Abstract
Background
Vitamin D deficiency and low bone mineral density (BMD) are complications of inflammatory bowel disease. Vitamin D deficiency is more prevalent among individuals of color compared to Caucasians. There is little data comparing differences in serum 25-hydroxyvitamin D (25OHD) concentrations and BMD between African American and Caucasian children with Crohn’s Disease (CD).
Methods
We compared serum 25OHD concentrations of African American children with CD (n=52) to Caucasian children with CD (n=64) and healthy African American controls (n=40). We also analyzed BMD using dual energy x-ray absorptiometry (DXA) results from our pediatric CD population.
Results
African American children with CD had lower serum 25OHD concentrations [16.1 (14.5-17.9, 95%CI) ng/mL] than Caucasians with CD [22.3 (20.2-24.6, 95%CI) ng/mL; p<0.001]. African Americans with CD and controls exhibited similar serum 25OHD concentration [16.1 (14.5-17.9, 95%CI) vs 16.3 (14.4-18.4, 95%CI) ng/mL; NS]. African Americans with CD exhibited no difference in serum 25OHD concentration when controlling for seasonality, disease severity and surgical history, though serum 25OHD concentration was significantly decreased in overweight children (BMI≥85%, p =0.003). Multiple regression analysis demonstrated that obese African American females with CD had the lowest serum 25OHD concentrations [9.6 (6.8-13.5, 95%CI) ng/mL]. BMD was comparable between African American and Caucasian children with CD (Z score −0.4 ± 0.9 vs −0.7 ± 1.2; NS).
Conclusions
African American children with CD are more likely to have vitamin D deficiency compared to Caucasian with CD, but have similar BMD. CD disease severity and history of surgery do not affect serum 25OHD concentrations among African American children with CD. African American children have low serum 25OHD concentrations, independent of CD, compared to Caucasian children. Future should research should focus on how race affects vitamin D status and BMD in children with CD.
Keywords: Vitamin D, Epidemiology, Crohn’s disease, IBD, children
Introduction
Vitamin D in humans is critical for calcium homeostasis and bone metabolism, but also may have important roles in other organ systems via local production of the hormonal form of vitamin D and subsequent binding to the nuclear vitamin D receptor1, 2. The potential role of vitamin D in the immune response has prompted an explosion of research in individuals with chronic disease, including inflammatory bowel disease (IBD).
Skin pigmentation is a major factor influencing vitamin D status in children 3. The most current National Health and Nutrition Examination Survey (NHANES III, 2001-2006) reports that up to 75% of all children have hypovitaminosis D with up to 95% of non-Hispanic black children having vitamin D insufficiency4. Despite the striking difference in vitamin D status between Caucasians and African Americans, the bone mineral density (BMD) of these two populations is not reflective of this difference with African Americans having higher BMD5-7. Although not fully understood, lower bone turnover, higher peak bone mass potential and longer periods of bone formation are proposed mechanisms to describe this paradox8. There is little evidence to suggest that this same paradox is true in IBD.
Crohn’s disease (CD) is a chronic inflammatory condition associated with both low serum 25OHD concentrations and decreased BMD9-11. The role of vitamin D in CD is not entirely understood, but CD patients with a more severe disease phenotype have lower serum 25OHD concentrations compared to those with milder phenotypes12. Although malnutrition and disease severity are associated with decreased BMD in CD13, 14, research in children with IBD shows that not only is vitamin D a poor predictor of low BMD15, 16, but that treatment with supplemental vitamin D did not accelerate accrual of BMD17. Important to consider is that the majority of the IBD population in these studies is Caucasian with minimal representation of other racial groups9, 11. Vitamin D status and bone health in African American children with CD is unknown.
The primary objective of this study was to compare vitamin D status between African American children with CD, Caucasian children with CD and a healthy African American control group. A secondary goal was to determine the effects of disease severity, history of surgery, body mass index (BMI) and seasonality on vitamin D status in African American children with CD. To evaluate differences in bone health between African American and Caucasian children with CD, we compared dual energy x-ray absorptiometry (DXA) of the pediatric CD population at our children’s hospital.
