Significance
We have shown that plectin, which we had previously identified as a biomarker for pancreatic ductal adenocarcinoma, has important function for tumor pathogenesis. We confirmed cell surface localization and identified the mechanism, exosomes, through which this occurs. Importantly, plectin is necessary for exosome formation and produces exosomes that are capable of promoting pancreatic tumor growth and aggression. Our results highlight the gain of function for plectin attributable to upregulation and exosomal localization in pancreatic cancer.
Keywords: migration, invasion, protein trafficking
Abstract
We recently demonstrated that plectin is a robust biomarker for pancreatic ductal adenocarcinoma (PDAC), one of the most aggressive malignancies. In normal physiology, plectin is an intracellular scaffolding protein, but we have demonstrated localization on the extracellular surface of PDAC cells. In this study, we confirmed cell surface localization. Interestingly, we found that plectin cell surface localization was attributable to its presence in exosomes secreted from PDAC cells, which is dependent on the expression of integrin β4, a protein known to interact with cytosolic plectin. Moreover, plectin expression was necessary for efficient exosome production and was required to sustain enhanced tumor growth in immunodeficient and in immunocompetent mice. It is now clear that this PDAC biomarker plays a role in PDAC, and further understanding of plectin’s contribution to PDAC could enable improved therapies.
In order for proteins to exert their regulated functions, they require appropriate spatiotemporal localization within a cell. Aberrant localization of proteins has been associated with the pathogenesis of many human diseases (1) resulting from deleterious gain-of-function or dominant-negative effects (2–4). For example, mislocalization of microtubule-associated protein tau to dendritic spines has recently been reported to mediate a synaptic dysfunction at the preclinical disease stages that immediately precede neurodegeneration (4). Additionally, deregulation of the spatiotemporal dynamics of signaling proteins can also promote tumorigenesis and metastasis (5, 6). In one of many examples, cytoplasmic mislocalization of the powerful tumor suppressor protein, p53 (6), interferes with its ability to regulate the cell cycle.
Recently, we used phage display and functional proteomics methods to identify biomarkers of pancreatic ductal adenocarcinoma (PDAC) (7). We were able to demonstrate overexpression and detect plectin on the surface of PDAC cells; however, in normal physiology, plectin is solely cytoplasmic (8). Given its aberrant localization in PDAC and expression in other cancers, the role of mislocalized plectin could have direct functional roles in the transition from preinvasive to invasive disease and/or be of importance for the established primary and metastatic tumors.
Plectin is a large (≥500-kDa) protein that is normally expressed in various tissues including skin, muscle, and brain (9). In normal cells, it plays a crucial role in cytoskeleton network organization (9, 10). In keratinocytes, plectin is concentrated at the basal surface (11), where it links intermediate filaments to the cytoplasmic domain of transmembrane glycoproteins such as integrin β4 (12). Although we have a good understanding of the role of plectin in normal cells through genetic studies (13, 14), the role of plectin in PDAC, the mechanisms behind its mislocalization and the importance of this mislocalization have not been studied.
In this study, we show that aberrant expression and mislocalization of plectin drives proliferation, migration, and invasion in PDAC cells. We also show that the mislocalization of plectin to the surface of PDAC cells arises from trafficking through exosomes. In addition, we demonstrate that plectin is necessary for efficient exosome production, on par with that of other proteins, such as Rab27a and -b, that are known to have prominent roles in exosome trafficking. Finally, in PDAC orthotopic mouse models, absence of plectin caused significant reduction of tumor volume and metastases. Our findings reveal a role for plectin expression and mislocalization in PDAC progression.
Results
Plectin Is Localized on the Cell Surface and Released Through Exosomes in PDAC.
Previous data indicated that plectin is a robust biomarker for pancreatic cancer and that it is overexpressed in PDAC specimens (7, 15), with a hint of cell surface localization. However, in normal physiology, plectin is exclusively cytoplasmic (9). Therefore, we sought to conclusively demonstrate cell surface localization and identify the path by which this protein localizes to the surface in PDAC. To confirm the cell surface localization of plectin, we performed a series of experiments including flow cytometry, immunogold TEM, and a plectin-binding assay that uses fluorescent-coupled plectin-targeting peptide (PTP) (sequence: KTLLPTP) (7, 15). Flow cytometric analysis using both plectin antibodies and PTP demonstrated specific cell surface binding to plectin in unfixed PDAC cells (Fig. 1A and Fig. S1A). Knockdown of plectin via shRNA diminished cell surface binding to levels equivalent to those observed with human pancreatic ductal epithelium (HPDE) cells, a normal control pancreas cell line.
