Significance
Agonistic antibodies targeting key TNF receptor (TNFR) molecules involved in antitumor responses have been demonstrated as potent antitumor therapies in preclinical studies. However, no effective agonistic anti-TNFR therapies have been successfully developed to date. In contrast, cytotoxic antitumor antibodies targeting tumor antigens or antagonistic antibodies blocking key inhibitory checkpoints are widely used in clinical settings. One explanation for this discrepancy has been recently provided by the finding that agonistic anti-TNFR antibodies have a previously unappreciated requirement for inhibitory Fcγ receptor FcγRIIB coengagement. Understanding the differential FcγRIIB coengagement requirement by different antibodies and the in vivo cross-linking function of FcγRIIB, as defined by this study, not only has implications for the development of potent agonistic anti-TNFR therapies but also for the understanding of TNFR activation mechanisms.
Keywords: anti-CD40, anti-DR5, ITIM, ITAM, Fc engineering
Abstract
Agonistic anti-TNF receptor (TNFR) superfamily member antibodies are a class of promising antitumor therapies in active clinical investigation. An unexpected requirement for inhibitory Fcγ receptor FcγRIIB coengagement has recently been described for their in vivo antitumor activities. Although these findings have informed the design of more potent antitumor agonistic, anti-TNFR therapies, the underlying mechanism has remained obscure. Through detailed genetic analysis of strains conditionally deleted for FcγRIIB on defined cellular populations or mutated in specific signaling components, we now demonstrate that different agonistic anti-TNFR antibodies have specific requirements for FcγRIIB expression on defined cellular populations and function in trans in the absence of FcγRIIB signaling components, thus supporting a general mechanism of FcγRIIB cross-linking in vivo for the activities of these antibodies.
Both mouse and human express several activating and one inhibitory Fcgamma receptors (FcγRs). These FcγRs are expressed broadly on lymphoid and myeloid cells such as B cells, dendritic cells, macrophages, neutrophils, and mast cells, where they regulate and mediate immune responses triggered by immune complexes. Whereas binding of immune complexes to activating FcγRs on dendritic cells and myeloid effector cells leads to cell activation, their binding to the coexpressed inhibitory FcγRIIB inhibits cell activation (1–4). In addition, FcγRIIB expression on B cells inhibits B-cell activation when coligated with B-cell antigen receptors. The opposing effects of activating and inhibitory FcγRs result from their different downstream signaling pathways (5). Typical activating human and mouse FcγRs either contain an immunoreceptor tyrosine-based activation motif (ITAM) or are associated with an ITAM-containing adaptor protein such as Fc receptor common γ-chain. Cross-linking of activating FcγRs by immune complexes results in ITAM phosphorylation, subsequent activation of phosphoinositide 3-kinase and generation of phosphatidylinositol-3,4,5-trisphosphate (PIP3), calcium mobilization, and further downstream signaling events that lead to cell activation. In contrast, FcγRIIB contains an immunoreceptor tyrosine-based inhibitory motif (ITIM), and its phosphorylation leads to the recruitment of SH2 domain-containing inositol 5-phosphatase (SHIP), which interferes with activating signaling pathways by hydrolyzing PIP3.
Activating FcγRs are essential mediators of antibody effector functions including cytotoxicity and phagocytosis by myeloid effector cells (5). It has been shown in both preclinical and clinical studies that interactions between the Fc domains of tumor antigen-specific effector antibodies and activating FcγRs are essential for their antitumor activities (6–9). Recently, αCTLA-4 antibodies that target a key negative immune checkpoint have also been demonstrated to mediate their antitumor activities through activating FcγR-dependent depletion of tumor-associated T regulatory cells that express high levels of CTLA-4 (10, 11). In addition, our previous studies have shown that the ratio of an Fc’s binding affinity to activating FcγRs relative to its binding affinity to the inhibitory FcγRIIB correlates with its ability to mediate antibody effector functions and antitumor responses (12). These findings highlight the importance of interactions between Fc and activating FcγRs in the activity of therapeutic effector antibodies, and have provided the basis for optimizing their antitumor activities by activating FcγR-targeted Fc engineering.
