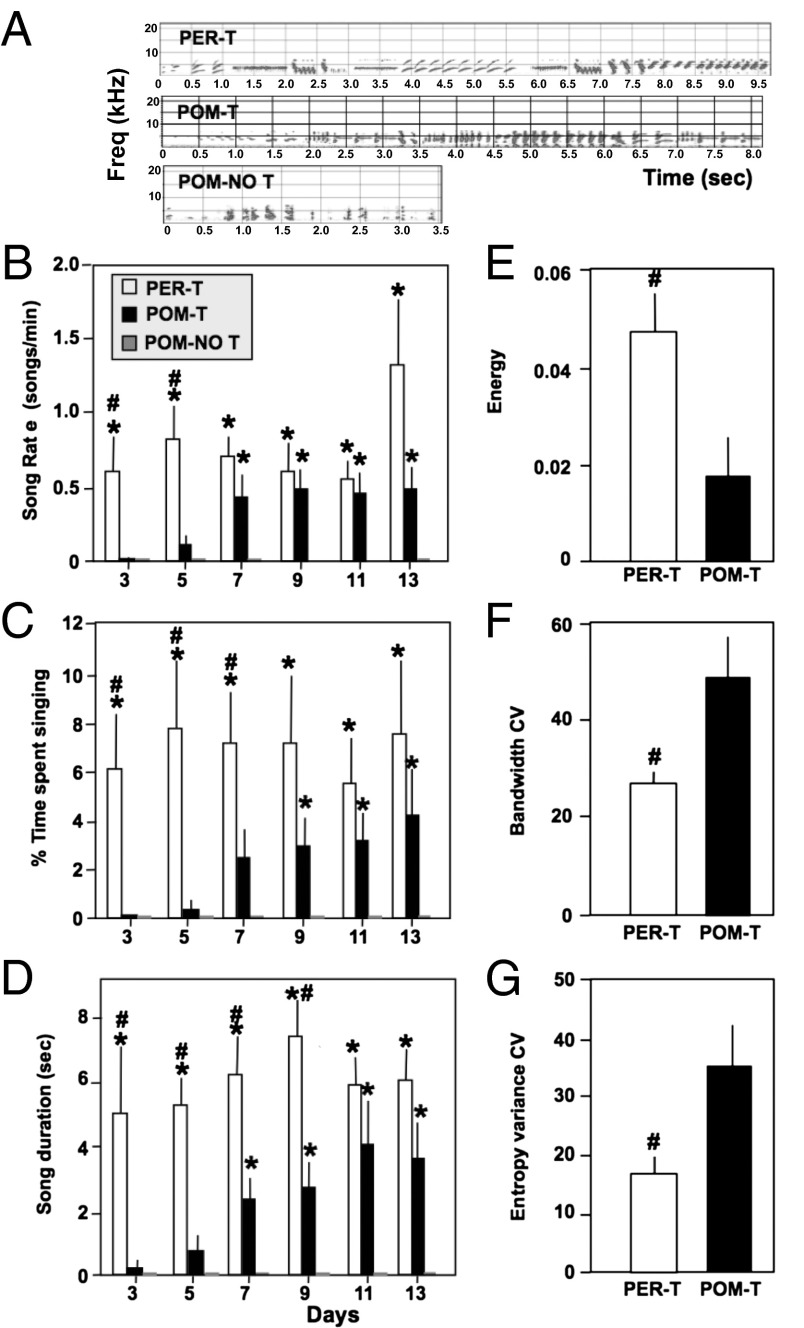
PER-T and POM-T birds produced long, complex songs compared with POM-NO T birds (Fig. 2A). Repeated-measures ANOVAs revealed a significant main effect of treatment on song rate measures (song rate, percentage time singing, and song duration; P < 0.001) and a significant interaction of day and treatment for these measures (P < 0.05). The results of post hoc analyses are in Fig. 2. PER-T birds began singing at high rate before the other groups and their songs had a longer song duration (Fig. 2 B–D). Eventually, POM-T birds began singing with indistinguishable song rate, percentage time singing, and song duration compared with PER-T birds, and both groups had larger values for these variables compared with POM-NO T in the latter half of the observations. Linear contrasts revealed that POM-T birds showed a linear increase in all of these measures (P < 0.05), whereas this was not exhibited by the other groups.
Fig. 2.
Effect of treatments on song rate, duration, and quality. (A) Representative spectrograms of song from each group. (B–G) Effects of T treatment on various song measures. B–D show the effects of treatment on measures of song rate and duration; E–G represent average quality features of song. *P < 0.05 vs. POM-NO T; #P < 0.05 vs. POM-T.