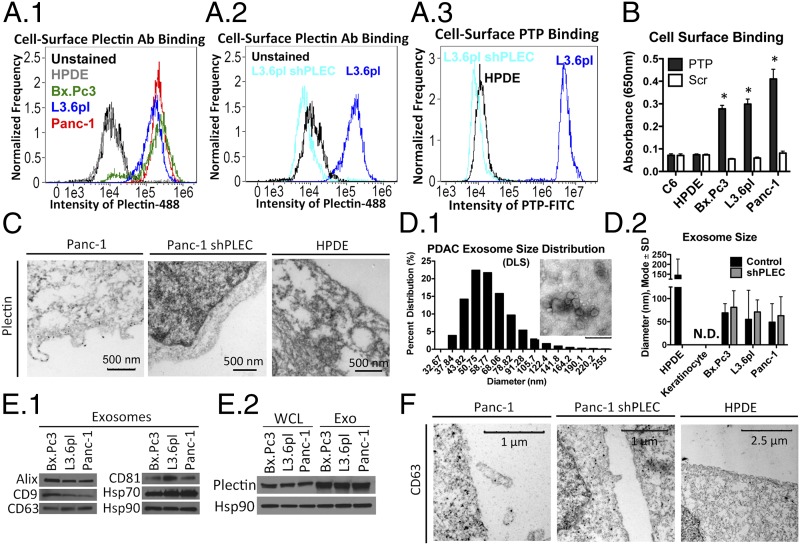
Fig. 1.
Plectin is localized on the cell surface and released through exosomes in PDAC. (A) Flow cytometry of unfixed HPDE and PDAC cells (A1), L3.6pl and L3.6pl shPLEC cells using plectin antibody (A2), and L3.6pl and L3.6pl shPLEC cells using PTP (10 µM) (A3). (B) PTP (1 µM) was used to determine cell surface plectin expression. *Significant to HPDE (P < 0.0001). (C) TEM of plectin immunogold staining. (D1) DLS analysis and TEM negative staining for visualization (Inset). (Inset scale bar: 200 nm.) (D2) NanoSight analysis of purified PDAC exosomes for their size in diameter (mode ± SD). (E) Immunoblot analysis showing the presence of various exosome markers in purified PDAC exosomes (E1) and plectin in both whole cell lysates and exosomes of PDAC cell lines (E2). (F) TEM of CD63 immunogold staining.
We also used a PTP-based binding assay, which was used instead of subcellular fractionation because it enables quantification of protein levels on the outer leaflet of the plasma membrane, whereas subcellular fractionation is indiscriminate between proteins that are extracellular or intracellularly bound to transmembrane proteins. Scrambled peptide (Scr) was used as a control for the binding assay. Consistent with our previous findings (7), we demonstrated strongly increased binding of PTP to the surface of PDAC cell lines, compared with binding to C6 glioma and HPDE controls (Fig. 1B). Immunogold analysis revealed the presence of plectin on PDAC cells, whereas plectin-knockdown cells and HPDE cells showed no plectin cell surface localization (Fig. 1C and Fig. S1B). Intriguingly, in the PDAC cell images, there were plectin-positive, nanometer-sized particles emanating from the plasma membrane.
Various types of cells are known to produce exosomes and these structures have been implicated in cellular movement, cell–cell signaling, tissue development, and cancer (16). To assess whether plectin-positive, nanometer-sized particles from PDAC cells were exosomes, we first purified the particles from conditioned medium of PDAC cells according to established protocols (17, 18) and subjected them to transmission electron microscopy (TEM), particle-size analysis using dynamic light scattering (DLS) (Fig. 1D1) and NanoSight (Fig. 1D2). DLS analysis revealed that the mean size of PDAC particles was 63.53 ± 4.46 nm in diameter and that the mode size was 50.75 nm. NanoSight analysis showed similar results (57.67 ± 20.00 nm). This size distribution was consistent with exosomes (19) and not microvesicles.
Purified particles were analyzed by Western blotting using well-characterized exosome markers (Alix, CD9, CD63, CD81, and Hsp70) (Fig. 1E1). In addition, immunoblot analysis revealed the presence of plectin in the exosomes (Fig. 1E2). Finally, immunogold staining showed that the plectin-positive particles initially observed by TEM were positive for the exosome marker CD63 (Fig. 1F). Together, these data identified the plectin-positive particles as exosomes. Intriguingly, plectin-rich exosomes could also be purified from the serum of mice with PDAC xenografts (Fig. S1C). Exosomes from the serum of healthy, nontumored mice were devoid of plectin. The presence of plectin in exosomes from the serum of animals with PDAC represents the potential for plectin as a serum marker, further indicating the importance of studying plectin in PDAC.
In assessing whether normal cells that produce abundant exosomes (JAWSII immature dendritic cells) (20) or cells that are rich in cytosolic plectin (keratinocyte, melanocyte, and C6 glioma) (21, 22) also have plectin on their cell surface (Fig. S1D), we found that, unlike PDAC cells, these types of cells did not exhibit PTP surface binding. This indicated that not all cell types rich in plectin or exosomes induce cell surface localization of plectin.
Plectin Silencing Reduces Exosome Secretion in PDAC.
Additional TEM analysis showed that L3.6pl cells exhibited changes to the membrane morphology upon plectin knockdown (Fig. S2A). Cells with plectin knockdown have membranes that, in appearance, resemble those of the more normal HPDE cells, than those of cancerous cells. Based on this observation, we investigated whether plectin affects exosome secretion from PDAC cells. Compared with HPDE, PDAC cells produced >10-fold higher amounts of exosomes as analyzed by NanoSight (Fig. 2A). Conditioned medium from keratinocytes did not yield enough exosomes to be detected via NanoSight. We also discovered that knockdown of plectin decreased exosome production by ∼fivefold in all three PDAC cell lines tested, indicating a possible role of plectin in efficient PDAC exosome production. We also analyzed exosomes from the indicated cell lines for protein content. The results were comparable to those obtained via NanoSight analysis (Fig. S2B). Interestingly, although HPDE cells produce less exosomes, the exosomes were larger in size than PDAC exosomes (Fig. 1D2).