Methods
Study Population and Variables
In this cross-sectional analysis of vitamin D status, we evaluated clinical data and serum samples of African American and Caucasian children with CD already enrolled in a prospective cohort study at our institution. The Emory University Institutional Review Board approved the protocol for this trial, and all patients gave informed consent and assent, wherever appropriate. Any child with a diagnosis of CD receiving care of Emory Children’s Center/Children’s Healthcare of Atlanta was asked to participate in the study. Samples were obtained from spring 2009 to winter 2011. African American controls were recruited from healthy volunteers or patients with functional gastrointestinal disorders, gastroesophageal reflux or constipation from our general gastroenterology clinic.
Chart review was used to determine race, history of surgery and disease severity. Patients were characterized as having severe disease if they fulfilled Paris classification for stricturing disease (B2), penetrating disease (B3), growth delay (G1) or perianal disease (p modifier)18. Surgical interventions included ileocolectomy, colectomy, or small bowel resection. Underweight was defined as a BMI ≤ 5th percentile for age and overweight was a BMI ≥ 85th percentile for age based on the 2000 Center for Disease Control 2-20 year old growth curves19. Season was determined based on the timing of the blood draw and described as winter (December-February), spring (March-May), summer (June-August) or fall (September-November).
All DXA scans performed on children with CD at Children’s Healthcare of Atlanta from 2006-2011 were included in our analysis. If a patient had multiple scans, only the initial DXA scan was included in the analysis. BMI and history of surgery in this group was determined by electronic chart review. DXA scan results are reported as Z scores of the total body minus head and spine, adjusted for age and sex. Patients with a Z score of <-1 for lumbar spine and/or total body met the definition of decreased BMD.
Laboratory Analysis
Serum 25OHD concentration (ng/mL) was assayed using the Immunodiagnostic Systems iSYS (IDS-iSYS) automated analyser (Immunodiagnostic Systems Inc, Fountain Hills, AZ), which uses a competitive binding chemiluminescence assay technique and correlates well with the liquid chromatography-tandem mass spectrometry method 20. Serum samples from the study participants were stored at −80°C prior to analysis. All samples were performed at one batch to reduce inter-assay variability. In our lab, the inter-assay CV for this method is 10.1 to 13.0%, and the intra-assay CV is 1.8 to 4.0%. The reportable range of the assay is 6-126 ng/mL. We participated in the vitamin D external quality assessment scheme to ensure the accuracy of the 25OHD measurements (site 606). For this study, we considered serum 25OHD concentrations ≤15 ng/mL as representing vitamin D deficiency and <32 ng/mL as vitamin D insufficiency in keeping with data from the Institute of Medicine as well as previous studies and skeletal health guidelines for children with inflammatory bowel disease 3, 9, 11, 21.
Statistics
All statistical analyses were performed using SAS 9.2 (Cary, NC) and statistical significance was assessed at the 0.05 significance level unless otherwise noted. Prior to analysis, the normality of age, serum 25OHD concentrations, and DXA normalized scores were assessed using the Anderson-Darling test for normality and by visual inspection of the histograms. The primary outcome measure, serum 25OHD concentration was positively skewed; therefore, a log transformation was applied to obtain an approximate normal distribution. All analysis related to serum 25OHD concentrations were performed on the log-transformed data (i.e., log(25OHD)) and then back transformed to the original units for reporting of the results. The results of 25OHD are presented using the geometric mean and its corresponding 95% confidence intervals22. Additionally, age was not normally distributed; therefore, we present the mean, 25th and 75th percentiles for age (years).