On average during days 5–11, PER-T birds sang songs that were more than twice as loud (energy, post hoc Tukey’s, P = 0.043) and of higher quality [bandwidth coefficient of variation (CV) and entropy variance CV, post hoc Tukey’s P < 0.05] compared with POM-T birds (Materials and Methods; Fig. 2 E–G). These results as a whole suggest that T in the POM is sufficient to increase song rate, but globally circulating T increases song rate more rapidly (i.e., within 1–3 d) and enhances the full suite of acoustic and quality measures characteristic of male canary song.
T in the POM Induces SCS Growth, and Singing Rate Predicts HVC and RA Volume.
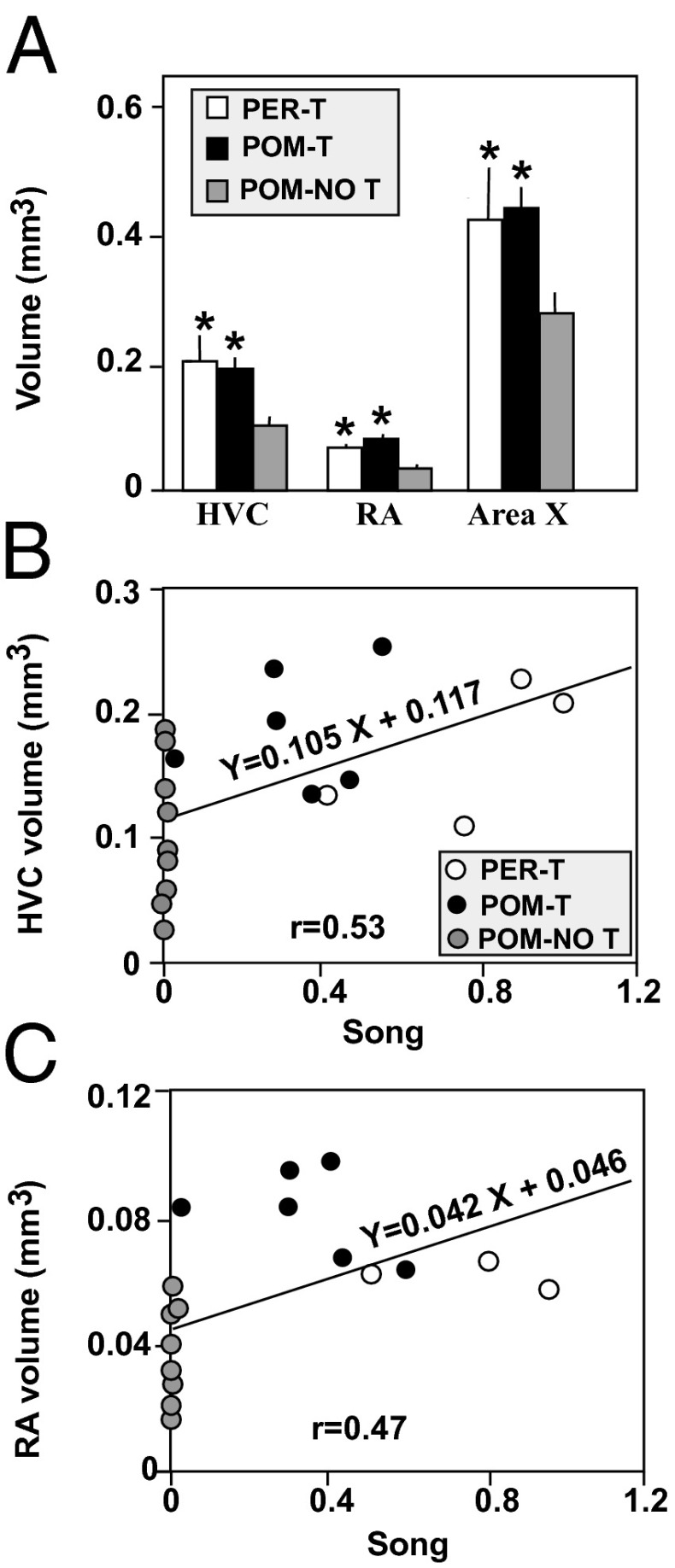
After observing the substantial induction of song rate in POM-T birds, we wondered whether the SCS had changed in response to this treatment. POM-NO T birds had smaller song control nuclei than both other groups (HVC, RA, and Area X; post hoc Tukey’s P < 0.05), whereas PER-T and POM-T were indistinguishable from each other (Fig. 3A). These results suggested that T action in the POM leads to song control nuclei growth indirectly via increased singing activity.