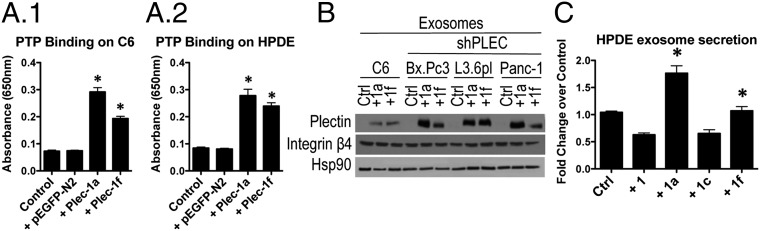
Fig. 2.
Plectin silencing reduces exosome production and plectin transfer via exosomes enhances tumor growth. (A) NanoSight analysis of secreted exosomes. *Significant to HPDE; **significant to controls. (B) Rab27a, -b, and plectin knockdown decreases exosome production. *Significant to control. (C) Rab27A knockdown decreases cell surface PTP binding. *Significant to control. (D) Cell surface PTP binding on NIH 3T3 cells with or without exosome treatment. *Significant to C6; **significant to C6, NIH 3T3, and NIH 3T3 plus PBS. (E) shRab27a decreases PDAC proliferation. (F) Plectin-rich exosomes enhanced the growth of L3.6pl shRab27a tumors, whereas plectin-negative exosomes did not have any effect on tumor growth (n = 10). For all significance, P < 0.0001.
Ostrowski et al. have shown that Rab27a and Rab27b are involved in the exosome secretory pathway (23). Therefore, we created Rab27a and Rab27b knockdown PDAC cell lines as controls to compare exosome formation and number of exosomes against plectin-knockdown cells. As expected, Rab27a- and Rab27b-knockdown PDAC cells both demonstrated a decrease in exosome production (Fig. 2B). Plectin knockdown resulted in a similar decrease in exosome production (Fig. 2A), again indicating that plectin plays a role in the formation and/or secretion of exosomes in PDAC.
Exosomal Plectin Results in Cell Surface Localization.
To investigate the potential of plectin cell surface localization through an exosome-mediated mechanism, we applied the PTP-binding assay to Rab27a knockdown cells. Rab27a knockdown resulted in significantly decreased exosome formation, as well as PTP binding (Fig. 2C). Indeed, the levels of PTP binding to Rab27a knockdown cells were similar to that of plectin-knockdown cells. However, knockdown of Rab27a and -b did not influence plectin expression (Fig. S2 C1 and C2). Similarly, plectin knockdown had no effect on Rab27a or -b expression (Fig. S2C3), indicating that lack of plectin cell surface localization attributable to Rab27a knockdown is not attributable to decreased plectin expression.
To further validate exosomes as a potential route for cell surface plectin localization, we sought to determine whether exosome treatment would transfer plectin to the surface of other cell types that are normally devoid of cell surface plectin. NIH 3T3 fibroblasts (Fig. 2D) and human umbilical vein endothelial cells (HUVECs) (Fig. S2D) were incubated with the same concentration of exosomes (10 µg/mL) from the various PDAC cell lines, their plectin-knockdown counterparts, or from C6 glioma cells, which do not have cell surface plectin or plectin in their exosomes (Fig. 1B and see Fig. 4B). Notably, treatment with PDAC exosomes resulted in a fourfold increase in PTP binding, suggesting that the surface of the fibroblasts and endothelial cells recruited the plectin from the exosomes to their cell surfaces. In contrast, there was no increase in PTP binding to cells treated with the same number of exosomes from C6 or from the plectin-knockdown PDAC cells. From these results, we conclude that the mechanism leading to plectin’s cell surface exposure involves exosomes.
Fig. 4.
Exogenous isoforms 1a and 1f colocalize with the plasma membrane and show trafficking through exosomes. (A) Exogenous plectin-1a and -1f increased PTP binding on the surface of C6 glioma (A1) and HPDE (A2) cells. *Significant to both control and control plus pEGFP-N2 (P < 0.0001). (B) Exosomes collected from C6 and plectin-knockdown PDAC cells transfected with plectin-1a or -1f contain plectin. (C) Exosome secretion from HPDE transfected with plectin-1, -1a, -1c, or -1f. *Significant to control (P < 0.0001).
Plectin Transfer via Exosomes Induces Enhanced Tumor Growth.