Agonistic antibodies represent another class of antitumor antibodies designed to mimic the activity of endogenous ligands, thereby activating the downstream signaling pathways of targeted molecules. Many tumor necrosis factor receptor (TNFR) superfamily members such as CD40 and DR5 control key signaling pathways involved in immune and antitumor responses, and agonistic antibodies targeting these molecules have shown promising antitumor activities in preclinical studies (13). We and others have recently found that both agonistic αCD40 and αDR5 antibodies require Fc–FcγR interactions for their in vivo activities and, in contrast to cytotoxic effector antitumor antibodies, these agonistic antibodies require no activating FcγRs, but inhibitory FcγRIIB (14–16). These studies, together with previous and other recent studies (17, 18), have established a general requirement of FcγRIIB for the in vivo activities of agonistic anti-TNFR antibodies (19). In addition, we have also demonstrated that Fcs that preferentially bind to inhibitory FcγRIIB are more potent for agonistic anti-TNFR antibodies, and that the potency of agonistic anti-TNFR antibodies can be enhanced through FcγRIIB-targeted Fc engineering (14, 15). Although these studies have provided a logical approach to designing potent agonistic anti-TNFR antibodies, the in vivo mechanism underlying this general FcγRIIB requirement remains to be determined. We now demonstrate through the use of genetically defined deletions of FcγRIIB on specific immune cell populations and targeted mutations in FcγRIIB signaling domains the mechanistic basis for this general requirement.
Results
trans-Coengagement with FcγRIIB Is both Necessary and Sufficient for the in Vivo Activities of Agonistic Anti-TNFR Antibodies.
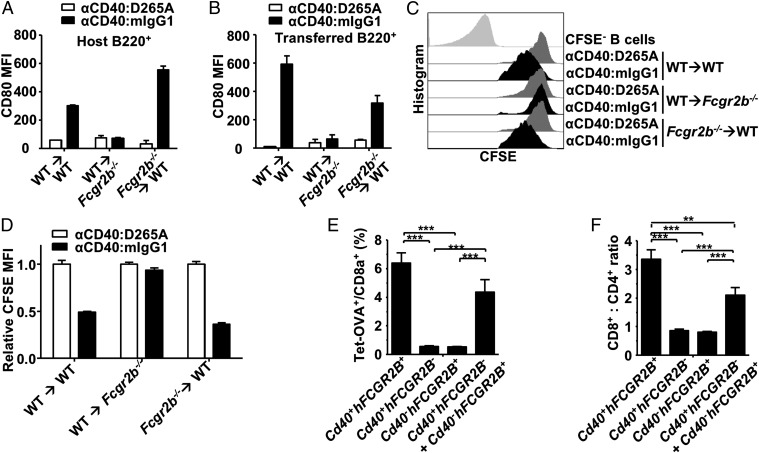
To define the mechanism of action underlying the requirement of FcγRIIB coengagement to drive the in vivo activity of agonistic anti-TNFR antibodies, we first determined whether FcγRIIB and agonistic anti-TNFR antibodies interact in cis or in trans. Some tumor cells targeted by agonistic αDR5 antibodies such as MC38 and 4T1.2 do not coexpress FcγRIIB with the targeted TNFR molecules (Fig. S1A), and thus these agonistic antibodies must function in trans in these models. However, there are also cells targeted by agonistic anti-TNFR antibodies that do coexpress FcγRIIB with the targeted TNFR molecules, such as B cells that coexpress FcγRIIB with CD40, which makes it possible for agonistic αCD40 antibodies to coengage FcγRIIB in cis or in trans. To investigate whether agonistic αCD40 antibodies are active in the presence of FcγRIIB cis-coengagement, 5-(6)-carboxyfluorescein diacetate succinimidyl diester (CFSE)-labeled CD45.1+ wild-type (WT) splenic cells were adoptively transferred into CD45.2+ WT or FcγRIIB-deficient (Fcgr2b−/−) mice and tested for B-cell stimulation in response to a previously described agonistic CD40 antibody, αCD40:mIgG1 (14). The D265A variant of αCD40:mIgG1 (αCD40:D265A) that does not bind to FcγRs and thus has no immunostimulatory activity (14) was used as a negative control. Because the transferred cells are very rare in the recipient mice, no trans-interactions between these transferred cells are expected, and the effect of agonistic αCD40 antibody on transferred B cells will be either due to its cis-coengagement with FcγRIIB on transferred cells or to its trans-coengagement with FcγRIIB on host cells. As expected, when WT B cells were transferred into WT recipient mice, both host and transferred B cells are stimulated by agonistic αCD40 antibodies, as shown by increased CD80 levels (Fig. 1 A and B) and diluted CFSE (Fig. 1 C and D). In contrast, WT B cells transferred into Fcgr2b−/− mice failed to respond (Fig. 1 B and D), suggesting agonistic αCD40 antibody requires trans-coengagement with FcγRIIB on the host cells to stimulate transferred B cells. It also suggests that cis-coengagement with FcγRIIB on transferred B cells is not sufficient for agonistic αCD40 antibody to stimulate transferred B cells. At the same time, when Fcgr2b−/− cells were transferred into WT mice, where only trans-coengagement with FcγRIIB on host cells is possible for agonistic αCD40 antibodies binding to the CD40 on transferred B cells, these transferred B cells are stimulated by agonistic αCD40 antibodies. Therefore, trans-coengagement with FcγRIIB is not only necessary but also sufficient to drive the immunostimulatory activities of agonistic αCD40 antibodies.