Fig. 3.
Effects of treatments on SCS volume (A) and relationship between song rate and HVC and RA volume (B and C). Trend lines indicate a significant regression. Different symbols were used to indicate data from the three experimental groups. In A, *P < 0.05 vs. POM-NO T.
Accordingly, a forward regression analysis identified song rate as a significant predictor of HVC and RA volume (Materials and Methods; r = 0.533 and 0.477, respectively; P < 0.05; Fig. 3 B and C). These results are fully consistent with the notion that the substantial increase in SCS volume in POM-T birds may be in part due to singing activity.
T in the POM Induces Copulation but Not Vocalizations in the Presence of a Female.
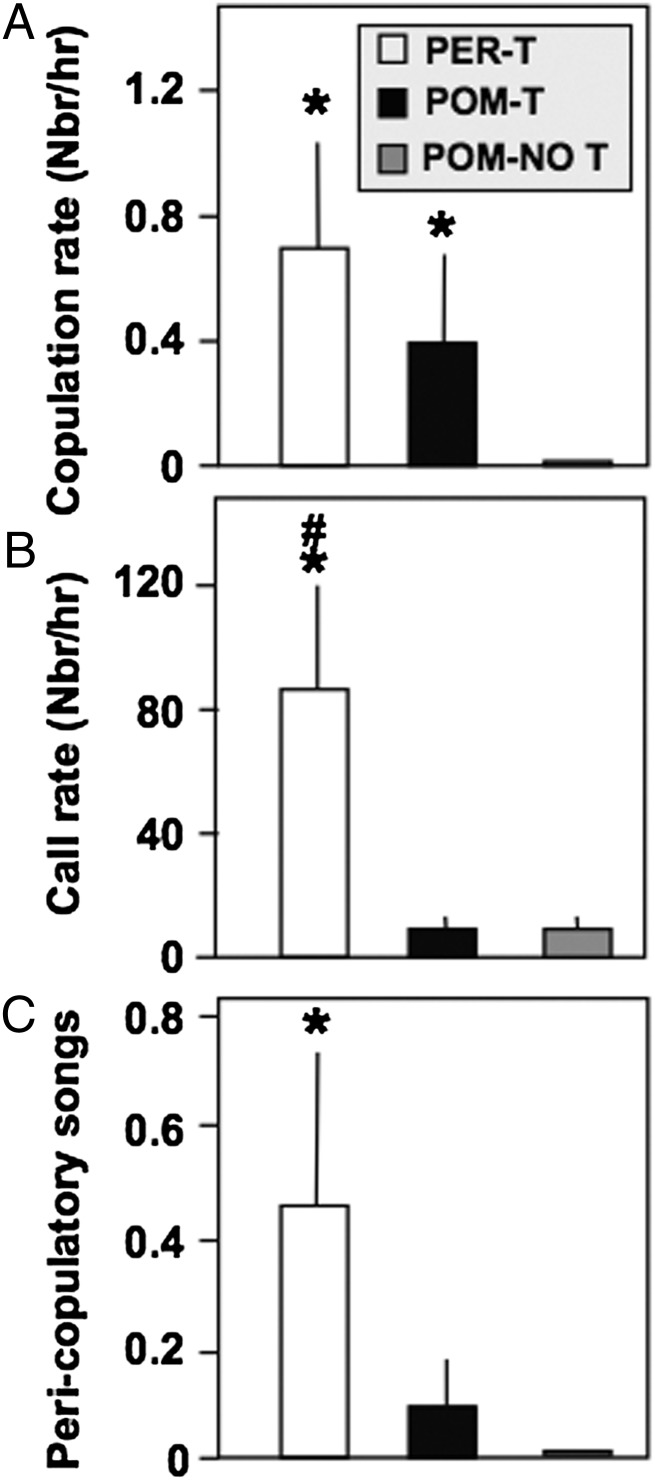
There was a main effect of treatment on the copulation rate (Kruskal–Wallis ANOVA, P = 0.02). Specifically, POM-T birds attempted to copulate more frequently compared with POM-NO T birds (post hoc Mann–Whitney, P = 0.038; Fig. 4A). Moreover, PER-T birds copulated more frequently than POM-NO T birds (post hoc Mann–Whitney, P = 0.007) and were not different from POM-T birds (post hoc Mann–Whitney, P > 0.05). There was also a main effect of treatment on call rate (P = 0.002) and pericopulatory songs (P = 0.05; see Materials and Methods for definition; Kruskal–Wallis ANOVA). Interestingly, POM-T birds were not different from POM-NO T birds for these two measures (call rate, P = 0.92; pericopulatory song, P = 0.20; post hoc Mann–Whitney; Fig. 4 B and C), whereas PER-T birds were significantly different from POM-NO T for both measures (call rate, P = 0.001; pericopulatory song, P = 0.02; post hoc Mann–Whitney). PER-T birds also had a higher call rate compared with POM-T birds (P = 0.006) but were not different from POM-T in terms of pericopulatory song (post hoc Mann–Whitney, P = 0.19). This suggests T acting in other areas of the brain is required to orchestrate vocalizations specific to the sexual context.