Descriptive statistics were calculated for all variables of interest for each cohort of patients. Serum 25OHD concentration in African Americans with CD, Caucasians with CD and African American controls were compared using one-way analysis of variance (ANOVA) with the Dunnet’s multiple comparison procedure (MCP) or the Tukey-Kramer MCP. Additionally, the distribution of age within each group was compared using the Kruskal-Wallis one-way ANOVA procedure. Two-sample t-tests were used to compare whole body BMD and spine BMD Z scores between African American and Caucasians with CD, while Pearson chi-square was used to compare the proportion of patients with Z scores ≤-1 and ≤-2 between races. Additionally, two-sample t-tests were used to compare the log-transformed serum 25OHD concentration mean and BMD standardized values among various demographics groups. Chi-square tests were used to compare the distributions of categorical variables (e.g., race, BMI, CD severity, history of surgery, and seasonality) among the groups of patients. Spearman’s rank correlations were used to assess the association between age (in years) with 25OHD and BMD. Finally, multiple linear regression was utilized to identify significant predictors of serum 25OHD concentration. The regression model, which included gender, race, disease severity, surgical history, BMI and season of 25OHD measurement as variables, was constructed on the log-transformed data and the resulting coefficients and confidence intervals were back-transformed to the original scale. Significant interactions were retained if they were significant at the 0.1 level.
Results
Demographics and Clinical Characteristics of Participants
A total of 156 subjects (70 female) were included in the 25OHD analysis (Table 1). There were 52 African American children with CD (9-21 yrs), 64 Caucasian children with CD (8-21 yrs) and 40 African American controls (1-21 yrs). On average, African Americans with CD were older than African American controls (17.0 vs 11.0 yrs; p < 0.001). There were predominately more females in the African American control group while the CD groups had predominately more males (p = 0.038). The majority of African American children with CD had 25OHD concentrations drawn in winter and spring, while in controls it was summer and fall (p<0.001).
Table 1.
Demographic and clinical data of the 25OHD study population
| African Americans with CD (n=52) |
Caucasians with CD (n=64) |
African American Controls (n=40) |
p-value | |
|---|---|---|---|---|
| Age | ||||
|
| ||||
| Median (25%-75%) |
17.0 (15.0-18.5) | 15.5 (13.0-18.0) | 11.0 (5.0-15.0) | 0.0901 <0.001 |
|
| ||||
| 25OHD (ng/mL) | ||||
|
| ||||
| Mean (95% CI) |
16.1 (14.5-17.9) | 22.3 (20.2-24.6) | 16.3 (14.4-18.4) | <0.0012 0.990 |
|
| ||||
| Gender – N (%) | ||||
|
| ||||
| Male | 32 (61.5) | 39 (60.9) | 15 (37.5) | 0.038 |
| Female | 20 (38.5) | 25 (39.1) | 25 (62.6) | |
|
| ||||
| BMI – N (%) | ||||
|
| ||||
| Underweight | 12 (23.1) | 6 (9.4) | - | 0.118 |
| Normal | 32 (61.5) | 44 (68.8) | - | |
| Overweight | 5 (9.6) | 10 (15.6) | - | |
|
| ||||
| CD Severity – N (%) | ||||
|
| ||||
| Non-complicated | 27 (51.9) | 43 (67.2) | - | 0.109 |
| Complicated | 21 (40.4) | 17 (26.6) | - | |
|
| ||||
| History of Surgery – N (%) | ||||
|
| ||||
| Yes | 9 (17.3) | 14 (21.9) | - | 0.642 |
| No | 43 (82.7) | 50 (78.1) | - | |
|
| ||||
| Seasonality – N (%) | ||||
|
| ||||
| Winter | 19 (36.5) | 23 (35.9) | 3 (7.5) | <0.001 |
| Spring | 15 (28.8) | 22 (34.4) | 8 (20.0) | |
| Summer | 8 (15.4) | 18 (28.1) | 19 (47.5) | |
| Fall | 10 (19.2) | 1 (1.6) | 10 (25.0) | |
25OHD values were not normally distributed. A log-transformation was applied prior to data analysis. The resulting means and confidence limits were then back-transformed to the original units (ng/mL).
p-values were calculated using Dunnett’s MCP test on log-transformed Vitamin D to account for non-normality. The first p-value is Underweight vs Normal. The second p-value is Underweight vs Overweight. The third p-value is Normal vs Overweight.