Fig. 1.
FcγRIIB works in trans to drive the in vivo activity of agonistic αCD40 antibodies. (A–D) CFSE-labeled WT or Fcgr2b−/− splenocytes were adoptively transferred into WT C57BL/6 or Fcgr2b−/− mice, which were then treated with an agonistic αCD40 antibody (αCD40:mIgG1) or an inactive αCD40 control antibody (αCD40:D265A). On day 3, CD80 levels were analyzed in host B cells (A) and transferred B cells (B) in blood and presented as CD80 mean fluorescence intensity (MFI); on day 5, CFSE levels expressed as MFI were analyzed in transferred B cells in spleen (C) and are summarized (D). (E and F) WT C57BL/6 mice reconstituted with bone marrow cells isolated from mice of the indicated genotypes were immunized with DEC-OVA and αCD40:hIgG1(S267E). Seven days later, the percentage of OVA-specific cells among CD8+ T cells (E) and the ratios of CD8+ to CD4+ cells (F) were analyzed. **P < 0.01, ***P < 0.001, ANOVA with Tukey’s post hoc. All error bars represent SEM. Representative of two experiments.
We have demonstrated previously that the immunostimulatory and antitumor activity of chimeric, mouse–human agonistic αCD40 antibodies can be enhanced by human FcγRIIB (hFcγRIIB)-targeted Fc engineering (14). To determine whether trans-coengagement with human FcγRIIB can support the activity of these hFcγRIIB-enhanced, agonistic αCD40 antibodies, lethally irradiated WT C57BL/6 recipient mice were reconstituted with bone marrow cells that express both CD40 and human FcγRIIB (Cd40+hFCGR2B+), CD40 alone (Cd40+hFCGR2B−), human FcγRIIB alone (Cd40−hFCGR2B+), or a 1:1 mixed bone marrow preparation that expresses only CD40 or only human FcγRIIB (Fig. S1B), and analyzed for activity of the human FcγRIIB-enhanced αCD40:hIgG1(S267E) antibody (14). As expected (14), mice reconstituted with Cd40+hFCGR2B+ donor cells developed a robust ovalbumin (OVA)-specific T-cell response and a high CD8+:CD4+ ratio when immunized with DEC-OVA (an OVA vaccine targeted to dendritic cells by αDEC-205 antibodies) and αCD40:hIgG1(S267E) (Fig. 1 E and F) and, in contrast, αCD40:hIgG1(S267E) displayed no activities when donor cells expressed only CD40 or human FcγRIIB. Importantly, a significant OVA-specific T-cell response and a high CD8+:CD4+ ratio were observed in mice reconstituted with a 1:1 mixed bone marrow preparation of cells that express either CD40 or human FcγRIIB (Fig. 1 E and F), suggesting that αCD40:hIgG1(S267E) can activate CD40 expressed on Cd40+hFCGR2B− cells when Cd40−hFCGR2B+ cells are present. Therefore, trans-coengagement is also sufficient to drive the immunostimulatory activity of this human FcγRIIB-enhanced agonistic CD40 antibody. Taken together, these studies suggest that regardless of whether cells targeted by agonistic anti-TNFR antibodies coexpress FcγRIIB with the target TNFR molecules, FcγRIIB provides trans-coengagement interactions to drive the in vivo activities of these antibodies.
The Impact of FcγRIIB on the in Vivo Activity of Agonistic Anti-TNFR Antibodies Is Independent of FcγRIIB Downstream Signaling.
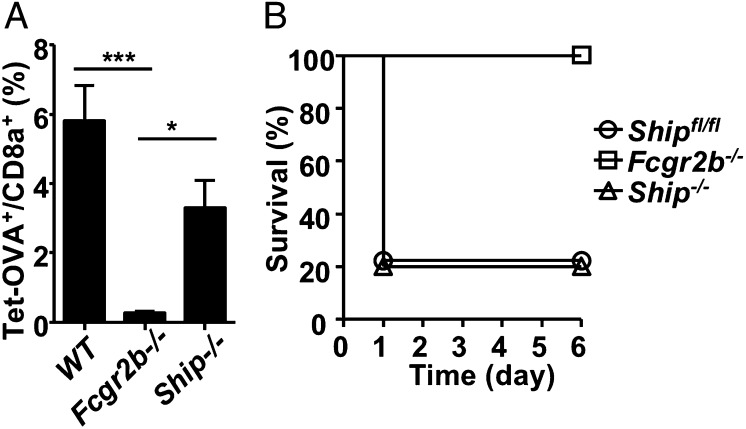
In previous studies on the mechanism of inhibitory signaling by FcγRIIB, cis-coengagement was found to be the dominant pathway based on the requirement that the ITIM be phosphorylated, which recruits SHIP and results in inhibitory signaling (1, 20–22). The findings that FcγRIIB functions in trans to drive the activities of agonistic anti-TNFR antibodies raised the question of whether the FcγRIIB inhibitory signaling pathway is required in this process. To address this question, we analyzed the activities of agonistic αCD40 and αFas antibodies in SHIP-deficient mice (Ship−/−). As shown in Fig. 2, SHIP is neither required for the immunostimulatory activity of agonistic αCD40 antibody nor for the hepatotoxic activity of agonistic αFas antibody (clone Jo2) that results in mortality.