Fig. 4.
Effects of treatments on copulation rate (A), call rate (B) and frequency of peri-copulatory songs (C) in the presence of a female. *P < 0.05 vs. POM-NO T; #P < 0.05 vs. POM-T.
Discussion
Steroid hormones can induce variation in physiological state that dramatically affects responses to socially relevant stimuli (1, 36, 37). Steroids can also modify cognitive processes ranging from attention (38) to learning (2, 3) that affect the probability and quality of a particular behavioral response. Our studies of the neural basis of how T regulates song behavior illustrate two important principles. The first is that T, like other steroid hormones, can regulate different aspects of the same behavior or the expression of a given behavior in different contexts by acting independently in different brain areas. The second is that T-induced changes in behavioral activity can feed back on the morphology and physiology of brain areas involved in the production or regulation of sensorimotor aspects of the behavior of interest. Studies of steroid action on courtship song are especially amenable to this sort of analysis.
One implication of the pioneering study by Nottebohm et al. (9) not widely appreciated at the time is that the SCS regulates the learning and production of song whereas areas outside of this system regulate the probability that song will occur. Canaries with HVC lesions tried to sing but could not produce the vocal output [what Nottebohm referred to as “silent song” (9)]. At that time, the discovery of steroid receptors in several song control nuclei (2, 39–42) drew attention to the action of steroids directly in the SCS (41, 42), and it is only later that research suggested that T is acting potentially at multiple sites to regulate distinct components of song behavior (2, 5, 29, 30). In this study, we conclusively identify a neural substrate outside of the SCS where T acts to modulate song. Specifically, T in POM was sufficient to enhance the rate of song production but did not lead to the production of fully stereotyped vocalizations typical of birds systemically exposed to T. Thanks to the availability of extensive video recording, we also confirm in songbirds the role of T action in the preoptic area on male-typical copulatory behavior, previously described in other avian and in mammalian species (33, 34, 43).
Dissociations similar to these observed here in the control of singing have been observed in castrated rodents implanted with T solely in their medial preoptic area, resulting in an enhancement in copulation but a lack of the vigor and performance present in intact animals (44). This study goes a step further, by demonstrating the dissociability between the motivation to perform a complex social behavior and its quality as well as its coordination with relevant stimuli (the female). It is likely that T acting at neural substrates in multiple areas of the social behavior network (45, 46) that includes the POM, as well as in the SCS itself (2), is required for the occurrence of the full suite of reproductive behaviors present in songbirds.
This study also provides credence to the hypothesis that singing in and of itself has stimulating effects on neuroplasticity (15, 16). Recently, it has been debated whether the effects of singing activity can be separated from the effects of circulating T on changes in SCS morphology (16, 47). Here, we show that with substantial increases in song rate in the absence of globally circulating T and in the absence of T in the brain outside of its implantation in the POM, the SCS undergoes robust changes in size, supporting the notion of activity-induced neural growth and plasticity. For instance, the volume of the contralateral POM, which is positively associated with exposure to T (48, 49), was about twice as large in birds exposed to global T compared with castrated birds with T solely in their POM. Hence, our study provides strong evidence that singing activity can lead to robust neuroplasticity in the SCS independently of T.
These results also corroborate models of T actions indicating that T works both directly and indirectly to change the SCS and song activation (2). Moreover, these data raise the question of how T in the POM exerts these effects: transsynaptic influences are possible, as indirect connections exist between the POM and HVC and RA, via the periaqueductal gray and the ventral tegmental area (50–52). It is, however, more likely that the observed plasticity is a direct consequence of the singing activity itself (15, 53). Future investigations on these respective and possibly interacting hypotheses are thus of the utmost importance.