Spearman’s rank correlation and associated 95% confidence interval.
Serum 25OHD Concentration
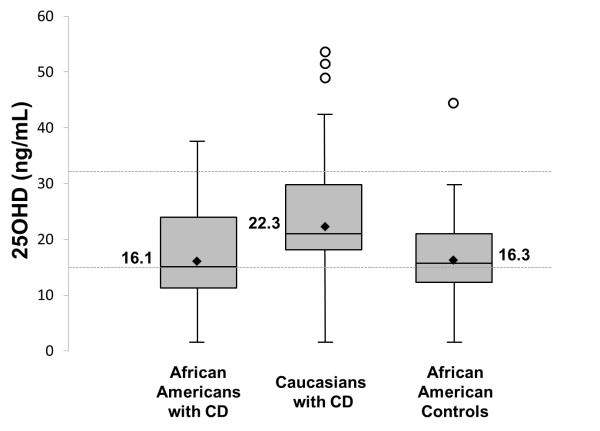
The mean (95% CI) serum 25OHD concentration of African American children with CD was 16.1 ng/mL (14.5-17.9) while in Caucasian children with CD, it was 22.3 (20.2-24.6; p<0.001). In the African American control group, the mean serum 25OHD concentration was 16.3 ng/mL (14.5-17.9; p =0.990; Figure 1). In our study, the prevalence of vitamin D deficiency and insufficiency in African American children with CD was 63% and 98%, compared to 32% and 83% of Caucasians with CD. The prevalence of vitamin D deficiency and insufficiency in our control population was 70% and 98%.
Figure 1.
Box plot graph of serum 25OHD concentrations (ng/mL) by race and disease. Boxes represent values within the 25th–75th percentiles, lines represent the range, open circles the outliers, diamonds the geographic mean and inner-box lines the median. Mean 25OHD in African Americans with CD was lower than Caucasians with CD (p<0.001) but similar in African American controls (p=0.990). Dashed line represents serum 25OHD concentration of 32 ng/mL (vitamin D insufficiency) and 15 ng/mL (vitamin D deficiency).
Table 3 presents the comparisons of serum 25OHD concentration among various demographic subgroups in African American patients with CD. Because the analysis was conducted on the log(25OHD) and then back-transformed to the original units, the results are presented using means and 95% confidence intervals. From these results, serum 25OHD concentration was found to be significantly different between BMI subgroups. Serum 25OHD concentrations in overweight African Americans with CD were significantly lower than patients with normal and underweight BMI values (9.9 vs. 16.8 ng/mL; p = 0.009 and 9.9 vs 17.8 ng/mL; p = 0.010). There was no difference in serum 25OHD concentrations among African American children with CD when stratified by disease severity (p=0.914) or history of surgery (p=0.372). In African American children with CD, those with non-complicated disease had a mean serum 25OHD concentration of 16.1 ng/mL (13.6-19.2) compared to 15.8 ng/mL(13.4-18.7) in those with complicated disease. African American children with CD and a history of surgery had a mean 25OHD concentration of 17.0 ng/mL (11.9-24.3) compared to 15.9 (14.2-17.9) in those with no surgical history.
Table 3.