Fig. 2.
SHIP is not required for the impact of FcγRIIB on the activities of agonistic αCD40 and αFas antibodies. (A) The percentage of OVA-specific cells among CD8+ T cells in spleen (expressed as mean ± SEM) in WT, Fcgr2b−/−, and Ship−/− mice. Mice were treated with DEC-OVA and agonistic αCD40 antibodies (1C10) and analyzed 7 d later for OVA-specific CD8+ T cells. *P < 0.05, ***P < 0.001, ANOVA Tukey’s post hoc. (B) Survival curves of WT (Shipfl/fl), Fcgr2b−/−, and Ship−/− mice treated with agonistic αFas antibodies. Representative of three experiments.
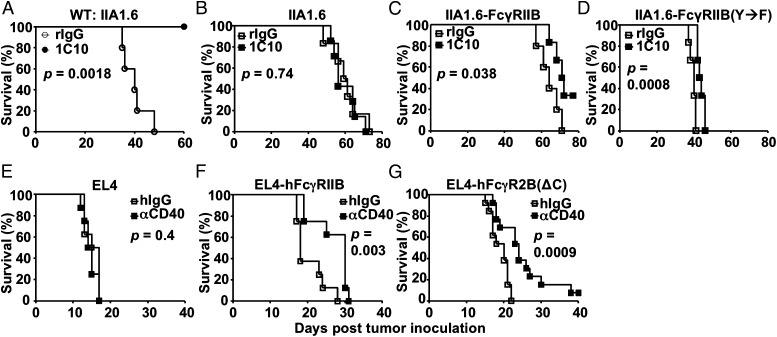
To directly determine the contribution of the FcγRIIB ITIM in the agonistic activity of these antibodies, we tested whether FcγRIIB carrying a Y→F mutation in the ITIM, and thus unable to recruit SHIP (21, 23) or mediate inhibitory signaling (1, 22), is able to support the antitumor activities of an agonistic αCD40 antibody. Using a syngeneic model based on IIA1.6 lymphoma cells, an FcγRIIB-deficient B-cell lymphoma cell line, we have generated lines expressing either wild-type or signaling-null (with a Y→F mutation in the ITIM) FcγRIIB-expressing cells, referred to as IIA1.6-FcγRIIB and IIA1.6-FcγRIIB(Y→F), respectively, for in vivo tumor challenge studies (Fig. S2 A and B) (1). As shown in Fig. 3A, agonistic αCD40 antibody 1C10 protected WT but not FcγRIIB-deficient mice in the IIA1.6 model (Fig. 3B), consistent with our previous finding that FcγRIIB is required for the antitumor activities of agonistic αCD40 antibodies. Interestingly, when IIA1.6-FcγRIIB cells were used, agonistic αCD40 antibodies displayed significant antitumor activities in FcγRIIB-deficient mice (Fig. 3C), although much weaker than in WT mice (Fig. 3A), suggesting that FcγRIIB expressed by IIA1.6 cells can partially replace endogenous FcγRIIB and drive significant antitumor activities of agonistic αCD40 antibodies in vivo. We exploited this feature to test whether signaling-null FcγRIIB with a Y→F mutation in the ITIM can drive the antitumor activities of agonistic αCD40 antibodies. As shown in Fig. 3D, significant antitumor activities were observed in FcγRIIB-deficient mice when IIA1.6-FcγRIIB(Y→F) cells were used, suggesting that mouse FcγRIIB ITIM signaling is not required for its ability to drive the antitumor activities of agonistic αCD40 antibodies.
Fig. 3.
FcγRIIB ITIM signaling is not required for the antitumor activity of agonistic αCD40 antibodies. (A and B) WT (A) or Fcgr2b−/− (B) BALB/c mice were inoculated i.v. with IIA1.6 lymphoma cells, treated with agonistic αCD40 antibodies (1C10) or rat control IgG, and monitored for survival. (C and D) Same as B except that IIA1.6 cells that express mouse FcγRIIB (IIA1.6-FcγRIIB) (C) or FcγRIIB with a Y→F mutation in the ITIM [IIA1.6-FcγRIIB(Y→F)] (D) were used. (E–G) WT C57BL/6 mice were inoculated with EL4 cells (E), EL4 cells that express human FcγRIIB (F), or EL4 cells that express truncated human FcγRIIB without the cytoplasmic domain (G), and treated with human FcγRIIB-dependent agonistic αCD40 antibodies [αCD40:hIgG1(S267E)] or control human IgG, and monitored for survival. Presented are all survival curves with P values calculated by log-rank tests. Representative of two experiments.