A basic principle of steroid hormone action that was identified early in the history of the field is that multiple functions must be coordinated to organize a functional response such as reproduction or stress (54). It is easy to assess such coordination when comparing actions in very different target tissues such as the brain vs. the periphery. However, when multiple functions are combined to a single target tissue such as the brain, it is more of a challenge. Despite the fact that steroids have been directly implanted into the brain since the 1960s, there are still few examples that illustrate specialization of steroid action in multiple brain sites for the coordination of a single behavior (6). Our study illustrates how steroids can act on distinct cell groups to regulate separate components of a single behavioral response. T acting in the POA clearly can motivate song but it cannot ensure that song performance is optimal. However, just increasing song activity via action in the POA results in enhanced volumes of the key song nucleus HVC. This type of coordination can be important in regulating many social behaviors, especially those depending on experience.
Materials and Methods
Animals and Preexperimental Manipulations.
Canaries (Serinus canaria) of the Border strain were used for this study. Male and female canaries were obtained from a local breeder (Maryland Exotic Birds). The protocols and procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Upon entry into the laboratory, birds were placed on a short day (SD) photoperiod [8 light (L):16 dark (D)] for 6 wk to induce photosensitivity (55). Birds in a photosensitive but not photostimulated state also possess regressed testes, which facilitates effective castration.
Male birds were castrated by deeply anesthetizing them with isoflurane gas (IsoSol isoflurane, Vedco; Isotec 4 anesthesia machine, Surgivet) and placed on their right side. The left testis was then removed through an incision below the last rib; immediately after, the bird was placed on its left side and the right testis was removed in an identical manner. After recovery from surgery, birds were placed under a heat lamp until they perched. Then, birds were placed back in their home cages and allowed to recover for 6 wk, to allow adequate time for the physiological and behavioral effects of T to disappear.
Experimental Groups and Stereotaxic Implantation.
Birds were anesthetized using isoflurane gas and placed in a stereotaxic apparatus modified for use in small birds such as canaries with the beak holder placed 45° below the horizontal axis of the apparatus. We used the following stereotaxic coordinates to target the POM: dorsoventral: −7 mm from the dorsal surface of the brain; anterior–posterior: 2.3 mm from the rostral tip of the cerebellum; and medial–lateral: ±0.15 mm from midline. Each bird received a unilateral implant aimed at the POM using a Hamilton syringe fashioned to hold the 27-gauge cannula filled with T or left empty. Cannulae were lowered to the target coordinates and dental cement was applied around the implant. Excess portion of the cannula was clipped off after the cement had dried. The skin was then sutured over the implant, and lidocaine and antibiotics were applied around the sutured portion of the skin using a Q-tip.
Implants were made using blunted 27-gauge needles filled over a length of 1 mm with crystalline T (ref. 20; Sigma T 1500). Implants were cleaned using acetone and a Kimwipe to remove any hormone that stuck to the outside of the tube. The side of the brain in which the implant was made was randomized across birds. Once birds recovered, they were returned to individual, sound-attenuated chambers set to 14L:10D to simulate breeding photoperiods.
Due to expected variation in implant sites (31), we implanted 13 birds with a T-filled cannula and 3 with an empty cannula. Pilot studies indicated when T missed the POM, no song or copulatory behavior was induced and accuracy of the implants was about 50%. Therefore, when T-filled cannulae missed the POM, we lumped the birds with T-filled cannulae that missed the POM and birds that received empty cannulae into the same group, called POM-NO T, reflecting that neither group received T contacting the POM. We confirmed by t tests that these groups did not differ significantly on any morphological (SCS nuclei volumes, P ≥ 0.40; contralateral POM volumes, P > 0.17), physiological (T concentrations, P ≥ 0.90), or behavioral measure (all P ≥ 0.84). In the end, 6 of 13 T-filled cannulae contacted the POM.
Overall, this experiment had three groups: group 1 was administered T peripherally, by implanting each subject s.c. with an 8-mm-long Silastic implant (Dow Corning; internal diameter, 0.76 mm; external diameter, 1.65 mm) filled with 6 mm of crystalline T (PER-T; n = 4); group 2 received a cannula filled with T that contacted the POM (POM-T; n = 6); group 3 received a cannula that was either empty (n = 3) or filled with T but missed the POM (n = 7) (POM-NO T; n = 10; see above). All birds received either a T-filled or empty brain cannula and a T-filled or empty Silastic implants; the contents of cannulae or implants varied based on the treatment group.
Song and Other Sexual Behaviors.
Each day, video and audio recordings were made from 800 to 930 hours (lights on at 800 hours), 1300 to 1430 hours, and 1600 to 1730 hours. The following song behaviors were quantified from recordings made every other day during 11 d, starting after 3 d of treatment: song rate (songs per hour), mean duration of each song, percentage of time spent singing, entropy variance, energy, fundamental frequency, and bandwidth (56, 57). Songs were defined as vocalizations being longer than or equal to 1 s in duration and separated by 500 ms of silence (58, 59).