25OHD comparisons among demographic and clinical variables in African American children with CD
| Characteristic | Level | Mean 25OHD (ng/mL) (95% CI)1 |
p-value |
|---|---|---|---|
|
| |||
| Gender | |||
|
| |||
| Male | 17.4 (15.2 – 20.0) | 0.065 | |
| Female | 14.2 (11.8 – 17.1) | ||
|
| |||
| CD Severity | |||
|
| |||
| Non-complicated | 16.1 (13.6 – 19.2) | 0.857 | |
| Complicated | 15.8 (13.4 – 18.7) | ||
|
| |||
| History of Surgery | |||
|
| |||
| No | 15.9 (14.2 – 17.9) | 0.659 | |
| Yes | 17.0 (11.9 – 24.3) | ||
|
| |||
| BMI | |||
| Underweight | 17.8 (14.3 – 22.1) | 0.8732 | |
| Normal | 16.8 (14.7 – 19.2) | 0.009 | |
| Overweight | 9.9 (7.1 – 13.8) | 0.010 | |
|
| |||
| Season | |||
|
| |||
| Winter | 14.8 (12.4 – 17.7) | 0.321 | |
| Spring | 16.9 (13.8 – 20.7) | ||
| Summer | 14.5 (11.0 – 19.1) | ||
| Fall | 19.1 (14.9 – 24.5) | ||
|
| |||
| Age (rs - 95% CI)3 | −0.04 (−0.31 – 0.24) | 0.783 | |
25OHD values were not normally distributed. A log-transformation was applied prior to data analysis. The resulting means and confidence limits were then back-transformed to the original units (ng/mL).
Spearman’s rank correlation and associated 95% confidence interval.
Multiple linear regression of serum 25OHD concentration using gender, race, disease severity, surgical history, BMI and season of 25OHD measurement revealed African American race (p<0.001) and male gender (p=0.002) to be significant predictors of lower serum 25OHD concentration. Additionally, an interaction between African American race and male gender (p=0.004) and African American race and overweight BMI (p=0.061) was found to be significant. Table 4 presents back transformed, model-based predicted means and 95% confidence intervals of 25OHD. The results from this table point toward lower serum 25OHD concentrations for African Americans in general, but an even larger deficiency in those that are overweight and female. For Caucasians, the largest predicted deficiency occurs in overweight males.
Table 4. Predicted means from multiple regression model and 95% CI.
Predicted serum 25OHD concentration and confidence intervals of specific demographic and clinical variables calculated based on the multiple linear regression model. The regression model was constructed on the log-transformed data and the resulting coefficients and confidence intervals were back-transformed to the original scale. Significant interactions were retained if they were significant at the 0.1 level.
| Clinical & Demographic Characteristics |
Predicted Mean 25OHD (ng/mL) |
95% CI |
|---|---|---|
|
| ||
| African American with CD | ||
|
| ||
| overweight, female | 9.6 | (6.8 – 13.5) |
| normal weight, female | 15.6 | (13.0 – 18.8) |
| overweight, male | 11.0 | (7.5 – 16.2) |
| normal weight, male | 17.9 | (15.6 – 20.7) |
|
| ||
| Caucasian with CD | ||
|
| ||
| overweight, female | 26.1 | (19.8 – 34.5) |
| normal weight, female | 27.5 | (23.6 – 32.1) |
| overweight, male | 19.0 | (14.9 – 24.3) |
| normal weight, male | 20.0 | (17.4 – 23.0) |
Bone Mineral Density DXA Z score
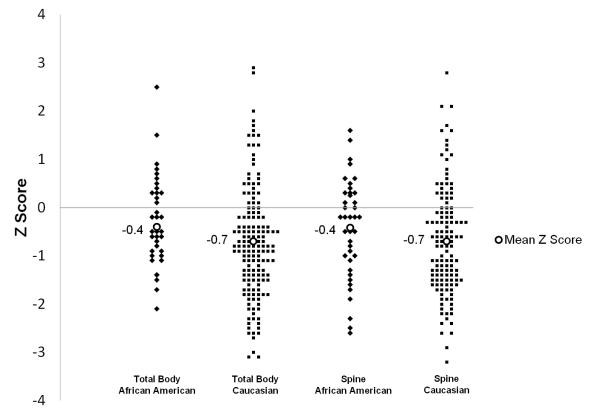
One hundred sixty-six DXA scans of pediatric patients with CD were included in the analysis of BMD. There were 38 African American patients and 128 Caucasian patients. The BMI and history of surgery were similar between the two groups (Table 2). The mean total body and spine Z score (± SD) was similar between African Americans with CD [−0.4 (±0.9), −0.4 (±1.0)] compared to Caucasians with CD [−0.7 (±1.2), −0.7 (±1.1), Figure 2]. The prevalence of decreased spine BMD in our population of African American children with CD was 32% and in Caucasians it was 43%, while for whole body BMD it was 29% and 39%, respectively.
