Abstract
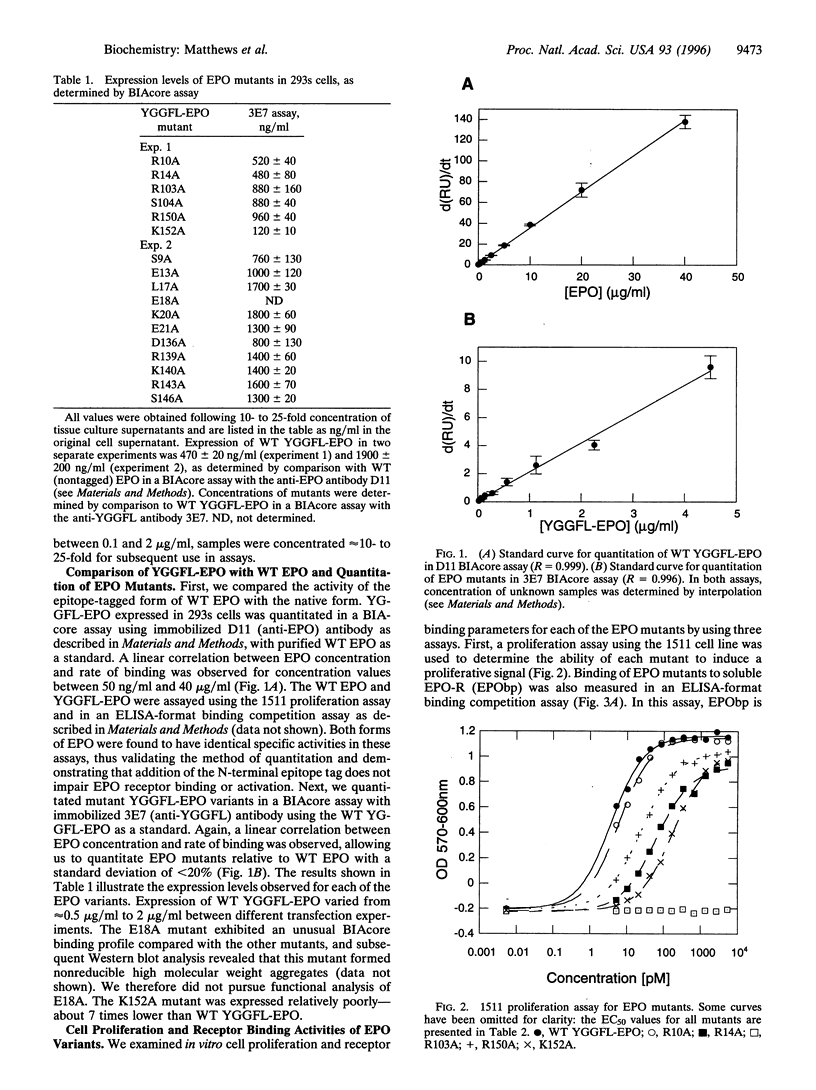
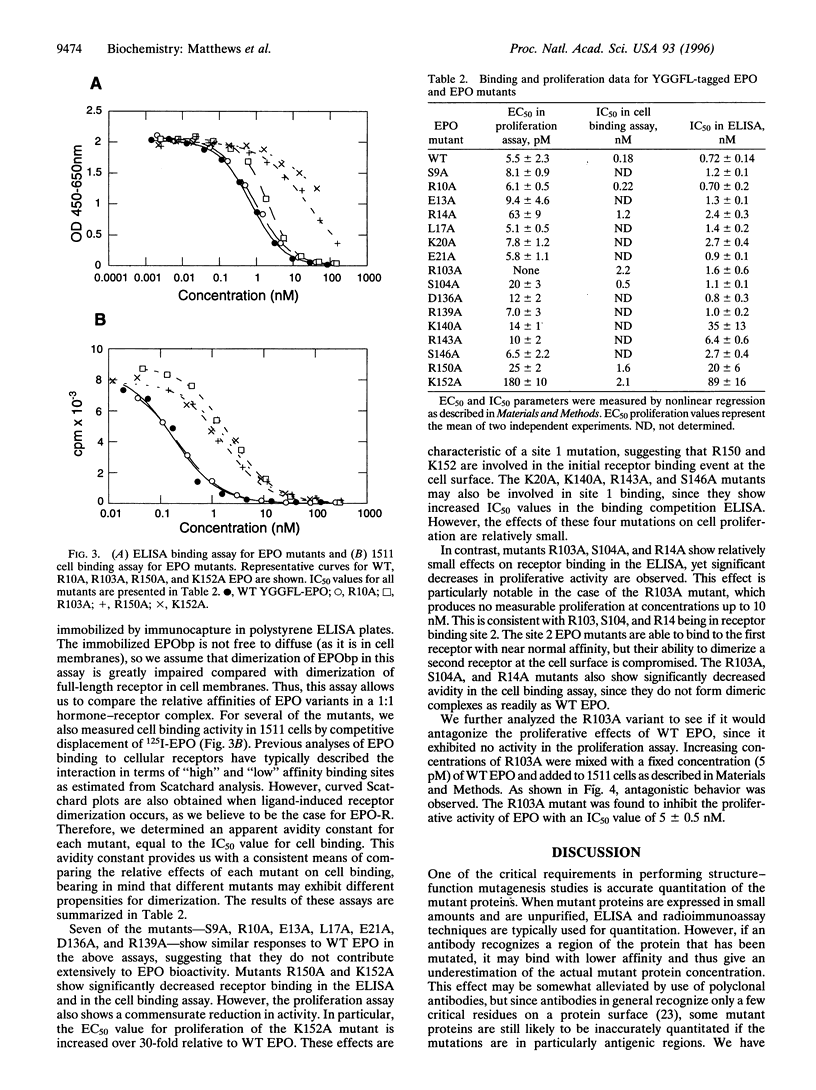
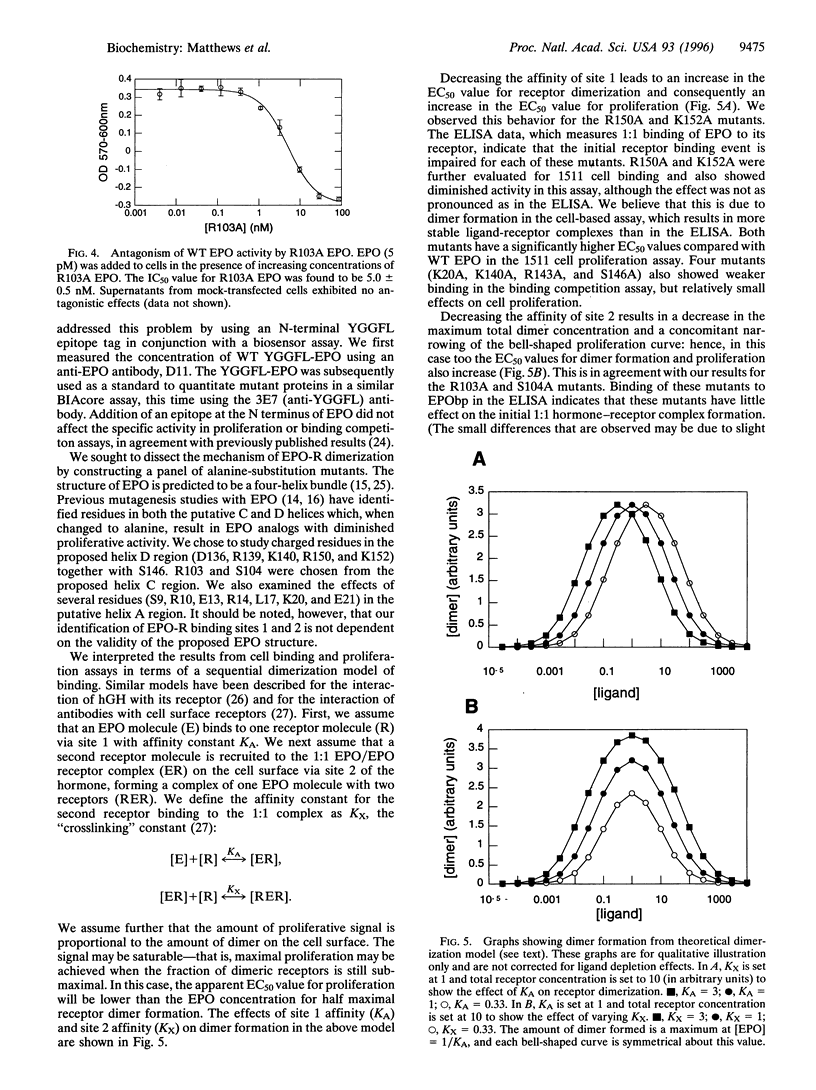
We have probed the interaction of human erythropoietin (EPO) with its receptor (EPO-R) by analyzing a panel of 17 EPO mutants in a variety of in vitro assays. Mutant proteins were expressed in 293s cells and quantified by using an N-terminal epitope tag in conjunction with a surface plasmon resonance assay. Receptor binding was studied using both a soluble form of the EPO-R extracellular domain in an ELISA-format binding competition assay and full-length EPO-R in transfected BaF3 cells. Proliferative activity of the mutants was also determined in the BaF3-derived cell line and was correlated with the results from binding assays. Based on the results of these assays, we identified two distinct receptor binding sites on the EPO molecule. We propose that one site, containing residues Arg-150 and Lys-152, binds initially to EPO receptor on the cell surface. A second site, containing Arg-103 and Ser-104 (and possibly Arg-14), is involved in binding a second EPO-R at the cell surface, thus forming a homodimeric receptor complex. Furthermore, we demonstrate that one EPO mutant (R103A), which has previously been shown to lack proliferative function, is in fact an EPO antagonist. Taken together, these data support a sequential dimerization mechanism of EPO-R activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber D. L., DeMartino J. C., Showers M. O., D'Andrea A. D. A dominant negative erythropoietin (EPO) receptor inhibits EPO-dependent growth and blocks F-gp55-dependent transformation. Mol Cell Biol. 1994 Apr;14(4):2257–2265. doi: 10.1128/mcb.14.4.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Haemopoietic receptors and helical cytokines. Immunol Today. 1990 Oct;11(10):350–354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel J. P., Lee W. R., Presnell S. R., Cohen F. E., Bunn H. F. Erythropoietin structure-function relationships. Mutant proteins that test a model of tertiary structure. J Biol Chem. 1993 Jul 25;268(21):15983–15993. [PubMed] [Google Scholar]