To test whether this is a phenotype specific for IIA1.6-derived cell lines and mouse FcγRIIB, we generated EL4 tumor cells that express either WT human FcγRIIB or a truncated version of human FcγRIIB (Fig. S2C), and tested the antitumor activity of a chimeric, agonistic αCD40 antibody that requires human FcγRIIB interactions, αCD40:hIgG1(S267E) (14). Consistent with our previous studies, αCD40:hIgG1(S267E) had no antitumor activities in WT C57BL/6 mice (Fig. 3E). When EL4 cells that express unmutated human FcγRIIB (EL4-hFcγRIIB) were used in WT C57BL/6 mice, αCD40:hIgG1(S267E) significantly prolonged mouse survival (Fig. 3F), consistent with the notion that human FcγRIIB expressed by tumor cells can support or partially support the antitumor activity of αCD40:hIgG1(S267E). Importantly, significant antitumor activities of αCD40:hIgG1(S267E) was also observed when EL4 cells that express a truncated human FcγRIIB without the cytoplasmic domain [EL4-FcγRIIB(ΔC)], and thus the ITIM, was used (Fig. 3G). These experiments demonstrated that both mouse and human FcγRIIB without ITIM signaling are able to drive the antitumor activity of agonistic αCD40 antibodies. Taken together, these studies suggest that the impact of FcγRIIB on the in vivo activity of agonistic anti-TNFR antibodies is independent of FcγRIIB downstream signaling.
Various Agonistic Anti-TNFR Antibodies Have Distinct Requirements for Cell-Specific FcγRIIB Expression.
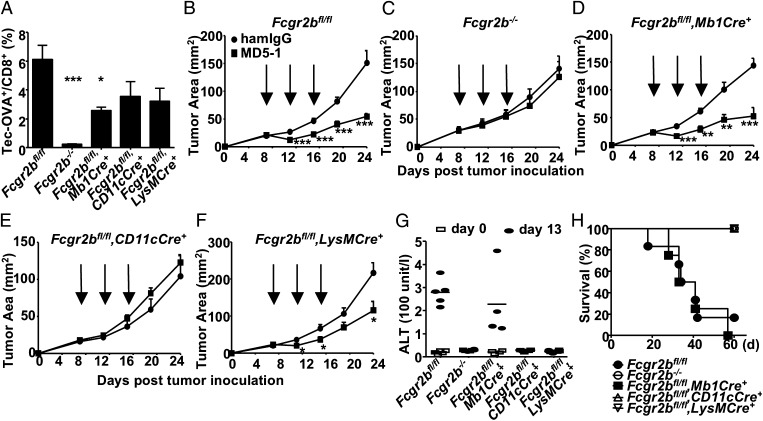
Given that signaling-independent trans-interactions are the general mode of action of FcγRIIB coengagement required to drive the in vivo activity of agonistic anti-TNFR antibodies, we investigated whether there was also a general cellular source of FcγRIIB involved in these trans-interactions. FcγRIIB is highly expressed on B cells, immature dendritic cells, and myeloid cells (5). FcγRIIB conditional knockout lines with selective deletion of FcγRIIB in these cells was achieved by cre:lox-mediated deletion of the floxed Fcgr2b gene crossed to Mb1-Cre–, CD11c-Cre–, and LysM-Cre–expressing lines, respectively (Fig. S3). As shown in Fig. 4A, examination of the immunostimulatory activity of an agonistic CD40 antibody (clone 1C10) in these mice showed that whereas none of these conditional knockout lines recapitulated the phenotype of the Fcgr2b germ-line knockout mouse, the activity of the agonistic αCD40 antibody was significantly reduced in mice with B cell-specific deletion of Fcgr2b. We also analyzed the antitumor and hepatotoxic activity of the agonistic αDR5 antibody MD5-1 (15) in these Fcgr2b conditional lines. In contrast to the significant contribution of B cell-specific FcγRIIB to the activity of agonistic αCD40 antibody, deletion of FcγRIIB in B cells had no effect on the antitumor activity of MD5-1 (Fig. 4 B–D). Instead, selective deletion of FcγRIIB in CD11c-cre+ and LysM-cre+ cells significantly reduced the antitumor activity of MD5-1 (Fig. 4 B, C, E, and F). In addition, the hepatotoxic activity of MD5-1 is completely dependent on FcγRIIB expression in CD11c-cre+ and LysM-cre+ cells, as neither elevated alanine aminotransferase (ALT) levels nor mortality due to MD5-1 treatment was observed in FcγRIIB conditional knockout mice that express either LysM-Cre or CD11c-Cre (Fig. 4 G and H). Therefore, different agonistic anti-TNFR antibodies have very different requirements for the source of FcγRIIB involved in their FcγRIIB trans-coengagement.