Table 2. Demographic and clinical data of the DXA study population.
Two-sample t-tests were used to compare whole body BMD and spine BMD Z scores between African Americans and Caucasians with CD, while Pearson Χ2 was used to compare the proportion of patients with Z scores ≤-1 and ≤-2 between races. Additionally, two-sample t-tests were used to compare the log-transformed BMD standardized values among various demographics groups.
| African Americans with CD (n=38) |
Caucasians with CD (n=128) |
p-value | |
|---|---|---|---|
| Age | |||
|
| |||
| Mean ± SD | 13.8±2.6 | 14.2±3.2 | 0.428 |
|
| |||
| Whole Body BMD | |||
|
| |||
| Mean ± SD | −0.4 ± 0.9 | −0.7 ± 1.2 | 0.104 |
| Z score ≤ −1 - N (%) | 11 (29) | 54 (39) | 0.142 |
| Z score ≤ −2 - N (%) | 1 (3) | 17 (12) | 0.064 |
|
| |||
| Spine BMD | |||
|
| |||
| Mean ± SD | −0.4 ± 1.0 | −0.7 ± 1.1 | 0.236 |
| Z score ≤ −1 - N (%) | 12 (32) | 59 (43) | 0.112 |
| Z score ≤ −2 - N (%) | 3 (8) | 13 (10) | 0.678 |
|
| |||
| Gender - N (%) | |||
|
| |||
| Male | 23 (60.5) | 66 (51.6) | 0.359 |
| Female | 15 (39.5) | 62 (48.4) | |
|
| |||
| BMI - N (%) | |||
|
| |||
| Underweight | 6 (15.8) | 25 (20.5) | 0.840 |
| Normal | 24 (63.2) | 72 (59.0) | |
| Overweight | 8 (21.0) | 25 (20.5) | |
|
| |||
| History of Surgery - N (%) | |||
|
| |||
| Yes | 28 (73.7) | 102 (79.7) | 0.502 |
| No | 10 (26.3) | 26 (20.3) | |
Figure 2.
Line plot graph of bone mineral density (DXA Z score) by race and total body or spine. Diamonds represent individual African American children with CD, squares are Caucasians with CD and open circles are the mean Z score in each group. Mean Z score was similar between races (−0.4 vs −0.7 for both total body and spine; p=0.104 and p=0.236).
Discussion
The results of our study add to a growing literature demonstrating differences between African American and Caucasian children with IBD. African American children with IBD less frequently have a positive family history and are more likely to present with anemia and nutritional failure23. Adult studies show that African Americans with CD more frequently have upper GI tract disease, colonic involvement and perianal disease compared to Caucasians24. This, in part, may be secondary to differences in genetic susceptibility25. Our study confirms previous data showing that vitamin D status is yet another difference between African American and Caucasians with CD.
Our results show that more than two-thirds of our African American controls have vitamin D deficiency, echoing the results of prior studies 26-28. We also found that more than 40% of African American children with CD are vitamin D deficient, a higher percentage than previously quoted studies that included only a small number of African-American patients 9, 11. Furthermore, our data reveals that the frequency of vitamin D deficiency in African Americans with CD is no different than healthy African American children. This is in sharp contrast to Caucasians, where published literature indicates 25OHD concentrations are significantly lower in patients with CD compared to healthy controls 9-11. Although our results do show a significant difference in serum 25OHD concentrations between African American and Caucasian children with CD, almost the entire cohort is vitamin D insufficient highlighting the importance of determining vitamin D status in children with CD.