- Cunningham B. C., Jhurani P., Ng P., Wells J. A. Receptor and antibody epitopes in human growth hormone identified by homolog-scanning mutagenesis. Science. 1989 Mar 10;243(4896):1330–1336. doi: 10.1126/science.2466339. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Ultsch M., De Vos A. M., Mulkerrin M. G., Clauser K. R., Wells J. A. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991 Nov 8;254(5033):821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. Comparison of a structural and a functional epitope. J Mol Biol. 1993 Dec 5;234(3):554–563. doi: 10.1006/jmbi.1993.1611. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Wlodawer A. Cytokines and their receptor complexes. FASEB J. 1995 Jan;9(1):50–56. [PubMed] [Google Scholar]
- Grasberger B., Minton A. P., DeLisi C., Metzger H. Interaction between proteins localized in membranes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6258–6262. doi: 10.1073/pnas.83.17.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodberg J., Davis K. L., Sykowski A. J. Alanine scanning mutagenesis of human erythropoietin identifies four amino acids which are critical for biological activity. Eur J Biochem. 1993 Dec 1;218(2):597–601. doi: 10.1111/j.1432-1033.1993.tb18413.x. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995 Jan 27;80(2):213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Ilondo M. M., Damholt A. B., Cunningham B. A., Wells J. A., De Meyts P., Shymko R. M. Receptor dimerization determines the effects of growth hormone in primary rat adipocytes and cultured human IM-9 lymphocytes. Endocrinology. 1994 Jun;134(6):2397–2403. doi: 10.1210/endo.134.6.8194466. [DOI] [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Jin L., Fendly B. M., Wells J. A. High resolution functional analysis of antibody-antigen interactions. J Mol Biol. 1992 Aug 5;226(3):851–865. doi: 10.1016/0022-2836(92)90636-x. [DOI] [PubMed] [Google Scholar]