Our findings that plectin is an exosomal protein and that exosomes have the ability to transfer cell surface plectin led us to investigate whether plectin-rich exosome secretion had phenotypic consequences on PDAC cells. Interfering with exosome secretion through Rab27a knockdown resulted in a delay in PDAC cell growth in vitro (Fig. 2E for L3.6pl and Fig. S2F for Bx.Pc3 and Panc-1) as well as in vivo (Fig. 2F). As shown in Fig. 2, these cells are still plectin-positive, do not display cell surface plectin, and produce significantly lower amounts of exosomes. We injected plectin-rich exosomes purified from control L3.6pl cells into L3.6pl-derived tumors as described in Bobrie et al. (5 µg resuspended in 50 µL of PBS) (16). Remarkably, plectin-rich exosomes increased tumor growth of Rab27a-knockdown tumors past that of unadulterated L3.6 tumors. Growth of Rab27a knockdown tumors injected with plectin-negative exosomes or PBS was indistinguishable from untreated Rab27a knockdown tumors (Fig. 2F). Overall, these results indicate that plectin-rich exosomes are important factors in pancreatic cancer growth.
Plectin-Positive and -Negative Exosomes Derived from PDAC Have Differential and Biologically Active Molecules.
Our results showed that plectin-positive exosomes have a functional role in the growth of pancreatic tumor. It is possible that plectin, as a scaffolding protein, complexes with various molecules to promote pathogenesis. Therefore, we performed a quantitative mass spectrometric analysis to compare the differences in the proteome of plectin-positive and -negative PDAC exosomes. In a comparison of exosomes from L3.6pl with those from L3.6pl transduced with short hairpin RNA against plectin (L3.6pl shPLEC), 345 nonredundant proteins were identified (ProteinProphet score ≥0.85; false-discovery rate, 1.5%). Among them, 52 proteins were differentially expressed in L3.6pl exosomes with twofold or greater changes compared with L3.6pl shPLEC exosomes (n = 34 overexpressed and n = 18 under-expressed). Plectin was significantly depleted in L3.6pl shPLEC exosomes with a eightfold decrease in abundance compared L3.6pl, which serves as an important internal control (Table S1). We have confirmed the differential expression of three proteins via Western blot (Fig. S3). Our data indicate that plectin is not only necessary for exosome formation it has important functions for protein content of the exosomes.
Plectin Deregulation Increases Proliferation, Migration, and Invasion of PDAC Cells.
The plectin locus has a complex organization, with 11 alternative first exons, 8 of which are coding, giving rise to at least eight different plectin isoforms (24). Based on the distinct roles played by different plectin isoforms in normal cells, the cytoplasmic expression of plectin in normal keratinocytes (21, 24, 25), and our previous findings of plectin overexpression and cell surface localization in PDAC (7), we sought to investigate the profile of plectin isoforms in PDAC cell lines. We found that HPDE cells and PDAC cell lines express comparable levels of total plectin (Fig. 3A1), but they exhibit distinct expression patterns of the individual plectin isoforms (Fig. 3A2 and Fig. S4A for C6, see Table S2 for primer sequences). Notably, plectin isoforms 1a and 1f isoforms were undetectable in HPDE cells but prominently expressed in the PDAC cell lines tested, whereas isoform 1 was restricted to HPDE cells. Human specimens of normal pancreas expressed significantly less plectin whereas PDAC had high levels of 1a and 1f, with specimen 2 having high levels of 1d, as well (Fig. 3A2). In our previous work (15), we were unable to detect protein expression in human specimens using immunohistochemistry. Thus, PDAC exhibits an increase in plectin and expresses different plectin isoforms.
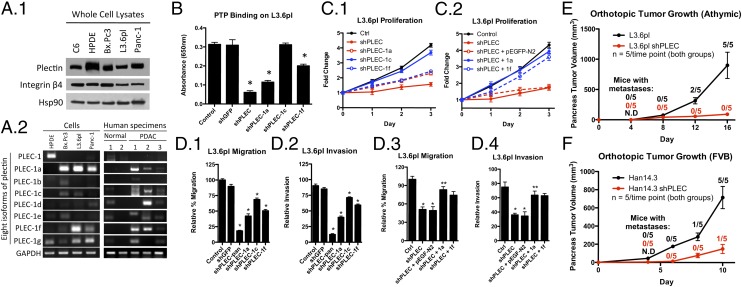
Fig. 3.
Plectin is deregulated and is associated with increased proliferation, migration, and invasion in PDAC. (A1) Immunoblot analysis showing total plectin expression in C6, HPDE, and PDAC cell lines. (A2) PCR analysis showing mRNAs of specific plectin isoforms. (B) shRNA knockdown of pan-plectin and plectin isoforms 1a and 1f resulted in a significant decrease in cell surface PTP binding. *Significant to both control and shGFP (P < 0.0001). (C1) Cell-viability assays showing increased ATP in control PDAC cells. (C2) Proliferation of plectin-knockdown cells overexpressing plectin-1a and -1f. (D) Isoform-specific knockdown of plectin-1a and -1f resulted in significant reduction in migration (D1) and invasion (D2) of PDAC cell lines. *Significant to control (P < 0.0001). Overexpression of plectin-1a and -1f increased migration (D3) and invasion (D4) in L3.6pl plectin-knockdown cells. *Significant to control (P < 0.0001); **significant to both shPLEC and shPLEC plus pEGFP-N2 (P < 0.0001). (E) Orthotopic growth of plectin-positive and plectin-negative L3.6pl tumors in athymic nude mice. N.D., not detectable/measurable via caliper; #/5 indicates the number of mice with metastases. (F) Orthotopic growth of plectin-positive and plectin-negative Han14.3 tumors in syngeneic FVB mice; #/5 indicates the number of mice with metastases.