We also quantified song stereotypy. Song stereotypy indicates how similar certain features of song are across song renditions. Song stereotypy was determined by calculating the CV [CV = (SD/AVG)*100)] using the SDs of song acoustic features (SD) described above and dividing this by the average (AVG) across the same values used to calculate the SD. CV is an inverse measure of song stereotypy (29).
Fourteen days after the beginning of treatment (3 d posttreatment plus the 11 d of song recording while males were alone), each male canary was presented with a female that had been implanted with a 14-mm Silastic implant filled with 12 mm of crystalline 17β-estradiol (Sigma). Notably, when male canaries are presented with a female, song production ceases (58). They were housed with the female for 3 d, during which time their behavior was recorded in the same way as described for the song behaviors. The following behaviors were quantified: proximity initiations (i.e., approaching the female to less than one body length), bill touches, copulation attempts, songs, calls, and pericopulatory songs. Calls are much shorter and simpler vocalizations compared with song (60). Pericopulatory songs are songs that are produced immediately before and during copulation in male canaries. We also quantified nonsocial behaviors such as feeding, drinking, and grooming.
Brain Collection and Verification of Implants and Castrations.
Sixteen days after treatment initiation, birds were deeply anesthetized (4% isoflurane in oxygen), weighed, and their brain was extracted and fixed in acrolein after collecting blood from the trunk region into 1.5-mL centrifuge tubes. Blood was spun down at 2,201 × g rpm for 6 min and serum was collected and placed at −20 °C. Brains were agitated in acrolein for 2 h, then washed for 15 min four times in PBS and placed in sucrose (30% solution in PBS) overnight until they sank to the bottom of the vial. After cryoprotection by sucrose, brains were flash frozen in dry ice for 5 min, and then placed into a −70 °C freezer. At autopsy, all birds were found to be completely castrated and no testicular remnants or regrowth could be detected.
Brain and Serum Analyses.
Brains were sectioned using a cryostat at 30 μm into four series of sections that were stored in cryoprotectant. These four series were placed into a −20 °C freezer. One series was later mounted on gelatin-coated slides and exposed to air for a day. Then, mounted sections were exposed to a standard Nissl staining procedure and coverslipped using Permount (Fisher Scientific). Based on these stained sections, the positions of the implant centers were drawn onto a series of modified atlas plates obtained from the canary atlas made by Stokes et al. (61).
Concentrations of serum T were determined using a standard ELISA (DRG Testosterone ELISA; DRG International). This allowed us to determine whether there was any detectable leakage of T from the brain cannula into the peripheral circulation as well as the efficacy of the Silastic implants and castrations.
SCS and POM Volume Reconstruction.
As stated above, T in the POM was found to induce song rate to levels of PER-T birds. We took advantage of this observation to assess whether high song rate in the absence of global T action could induce increases in the volume of SCS nuclei. To this aim, photomicrographs of Area X, HVC, and RA were taken at 2.5× magnification in the Nissl-stained sections. The area of each nucleus was determined in both hemispheres of each section where it appeared using NIH ImageJ, and volumes were determined by multiplying areas by the section thickness, summing these values, and then multiplying this value by 4, because only every fourth section was Nissl stained (62). In line with previous work on canaries, no systematic asymmetries were found between the hemispheres (62, 63). This would not be expected as the song nuclei in both hemispheres are active during song production (64). One bird in the POM-NO T group was an outlier for HVC and RA volumes (>2.5 SDs from the mean) and was thus removed from the ANOVA analysis and from the regression of singing activity on RA volumes (its RA volume was extraordinarily high and unexplainable based on our treatment groups and all other measures; it should be noted that multiple factors, including age, can influence SCS volume size; see Introduction and ref. 65). One PER-T bird had damaged sections that precluded analysis of RA and was therefore excluded from analysis of this brain region. We also quantified the volume of the POM in one section at the level of the anterior commissure contralateral to the implant sites using the same method as above in each bird. The volume of the POM is highly sensitive to the concentrations of T (48, 49), with T correlating positively with POM volume. Thus, the volume of the POM is a critically sensitive marker of T present in the cerebrospinal fluid and general circulation, and was thus used as a proxy for the efficacy and specificity of our central and peripheral T implants.
Statistical Analyses.