- Johnsson B., Löfås S., Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991 Nov 1;198(2):268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. The molecular mechanism of erythropoietin action. Eur J Biochem. 1992 Dec 15;210(3):649–663. doi: 10.1111/j.1432-1033.1992.tb17466.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lin F. K., Suggs S., Lin C. H., Browne J. K., Smalling R., Egrie J. C., Chen K. K., Fox G. M., Martin F., Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo J. S., Aoki K. H., Arakawa T., Narhi L. O., Wen J. Dimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: one high-affinity and one low-affinity interaction. Biochemistry. 1996 Feb 6;35(5):1681–1691. doi: 10.1021/bi9524272. [DOI] [PubMed] [Google Scholar]
- Quelle D. E., Lynch K. J., Burkert-Smith R. E., Weiss S., Whitford W., Wojchowski D. M. Phosphorylatable and epitope-tagged human erythropoietins: utility and purification of native baculovirus-derived forms. Protein Expr Purif. 1992 Dec;3(6):461–469. doi: 10.1016/1046-5928(92)90063-3. [DOI] [PubMed] [Google Scholar]
- Watowich S. S., Hilton D. J., Lodish H. F. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994 Jun;14(6):3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich S. S., Yoshimura A., Longmore G. D., Hilton D. J., Yoshimura Y., Lodish H. F. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A. Structural and functional basis for hormone binding and receptor oligomerization. Curr Opin Cell Biol. 1994 Apr;6(2):163–173. doi: 10.1016/0955-0674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Wen D., Boissel J. P., Showers M., Ruch B. C., Bunn H. F. Erythropoietin structure-function relationships. Identification of functionally important domains. J Biol Chem. 1994 Sep 9;269(36):22839–22846. [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- Youssoufian H., Longmore G., Neumann D., Yoshimura A., Lodish H. F. Structure, function, and activation of the erythropoietin receptor. Blood. 1993 May 1;81(9):2223–2236. [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]