We sought to determine whether the altered plectin expression generated a measurable impact on PDAC cells. Using the PTP-binding assay, we found that knockdown of pan-plectin, plectin-1a, and -1f significantly decreased cell surface plectin (Fig. 3B for L3.6pl and Fig. S4C for Bx.Pc3 and Panc-1), suggesting that plectin-1a and -1f are important isoforms in PDAC. Knockdown of plectin 1c, which is the major isoform found in C6 cells, did not alter PTP binding, indicating that plectin-1c may not be on the surface of PDAC cells. Although C6 cells express plectin and produce exosomes, the presence of 1c and the exclusion of 1a and 1f isoforms may indicate that 1a and 1f expression is necessary for incorporation into exosomes.
To study the impact of plectin expression on PDAC cell proliferation, we used cell-viability (Fig. 3C) and APO-BrdU TUNEL (Fig. S5) assays. We found that knockdown of pan-plectin expression all but eliminated proliferation of the rapidly growing PDAC cells (Fig. 3C for L3.6pl, and Fig. S4 D and E for Bx.Pc3 and Panc-1). For lentiviral experiments, pLKO.1 (backbone vector) containing shGFP was used as a control. The effect on proliferation appears to be attributable to reduction in plectin-1a and -1f because selective knockdown of either isoform reduced proliferation (Fig. 3C1 for L3.6pl and Fig. S4D for Bx.Pc3 and Panc-1) and selective expression rescued proliferation in the pan–plectin-knockdown cells (Fig. 3C2 for L3.6pl and Fig. S4E for Bx.Pc3 and Panc-1). The decrease in proliferation was not the result of modulation of apoptosis because the apoptotic rates were identical between plectin-positive and plectin-knockdown cells (Fig. S5). Interestingly, plectin 1c reduction did not result in a significant decrease in proliferation. Therefore, the expression in PDAC cells of plectin isoforms 1a and 1f, which are not present in nontransformed pancreatic cells, enhances the proliferation of PDAC cells. Likewise, in transwell-migration assays, we found that pan-plectin knockdown resulted in significant decreases in migration and invasion in three different PDAC cell lines (Fig. 3 D1 and D2 for L3.6pl, Fig. S4F for Bx.Pc3 and Panc-1, and Fig. S6A). Again, the reduction was partially rescued by selective expression of plectin-1a or -1f (Fig. 3 D3 and D4 for L3.6pl, Fig. S4G for Bx.Pc3 and Panc-1, and Fig. S6B). These results suggest that the plectin-1a and -1f isoforms have important and overlapping roles in the malignant growth of PDAC cells. Unlike proliferation, plectin-1c knockdown resulted in a significant decrease in migration and invasion (21%) but to a lesser extent than the plectin-1a (47.6%) and plectin-1f (39.1%) knockdowns.
We also evaluated the role of plectin on the growth and metastases of orthotopically implanted PDAC in immunocompromised (Fig. 3E) and immunocompetent animals (Fig. 4F). For both animal models, pancreata were injected with either plectin-positive or -knockdown cells. Animals at each time point were euthanized, tumor volumes were determined, and the presence of metastases were noted (fraction in graph represents the number of animals from the time point that had metastases). For the immunocompromised animals, the volume for plectin-positive and -negative tumors began to diverge at day 8 postinjection. By day 16, the plectin positive tumor-bearing animals had to be euthanized because of moribund conditions. At day 16, five of five animals had metastatic deposits. In contrast, plectin-knockdown, tumor-bearing animals had minimal tumor burden with no detectable metastases. Furthermore, the immune system has been shown to play a key role in tumor development and progression. Therefore, we assessed the role of plectin in PDAC in an immunocompetent, syngeneic mouse model using Han14.3 cells, which were derived from spontaneous PDAC arising in Pft1-Cre; LSL-K-RasG12D; p53+/− mice in the background of an inbred Friend Virus B NIH Jackson (FVB/NJ) strain (15). As in the immunocompromised animals, the growth of plectin-positive tumors was faster than that of plectin-knockdown tumors in immunocompetent animals. By day 10, Han14.3 tumor volume was fivefold larger compared with those of Han14.3 shPLEC tumors. Because of the aggressive nature of this tumor and according to the rules of the University of Virginia Animal Care and Use Committee, we were required to euthanize FVB mice bearing Han14.3 and Han14.3 shPLEC 10 d after tumor injection. All five Han14.3 tumors resulted in metastases, whereas only one mouse bearing Han14.3 shPLEC tumor had metastasis. This further strengthens our hypothesis that plectin is important in PDAC growth.
Exogenous Plectin Isoforms 1a and 1f Are Localized to the Plasma Membrane of the Cells Lacking Cell Surface Plectin and Show Trafficking Through Exosomes.