ANOVAs (Kruskal–Wallis if homogeneity of variance was not met) were used to assess the effects of treatment and/or day on all measures. A two-way (day by treatment) ANOVA was used to assess how treatment affected song rate, percentage time spent singing, and duration over time. ANOVAs were conducted on individual days when an interaction was observed. Five or 6 of 6 POM-T birds consistently sang from day 5 to 11; before this, a very small number of birds sang and a maximum of 2 of 10 birds in the POM-NO T group sang throughout the whole experiment. To avoid arbitrary value assignments to acoustic/stereotypic measures and removing birds from these analyses, we compared the PER-T and POM-T birds in terms of these features collapsed over days 5–11. A t test was used to make these comparisons. Tukey’s or Mann–Whitney tests were used for post hoc pairwise comparisons following significant omnibus parametric and nonparametric ANOVAs, respectively. A linear regression was also used to test whether singing activity predicted the volumes of the SCS nuclei. Specifically, song rate and percentage time spent singing were entered into a model as predictors for the volumes of HVC, RA, and Area X. Stepwise regression was used to determine which, if any, of these two variables are significant predictors of SCS nuclei volume.
Acknowledgments
We thank Dr. Farrah Madison for running the T assay, and Adam Podlisky, Kathryn Rownd, Hayley Weidenbenner, and Trevor Chan for technical assistance. This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant R01 35467 (to G.F.B.) and Grant SSTC PAI P7/17 from the Belgian Science Policy (to J.B. and G.F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Adkins-Regan E. Neuroendocrinology of social behavior. ILAR J. 2009;50(1):5–14. doi: 10.1093/ilar.50.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23(2):137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- 3.Bottjer SW, Johnson F. Circuits, hormones, and learning: Vocal behavior in songbirds. J Neurobiol. 1997;33(5):602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Pfaff DW. Hormone effects on specific and global brain functions. J Physiol Sci. 2008;58(4):213–220. doi: 10.2170/physiolsci.RV007008. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AP. Logical levels of steroid hormone action in the control of vertebrate behavior. American Zoologist. 1981;21:233–242. [Google Scholar]
- 6.Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm Behav. 2008;54(3):347–354. doi: 10.1016/j.yhbeh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GM, Sherwin BB. The association between testosterone, sexual arousal, and selective attention for erotic stimuli in men. Horm Behav. 1991;25(3):367–381. doi: 10.1016/0018-506x(91)90008-6. [DOI] [PubMed] [Google Scholar]
- 8.Ball GF, Hulse SH. Birdsong. Am Psychol. 1998;53(1):37–58. doi: 10.1037//0003-066x.53.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 10.Vicario DS, Simpson HB. Electrical stimulation in forebrain nuclei elicits learned vocal patterns in songbirds. J Neurophysiol. 1995;73(6):2602–2607. doi: 10.1152/jn.1995.73.6.2602. [DOI] [PubMed] [Google Scholar]
- 11.Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14(11 Pt 2):6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc Natl Acad Sci USA. 2007;104(39):15520–15525. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nottebohm F. A brain for all seasons: Cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214(4527):1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 14.Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23(6):251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22(19):8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119(1):233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- 17.Larson TA, et al. Postsynaptic neural activity regulates neuronal addition in the adult avian song control system. Proc Natl Acad Sci USA. 2013;110(41):16640–16644. doi: 10.1073/pnas.1310237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nottebohm F. Why are some neurons replaced in adult brain? J Neurosci. 2002;22(3):624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ball GF, et al. Seasonal plasticity in the song control system: Multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- 20.Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J Neurobiol. 2006;66(10):1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- 21.Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19(1):476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard DJ, Wilson FE, Ball GF. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Res. 1997;760(1-2):163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- 23.Gulledge CC, Deviche P. Photoperiod and testosterone independently affect vocal control region volumes in adolescent male songbirds. J Neurobiol. 1998;36(4):550–558. [PubMed] [Google Scholar]
- 24.Fee MS, Scharff C. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J. 2010;51(4):362–377. doi: 10.1093/ilar.51.4.362. [DOI] [PubMed] [Google Scholar]
- 25.Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140(10):4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- 26.DeVoogd TJ. Steroid interactions with structure and function of avian song control regions. J Neurobiol. 1986;17(3):177–201. doi: 10.1002/neu.480170305. [DOI] [PubMed] [Google Scholar]