Fig. 4.
Agonistic αCD40 and αDR5 antibodies require different cell-specific FcγRIIB for their in vivo activities. (A) The percentage of OVA-specific cells among CD8+ T cells (expressed as mean ± SEM) in WT and mutant mice with germ-line or conditional knockout of Fcgr2b. Mice were treated with DEC-OVA and agonistic αCD40 antibodies (1C10), and analyzed for OVA-specific CD8+ T cells in blood 7 d later. *P < 0.05, ***P < 0.001, ANOVA with Dunnett’s post hoc comparing each group with the Fcgr2bfl/fl group. (B–F) MC38 tumor growth curves (expressed as mean ± SEM). MC38 cells were implanted in mice of the indicated genotypes, treated with 100 µg of MD5-1 antibodies at the time points indicated by the arrows, and monitored for tumor growth. *P < 0.05, **P < 0.01, ***P < 0.001, two-tailed t test. (G and H) Mice of the indicated genotypes were treated with high doses of MD5-1 or hamster control IgG (300 µg per mouse repeated at 3-d intervals for a total of 1.2 mg per mouse), analyzed for serum ALT levels on days 0 and 13, and monitored for survival. Serum ALT levels are presented in G and survival curves are presented in H. Representative of two experiments.
FcγRIIB Expression Levels Are Important for Its Impact on the in Vivo Activity of Agonistic Anti-TNFR Antibodies.
We next tested whether the absolute level of FcγRIIB expression is important for its impact on the in vivo activity of agonistic anti-TNFR antibodies. FcγRIIB is expressed at high levels and is the most widely expressed FcγR (5). Strikingly, in the DEC-OVA model, agonistic CD40 antibody (clone 1C10) lost its immunostimulatory activity in heterozygous Fcgr2b knockout mice with reduced FcγRIIB expression (Fig. S4), to the same extent as in FcγRIIB knockout mice (Fig. 5A), thus suggesting that there is a minimal FcγRIIB expression level required to drive the in vivo activity of agonistic αCD40 antibodies. The expression level in the heterozygous Fcgr2b knockout mice is thus below the threshold required to support the agonistic activity of the αCD40 antibody. We found that this was also true for the agonistic αDR5 antibody (clone MD5-1). As shown in Fig. 5 B and C, the hepatotoxic and antitumor activity of MD5-1 is completely lost in heterozygous Fcgr2b knockout mice, equivalent to that observed in homozygous Fcg2b knockout mice, suggesting that there is a similar FcγRIIB expression-level threshold for agonistic αDR5 antibodies. However, the agonistic αFas antibody Jo2 is active in both WT mice and heterozygous Fcgr2b knockout mice but not in homozygous Fcgr2b knockout mice (Fig. 5D). Therefore, FcγRIIB expression levels are critical for its impact on the activities of agonistic anti-TNFR antibodies, and there seems to be expression-level thresholds for FcγRIIB to drive the activities of certain classes of agonistic anti-TNFR antibodies. At the same time, various agonistic anti-TNFR antibodies seem to have very different thresholds, which are above the FcγRIIB expression level in heterozygous Fcgr2b knockout mice for agonistic αCD40 and αDR5 antibodies and below this level for agonistic αFas antibodies.
Fig. 5.
Different FcγRIIB expression levels are required for the in vivo activities of various agonistic anti-TNFR antibodies. (A) The percentage of OVA-specific cells among CD8+ T cells in spleen (expressed as mean ± SEM) in WT, Fcgr2bfl/−, and Fcgr2b−/− mice was analyzed and is presented as in Fig. 2A. **P < 0.01; n.s., not significant, ANOVA Tukey’s post hoc. (B) Serum ALT levels in WT, Fcgr2bfl/−, and Fcgr2b−/− mice in response to agonistic αDR5 antibody (MD5-1) were analyzed and are presented as in Fig. 4G. (C) Tumor growth curves (expressed as mean ± SEM). Fcgr2bfl/− mice were inoculated with MC38 tumor cells, treated with MD5-1 or hamster control IgG, and analyzed as in Fig. 4 B–F. (D) The survival of WT (Shipfl/fl), Fcgr2bfl/−, and Fcgr2b−/− mice in response to agonistic αFas antibody was analyzed and is presented as in Fig. 2B. Representative of three experiments.