Results shown in Fig. 2D demonstrate that cells lacking surface plectin expression can be induced to localize plectin on the cell surface by treatment with plectin-containing exosomes. In light of these results and those from Figs. 3B, we determined whether exogenous expression of plectin-1a or -1f in C6 and HPDE cells, which both lack cell surface plectin, could induce cell surface localization of plectin. For this, the cells were transfected with pGR244 (EGFP-tagged mouse plectin-1a), pGR258 (EGFP-tagged mouse plectin-1f), or pEGFP-N2 (backbone vector), and then subjected to the PTP-binding assay to determine whether the plasma membrane-localized plectin was on the cell surface. The cells expressing plectin-1a and -1f showed increased binding of PTP on the cell surface (Fig. 4A) compared with the cells transfected with the pEGFP-N2 controls. Thus, the expressed plectin was localized to the cell surface.
These results led us to investigate whether plectin can traffic through exosomes in cells lacking plectin-1a or -1f, isoforms important in PDAC and shown to bind to integrin β4 (25). For this, we transfected C6 cells (expressing isoform 1c but not 1a or 1f) and plectin-knockdown PDAC cell lines with pGR244 and pGR258, purified exosomes from these transfected cells, and performed immunoblot analysis to evaluate plectin expression in the exosomes (Fig. 4B). In support of our hypothesis that plectin is trafficking via exosomes, expression of plectin-1a and -1f both resulted in accumulation of the protein in the C6 exosomes. Such accumulation of exogenously expressed plectin was also observed in PDAC exosomes in which endogenous expression was silenced. Therefore, plectin-1a and -1f show trafficking through exosomes leading to cell surface localization of plectin, as assessed through PTP binding.
Finally, we tested whether plectin isoforms affected exosome secretion in HPDE cells (Fig. 4C), which produce significantly less exosomes compared with PDAC cells. We found that although plectin-1a overexpression led to a 1.6-fold increase in exosome production, it was not to the level of PDAC cells, which were >10-fold higher than HPDE (Fig. 2A). Similarly, plectin-1f overexpression resulted in only a 1.2-fold increase. Our results indicate that plectin-1a overexpression can induce HPDE cells to produce more exosomes, but plectin-1a alone is not sufficient to produce exosomes to the level of cancer cells, because no exosomes were detectable in the case of keratinocytes that express high levels of plectin-1a (Fig. 2A) (21).
Integrin β4 Is Required for Plectin Mislocalization in PDAC, and Plectin–Integrin Interaction Have an Important Role in PDAC Growth and Migration.
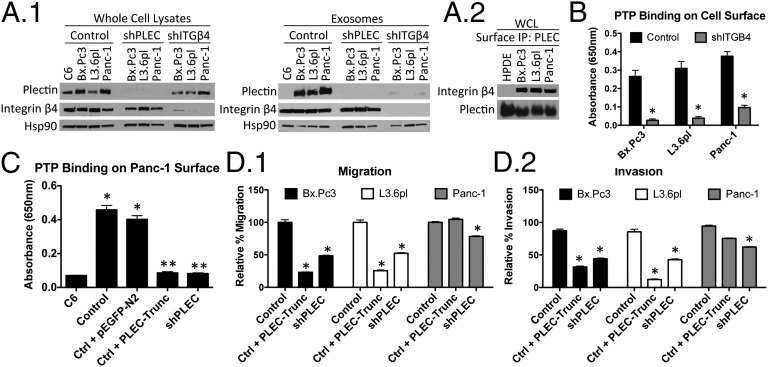
To further understand the mechanism of how plectin is trafficking in exosomes, we focused on the role of integrin β4, a transmembrane protein that is a direct binding partner of plectin in keratinocytes (12, 26) and pancreatic cancer cells (27), and a known constituent of exosomes (28). Western blot analysis of PDAC cell lysates and purified exosomes revealed the presence of plectin as well as integrin β4 in exosome samples from all PDAC cell lines (Fig. 5A1). Hsp90, a known component of secreted PDAC exosomes (17), was used as a loading control in these studies. Notably, shRNA-mediated knockdown of integrin β4 resulted in an almost complete loss of plectin in PDAC-secreted exosomes, whereas plectin expression was still detectable in whole-cell lysates, indicating that the presence of plectin in exosomes requires integrin β4 anchorage. We also confirmed via immunoprecipitation that plectin and integrin β4 directly interact with each other (Fig. 5A2). Additional analysis showed that there was a 5- to 10-fold decrease (depending on the cell line) in PTP binding of the integrin β4-knockdown cells compared with controls (Fig. 5B), equivalent to background levels, illustrating the importance of the β4–plectin interaction for plectin cell surface localization.
Fig. 5.
Interaction of plectin with integrin β4. (A1) Plectin is absent in exosomes produced from plectin- and integrin β4-knockdown cells. (A2) Coimmunoprecipitation of integrin and plectin. (B) PTP binding was assessed on Integrin β4-knockdown. *Significant to control (P < 0.0001 for Bx.Pc3 and Panc-1; P = 0.0003 for L3.6pl). (C) Transfection with PLEC-Trunc led to reduced plectin expression on the cell surface. C6 and shPLEC-transfected L3.6pl served as negative controls. *Significant to C6 (P < 0.0001); **significant to both control and control plus pEGFP-N2 (P < 0.0001). (D) PLEC-Trunc transfection decreased migration (D1), as well as invasion (D2), of PDAC cells. *Significant to control (P < 0.0001).