- 27.Pintér O, Péczely P, Zsebok S, Zelena D. Seasonal changes in courtship behavior, plasma androgen levels and in hypothalamic aromatase immunoreactivity in male free-living European starlings (Sturnus vulgaris) Gen Comp Endocrinol. 2011;172(1):151–157. doi: 10.1016/j.ygcen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Thompson CK. Cell death and the song control system: A model for how sex steroid hormones regulate naturally-occurring neurodegeneration. Dev Growth Differ. 2011;53(2):213–224. doi: 10.1111/j.1440-169X.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 29.Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27(44):12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenowitz EA, Lent K. Act locally and think globally: Intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci USA. 2002;99(19):12421–12426. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990;48(5):599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- 32.Davidson JM. Activation of the male rat’s sexual behavior by intracerebral implantation of androgen. Endocrinology. 1966;79(4):783–794. doi: 10.1210/endo-79-4-783. [DOI] [PubMed] [Google Scholar]
- 33.Paredes RG. Medial preoptic area/anterior hypothalamus and sexual motivation. Scand J Psychol. 2003;44(3):203–212. doi: 10.1111/1467-9450.00337. [DOI] [PubMed] [Google Scholar]
- 34.Watson JT, Adkins-Regan E. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm Behav. 1989;23(3):432–447. doi: 10.1016/0018-506x(89)90055-x. [DOI] [PubMed] [Google Scholar]
- 35.Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36(3):276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- 36.Harding CF. Hormonal modulation of singing: Hormonal modulation of the songbird brain and singing behavior. Ann N Y Acad Sci. 2004;1016:524–539. doi: 10.1196/annals.1298.030. [DOI] [PubMed] [Google Scholar]
- 37.Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011;32(3):287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15(5):681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- 39.Schlinger BA. Sex steroids and their actions on the birdsong system. J Neurobiol. 1997;33(5):619–631. [PubMed] [Google Scholar]
- 40.Lieberburg I, Nottebohm F. High-affinity androgen binding proteins in syringeal tissues of songbirds. Gen Comp Endocrinol. 1979;37(3):286–293. doi: 10.1016/0016-6480(79)90002-9. [DOI] [PubMed] [Google Scholar]
- 41.Arnold AP, Nottebohm F, Pfaff DW. Hormone concentrating cells in vocal control and other areas of the brain of the zebra finch (Poephila guttata) J Comp Neurol. 1976;165(4):487–511. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- 42.Gahr M. Distribution of sex steroid hormone receptors in the avian brain: Functional implications for neural sex differences and sexual behaviors. Microsc Res Tech. 2001;55(1):1–11. doi: 10.1002/jemt.1151. [DOI] [PubMed] [Google Scholar]
- 43.Riters LV, Absil P, Balthazart J. Effects of brain testosterone implants on appetitive and consummatory components of male sexual behavior in Japanese quail. Brain Res Bull. 1998;47(1):69–79. doi: 10.1016/s0361-9230(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 44.Wood RI, Newman SW. The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm Behav. 1995;29(3):338–353. doi: 10.1006/hbeh.1995.1024. [DOI] [PubMed] [Google Scholar]
- 45.Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One. 2011;6(6):e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 47.Adkins-Regan E. Activity dependent brain plasticity: Does singing increase the volume of a song system nucleus? Theoretical comment on Sartor and Ball (2005) Behav Neurosci. 2005;119(1):346–348. doi: 10.1037/0735-7044.119.1.346. [DOI] [PubMed] [Google Scholar]
- 48.Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm Behav. 2008;54(4):488–495. doi: 10.1016/j.yhbeh.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riters LV, et al. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38(4):250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 50.Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18(3):117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 51.Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13(5):649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- 52.Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: Possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316(1):35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- 53.Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci USA. 2000;97(15):8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beach FA. Hormones and Behavior. New York: Paul B. Hoeber; 1948. [Google Scholar]
- 55.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16(4):365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- 56.Derégnaucourt S, Mitra PP, Fehér O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433(7027):710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- 57.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59(6):1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 58.Alward BA, Rouse ML, Stevenson TJ, Ball GF. Photoperiodic and social regulation of song rate and structure in male border canaries (Serinus canaria) Integrative and Comparative Biology. 2012 52:E5 (abstr) [Google Scholar]
- 59.Voigt C, Leitner S. Seasonality in song behaviour revisited: Seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria) Horm Behav. 2008;54(3):373–378. doi: 10.1016/j.yhbeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Marler PR, Slabbekoorn H. Nature's Music: The Science of Birdsong. San Diego: Academic; 2004. [Google Scholar]
- 61.Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156(3):337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- 62.Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Horm Behav. 2005;47(4):467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194(4261):211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 64.McCasland JS. Neuronal control of bird song production. J Neurosci. 1987;7(1):23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernard DJ, Eens M, Ball GF. Age- and behavior-related variation in volumes of song control nuclei in male European starlings. J Neurobiol. 1996;30(3):329–339. doi: 10.1002/(SICI)1097-4695(199607)30:3<329::AID-NEU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]