Discussion
Our analysis of multiple representative agonistic anti-TNFR antibodies using in vivo models has revealed important features of the FcγRIIB coengagement required by these antibodies: (i) Signaling-independent, trans-coengagement with FcγRIIB is the general mode of action; and (ii) FcγRIIB distribution and expression levels are critical for its impact on the activities of agonistic anti-TNFR antibodies. Based on these studies, a general model of FcγRIIB-mediated activation of agonistic anti-TNFR antibodies has emerged, as summarized in Fig. S5. Because trans-coengagement is necessary and sufficient, and signaling by FcγRIIB is not required, we can postulate that FcγRIIB acts as a scaffold to provide the clustering of TNFR molecules on the membrane and thereby mimic the effect of multimeric ligands engaging these receptors. Clustering of TNFRs by the two arms of each single agonistic anti-TNFR antibody is insufficient to mimic the effect of the endogenous multimeric ligands for these receptors to trigger downstream signaling. This signaling-independent, in vivo cross-linking mechanism of FcγRIIB can be applied to all agonistic antibodies that target multimeric ligand-dependent TNFR molecules and possibly non-TNFR molecules regardless of the nature of their downstream signaling.
Consistently, our study has also showed that FcγRIIB distribution and expression levels are critical for the activities of agonistic anti-TNFR antibodies as they control whether FcγRIIB can interact with these antibodies and provide sufficient clustering of the targeted TNFR molecules. For instance, FcγRIIB expression in B cells plays a significant role in driving the immunostimulatory activity of agonistic αCD40 antibodies, likely because B cells are the most abundant FcγRIIB-expressing cells in lymphoid tissue, where CD40 on antigen-presenting cells is targeted. In contrast, FcγRIIB expression in CD11c+ and LysM+ cells, not B cells, is required for the hepatotoxic effect of agonistic αDR5 antibodies, which could be explained by the fact that residential macrophages and dendritic cells are rich and B cells are rare in liver, where DR5 on cholangiocytes is targeted (24, 25). Similarly, most immune cells infiltrated into tumor tissue are CD11b+ myeloid cells (10), which may explain a dominant role of FcγRIIB expression in these cells in driving the antitumor activity of agonistic αDR5 antibodies. In addition, different agonistic anti-TNFR antibodies require different FcγRIIB expression levels, which may be related to several factors, including the efficiency of the involved antibody being cross-linked by FcγRIIB, the expression levels of the targeted TNFR, and the amount of cross-linking required to activate the targeted TNFR in the targeted cells. Both agonistic αFas and αDR5 antibodies mediate their hepatotoxic effects by triggering a Fas-associated protein with death domain–dependent apoptotic signaling pathway, but liver cells appear to be more sensitive to apoptosis mediated by Fas than by DR5, as hepatocytes are more sensitive to FasL than TRAIL even though both Fas and DR5 are expressed (24). Therefore, it is reasonable to hypothesize that, to be activated in liver cells, Fas requires less cross-linking and thus lower FcγRIIB expression than DR5.
Whereas previous studies using in vitro cell-culture systems also supported a cross-linking function of FcγRIIB that is signaling-independent (16, 26), these and other (18) in vitro studies also showed that both mouse and human activating FcγRs can effectively cross-link agonistic αCD40, αDR4, and αDR5 antibodies. In contrast, activating FcγRs are clearly not sufficient, and even detrimental, in supporting the in vivo activities of these agonistic anti-TNFR antibodies (14–16, 18). Among many possible reasons, the inconsistency between in vitro and in vivo studies could be due to oversimplification or fundamentally a lack of tissue structure in the in vitro systems, highlighting the importance of using in vivo models. While the in vivo cross-linking function of FcγRIIB required by agonistic anti-TNFR antibodies has been hypothesized before (16, 17), this study provided the necessary in vivo evidence to establish it.
The in vivo cross-linking model also suggests that FcγRIIB can mediate this function uniquely among FcγRs, presumably because its engagement in trans would not induce antibody-dependent cell-mediated cytotoxicity, and thus result in depletion of the targeted TNFR-expressing cell. Although it is not clear whether trans-coengagement interactions with activating FcγRs by agonistic anti-TNFR antibodies always induce antibody-dependent cell-mediated cytotoxicity, it is reasonable to postulate that the targeted TNFR-expressing cells will be depleted when the strength and density of these interactions reach the levels required to effectively cluster TNFR molecules.
Finally, our findings have implications in the design of potent agonistic anti-TNFR antibodies or other TNFR-binding molecules such as TNFR ligands. It predicts that designs leading to increased clustering of TNFR molecules will result in increased potency in activating targeted TNFR molecules. FcγRIIB-targeted Fc engineering is an effective approach for agonistic anti-TNFR antibodies to achieve this goal, and permit the advancement of these promising therapeutics into clinical application.
Materials and Methods
Detailed materials and methods are described in SI Materials and Methods.
Mice and Antibodies.