To investigate the contribution of plectin-integrin β4 interactions to the exosomal and cell surface localization of plectin, we generated a truncation mutant of plectin, PLEC-Trunc covering plectin exons 1a through 9, with the anticipation that its overexpression would interfere with endogenous plectin binding to integrin β4. Previous studies have shown that it is the complete N-terminal end of plectin (including first exon 1a and the ensuing exons 2-to-8-encoded sequences) that is mainly responsible for integrin β4 interaction. Indeed, PDAC cell lines transfected with PLEC-Trunc showed a pronounced decrease in PTP binding, indicating reduced plectin on the cell surface (Fig. 5C for L3.6pl and Fig. S7 for Bx.Pc3 and Panc-1). Our results show that the truncated form of plectin, having a high affinity to integrin β4, interfered with the localization of endogenous plectin on the PDAC cell surface by hindering plectin–integrin β4 interaction.
To further investigate the role of plectin–integrin β4 interaction, we transfected PLEC-Trunc into PDAC cells and evaluated their migration and invasion. PLEC-Trunc–transfected cells demonstrated strong reductions in migration and invasion, comparable to those seen upon plectin knockdown (Fig. 5D and Fig. S6C). The effects of PLEC-Trunc on migration and invasion indicate that the interaction of plectin with integrin and/or mislocalization of plectin play an important role in the mobility of pancreatic cancer cells.
Discussion
Previously, we determined plectin to be a highly specific biomarker for the transition of noninvasive to invasive pancreatic cancer (7). We demonstrated the feasibility of targeting plectin with imaging agents as a method for the early detection that is critically needed for this terminal disease (15). Here, we confirmed the cell surface localization of plectin and illuminated a mechanism for plectin deregulation in cancer, involving isoform-specific up-regulation, mislocalization, and extracellular trafficking via integrin β4-binding and exosome secretion. Various types of normal and cancerous cells are known to produce exosomes. We demonstrate that plectin-rich exosomes can be isolated from tissue culture medium and, remarkably, from serum of tumor-bearing mice. Additionally, incubation of cells with plectin-rich exosomes could confer cell surface plectin on cells that were previously devoid of such expression. We also showed that integrin β4, a known binding partner of plectin, is also found in the PDAC exosomes and necessary for plectin inclusion in the exosomes. Furthermore, transient expression of plectin-1a and -1f in C6 and HPDE cells reveals that translocation of these isoforms to the cell surface, when abnormally up-regulated, is not unique to PDAC. However, keratinocytes, which express plectin-1a and -1f, as well as integrin β4, do not produce detectable amounts of exosomes, suggesting that the expression of these proteins is not sufficient to induce exosome secretion and plectin surface localization.
Plectin up-regulation coupled with mislocalization has profound effects on PDAC. When plectin expression is silenced in PDAC cells, proliferation, invasion, and migration are negatively impacted. Furthermore, orthotopic tumors generated with plectin-knockdown cells were 5- to 10-fold smaller compared with control tumors. Importantly, the mice bearing plectin-knockdown tumors showed almost no metastases whereas 100% of the control tumors generated metastases. However, because plectin knockdown inhibits exosome production, it is difficult to determine whether the effect is from intracellular or exosomal plectin. Therefore, we examined whether isolated, PDAC-derived exosomes from either plectin positive or plectin-knockdown cells had physiological effects on the tumor cells. Similar to the reports of Peinado et al. (29), inhibition of exosome formation by Rab27a knockdown also decreased tumor growth. However, although cytoplasmic levels of plectin remained unchanged in the Rab27a knockdown tumors, intratumoral injection of plectin-rich exosomes, but not exosomes from plectin-knockdown cells, overcame the growth inhibition attributable to Rab27a knockdown. Plectin-rich exosomes not only rescued tumor growth but also promoted a growth rate that was faster than that of control tumors. In addition, incubation of plectin-rich exosomes with surface plectin-negative cell lines, NIH 3T3 and C6, increased invasion and migration of these cell lines (Fig. S2E).
As a scaffolding protein, plectin likely carries a complex of signaling molecules in the exosomes. Knockdown of intracellular plectin may influence protein levels in the cell, resulting in altered proteome in the exosomes. Indeed, proteomic analysis of plectin-positive and -negative exosomes indicate that plectin modulates exosome content. The largest change in plectin-negative exosomes was a 20-fold loss of SACM1L, a phosphatidylinositide phosphatase, a protein important in vesicle trafficking and membrane dynamics. The second most decreased protein is a 60S ribosomal protein important to protein biosynthesis. This change may be attributable to reduction in plectin’s ability to scaffold proteins into the exosomes, as well as loss of signaling and cytoskeletal stabilization attributable to reduced intracellular plectin levels. Further studies should be conducted to investigate whether hindering plectin localization on the cell surface by inhibition of exosome formation in early stage pancreatic intraepithelial neoplasia would halt progression into PDAC.
Materials and Methods
Exosome characterization was performed with NS300 (NanoSight). The samples were injected in the sample chamber with sterile syringes (BD) until the liquid reached the tip of the nozzle. The software used for capturing and analyzing the data was Nanoparticle Tracking Analysis (NTA) Version 2.3. The samples were measured for 90 s. Three measurements of the same sample were performed for all exosome samples. Further details regarding materials and methods are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Kevin Janes for helpful discussion concerning various aspects of this work, Dr. Nabeel Bardeesy for the Han14.3 cells, and Marc Seaman for performing orthotopic surgeries. This work was supported by National Institutes of Health Grant R01 CA137071, National Science Foundation Graduate Research Fellowship Grant DGE-0809128 (to S.J.S.), and Austrian Science Research Fund Grant P23729-B11.
Footnotes
Conflict of interest statement: K.A.K. is affiliated with iTi Health, where the plectin-targeting peptide imaging agent is licensed.
This article is a PNAS Direct Submission. D.A.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309720110/-/DCSupplemental.
References
- 1.Hung MC, Link W. Protein localization in disease and therapy. J Cell Sci. 2011;124(Pt 20):3381–3392. doi: 10.1242/jcs.089110. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka AR, et al. Effects of mutations of ABCA1 in the first extracellular domain on subcellular trafficking and ATP binding/hydrolysis. J Biol Chem. 2003;278(10):8815–8819. doi: 10.1074/jbc.M206885200. [DOI] [PubMed] [Google Scholar]
- 3.Robben JH, Knoers NV, Deen PM. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2006;291(2):F257–F270. doi: 10.1152/ajprenal.00491.2005. [DOI] [PubMed] [Google Scholar]
- 4.Hoover BR, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu MC, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 6.Fabbro M, Henderson BR. Regulation of tumor suppressors by nuclear-cytoplasmic shuttling. Exp Cell Res. 2003;282(2):59–69. doi: 10.1016/s0014-4827(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 7.Kelly KA, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5(4):e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buijsrogge JJ, et al. Antiplectin autoantibodies in subepidermal blistering diseases. Br J Dermatol. 2009;161(4):762–771. doi: 10.1111/j.1365-2133.2009.09206.x. [DOI] [PubMed] [Google Scholar]
- 9.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111(Pt 17):2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- 10.Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313(10):2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Wiche G, Krepler R, Artlieb U, Pytela R, Aberer W. Identification of plectin in different human cell types and immunolocalization at epithelial basal cell surface membranes. Exp Cell Res. 1984;155(1):43–49. doi: 10.1016/0014-4827(84)90766-3. [DOI] [PubMed] [Google Scholar]
- 12.Rezniczek GA, de Pereda JM, Reipert S, Wiche G. Linking integrin alpha6beta4-based cell adhesion to the intermediate filament cytoskeleton: Direct interaction between the beta4 subunit and plectin at multiple molecular sites. J Cell Biol. 1998;141(1):209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrä K, et al. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev. 1997;11(23):3143–3156. doi: 10.1101/gad.11.23.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezniczek GA, Walko G, Wiche G. Plectin gene defects lead to various forms of epidermolysis bullosa simplex. Dermatol Clin. 2010;28(1):33–41. doi: 10.1016/j.det.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Bausch D, et al. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res. 2011;17(2):302–309. doi: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobrie A, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 17.Ristorcelli E, et al. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008;22(9):3358–3369. doi: 10.1096/fj.07-102855. [DOI] [PubMed] [Google Scholar]
- 18.Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 19.Zomer A, et al. Exosomes: Fit to deliver small RNA. Commun Integr Biol. 2010;3(5):447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin W, Ouyang S, Li Y, Xiao B, Yang H. Immature dendritic cell-derived exosomes: A promise subcellular vaccine for autoimmunity. Inflammation. 2013;36(1):232–240. doi: 10.1007/s10753-012-9539-1. [DOI] [PubMed] [Google Scholar]
- 21.Andrä K, et al. Plectin-isoform-specific rescue of hemidesmosomal defects in plectin (-/-) keratinocytes. J Invest Dermatol. 2003;120(2):189–197. doi: 10.1046/j.1523-1747.2003.12027.x. [DOI] [PubMed] [Google Scholar]
- 22.Foisner R, Bohn W, Mannweiler K, Wiche G. Distribution and ultrastructure of plectin arrays in subclones of rat glioma C6 cells differing in intermediate filament protein (vimentin) expression. J Struct Biol. 1995;115(3):304–317. doi: 10.1006/jsbi.1995.1055. [DOI] [PubMed] [Google Scholar]
- 23.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30, 1–13. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs P, et al. Unusual 5′ transcript complexity of plectin isoforms: Novel tissue-specific exons modulate actin binding activity. Hum Mol Genet. 1999;8(13):2461–2472. doi: 10.1093/hmg/8.13.2461. [DOI] [PubMed] [Google Scholar]
- 25.Kostan J, Gregor M, Walko G, Wiche G. Plectin isoform-dependent regulation of keratin-integrin alpha6beta4 anchorage via Ca2+/calmodulin. J Biol Chem. 2009;284(27):18525–18536. doi: 10.1074/jbc.M109.008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geerts D, et al. Binding of integrin alpha6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J Cell Biol. 1999;147(2):417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu PT, et al. The RON-receptor regulates pancreatic cancer cell migration through phosphorylation-dependent breakdown of the hemidesmosome. Int J Cancer. 2012;131(8):1744–1754. doi: 10.1002/ijc.27447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.