Fcgr2bfl/fl mice were generated from B6 ES cells and crossed to Cag-Cre, Mb1-Cre, Cg1-Cre, CD11c-Cre, and LysM-Cre to generate germ-line and conditional knockout stains. Cd40−/− mice (The Jackson Laboratory) were crossed to human FCGR2B transgenic mice (14) to generate Cd40−hFCGR2B+ mice. All mouse studies had been approved by The Rockefeller University Institutional Animal Care and Use Committee. Agonistic antibodies against mouse CD40 [1C10, and 1C10-derived αCD40:mIgG1, αCD40:mIgG1(D265A), and αCD40:hIgG1(S267E)], DR5 (MD5-1), and Fas (Jo2) have been described previously (14, 15, 17).
Agonistic αCD40 Antibody-Induced B-Cell Activation.
CFSE-labeled WT or Fcgr2b−/− splenocytes were adoptively transferred into recipient mice that express different CD45 congenic markers on day −1. Recipient mice were treated with 30 µg of αCD40:mIgG1 or αCD40:mIgG1(D265A) on day 0, and analyzed for CD80 levels in blood B cells on day 3 and CFSE levels in transferred B cells in spleen on day 5.
OVA-Specific T-Cell Response.
Mice were i.p. injected with 5 µg of DEC-OVA and 30 µg of αCD40 antibodies, and analyzed 7 d later for OVA-specific CD8+ T cells in blood or spleen by OVA peptide SIINFEKL H-2b tetramer staining as previously described (14). In the bone marrow chimeric experiment, lethally irradiated WT C57BL/6 recipient mice were reconstituted with 106 bone marrow cells for 3 mo and then analyzed for OVA-specific CD8+ T-cell response.
Tumor Models.
MC38 cells (106 per mouse) were implanted s.c. After 5–7 d, mice with palpable tumors were treated with 100 µg per mouse of MD5-1 or control hamster IgG i.v. three times at 4-d intervals, and monitored for tumor growth as described previously (15). In IIA1.6 and IIA1.6-derived B-cell lymphoma models, mice received 2.5 × 107 tumor cells on day 0 and 200 µg of 1C10 or control antibodies on day 7 and 10 through i.v. injection, and monitored for survival. In EL4, EL4-hFcγRIIB, and EL4-hFcγRIIB(ΔC) (generated by transducing EL4 cells with retroviruses containing pFB-Neo vectors expressing unmutated or truncated human FcγRIIB and Geneticin selection) models, mice received 1.5 × 107 tumor cells on day 0 and 200 µg of αCD40:hIgG1(S267E) or control antibodies on days 3 and 5 through i.v. injection, and monitored for survival.
Supplementary Material
Acknowledgments
We thank P. Smith for excellent technical support. This work was performed with support from National Institutes of Health grants (to J.V.R.). F.L. is supported in part by the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319502110/-/DCSupplemental.
References
- 1.Muta T, et al. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;369(6478):340. doi: 10.1038/369340a0. [DOI] [PubMed] [Google Scholar]
- 2.Daëron M, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3(5):635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 3.Dhodapkar KM, et al. Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204(6):1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhodapkar KM, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci USA. 2005;102(8):2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 6.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 7.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 9.Musolino A, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 10.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulliard Y, et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210(9):1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science. 2005;310(5753):1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 13.Grewal IS, editor. Therapeutic Targets of the TNF Superfamily. New York: Springer; 2009. pp 1–220. [Google Scholar]
- 14.Li F, Ravetch JV. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333(6045):1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Ravetch JV. Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcγ receptor engagement. Proc Natl Acad Sci USA. 2012;109(27):10966–10971. doi: 10.1073/pnas.1208698109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White AL, et al. Interaction with FcγRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187(4):1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, et al. Fc gamma Rs modulate cytotoxicity of anti-Fas antibodies: Implications for agonistic antibody-based therapeutics. J Immunol. 2003;171(2):562–568. doi: 10.4049/jimmunol.171.2.562. [DOI] [PubMed] [Google Scholar]
- 18.Wilson NS, et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19(1):101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Ravetch JV. A general requirement for FcγRIIB co-engagement of agonistic anti-TNFR antibodies. Cell Cycle. 2012;11(18):3343–3344. doi: 10.4161/cc.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daëron M. Intracytoplasmic sequences involved in the biological properties of low-affinity receptors for IgG expressed by murine macrophages. Braz J Med Biol Res. 1995;28(3):263–274. [PubMed] [Google Scholar]
- 21.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383(6597):263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, et al. The inositol polyphosphate 5-phosphatase Ship is a crucial negative regulator of B cell antigen receptor signaling. J Exp Med. 1998;188(7):1333–1342. doi: 10.1084/jem.188.7.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong DC, et al. Mutational analysis reveals multiple distinct sites within Fc gamma receptor IIB that function in inhibitory signaling. J Immunol. 2000;165(8):4453–4462. doi: 10.4049/jimmunol.165.8.4453. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci USA. 2008;105(31):10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(Suppl 1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199(4):437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.