Our data also suggests that serum 25OHD concentrations in obese African American children with CD are lower than normal or underweight patients. This observation is seen in larger population studies as well 29. That obese African American females in our study had the lowest serum 25OHD concentrations was also expected, as female children are more likely to have hypovitaminosis D27, 30. This data highlights a potential high risk population that warrants close monitoring. As with other studies comparing 25OHD concentrations in individuals with pigmented skin, our data demonstrates that seasonality does not affect 25OHD concentrations in African Americans with or without CD disease 7, 26, 31. We also found that, unlike previous studies, vitamin D status in African American children with CD is independent of disease severity 12, 32. The reason for this dissimilarity is unknown, but possibilities may include racial differences in the role of vitamin D pathophysiology in CD, or that universally low 25OHD concentrations in the African American population prevents detecting a difference in individuals with CD.
The BMD of African American children with CD was similar to Caucasians with CD. That these findings are different from population based studies demonstrating healthy African American children have higher bone density than Caucasians33-35 may be due to our relatively small sample size. The high percentage of Caucasian patients with DXA scans is more likely secondary to differences in the patient population of the practices that refer to the Children’s Healthcare of Atlanta radiology department than from racial disparity. The paradox of lower serum 25OH concentration in African Americans despite higher bone mineral density does not hold true in our population. One possible explanation is that both African American and Caucasian children with CD have sufficiently decreased serum 25OHD concentrations to lower BMD. Another possibility, demonstrated by several previous studies, is that vitamin D status in children with CD may play less of a role in BMD.15, 36 Given our small cohort, larger, multi-center studies are necessary to determine how race and vitamin D supplementation affects bone mineral density in children with CD. As research continues to identify links between vitamin D and inflammation, perhaps learning more about the differences of vitamin D absorption and metabolism in Caucasian and African American children with CD can lead to further clues about the role of vitamin D in inflammation.
Although this study has several limitations, we have a large cohort of African American children with CD to determine the prevalence of vitamin D deficiency. Our study did not consider vitamin D supplementation or dietary consumption when comparing serum 25OHD concentration. We feel that our results may still be valid as the two previous studies evaluating vitamin D status in children with IBD found no difference in 25OHD when considering dietary or supplemental vitamin D 9, 11. Although classification of disease severity did not use a standardized clinical activity index, we feel that characterizing patients by Paris classification acts as reasonable surrogate for severity. Children in our control group were overly proportioned with females (62% vs 38%) and had a mean age that was significantly less than the study group (10.3 vs 16.5). Despite these differences, other studies have shown a similar percentage of vitamin D deficiency and insufficiency among healthy African American children 37, 38. Many of the children in the African American control group consisted of patients visiting our pediatric GI clinic. Although these patients may not be considered “healthy” control subjects, we limited our selection to individuals with issues unlikely affecting vitamin D status. Although recent literature suggests that characterization of BMD should consider bone age, our data relies solely on chronologic age39. Because our study relied on separate cohorts for the comparisons of serum 25OHD concentration and bone density, our conclusions regarding vitamin D status and bone health should be interpreted with caution, and attempts to replicate this interesting finding in a single cohort would be warranted. Despite the limitations of our study, we feel it adds to a growing body of literature demonstrating differences between African American and Caucasian children with CD.
Conclusions
African American children, regardless if they have CD, have a higher likelihood of being vitamin D deficient compared to Caucasians with CD. Unlike Caucasians with CD, disease severity and history of surgery do not affect serum 25OHD concentrations in African American children. Obese African American females with CD are at highest risk of vitamin D deficiency. The paradox between low serum 25OHD concentration and decreased bone mineral density may not hold true in African American children with CD and further research evaluating differences in vitamin D metabolism and effects of supplementation between African American and Caucasians may help determine the role of vitamin D in CD.
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health: T32 DK007734 (to JAA), K23 AR054334 (to VT), K24 DK096574 (to TRZ), R01 DK 087694 (to SK) and UL1 TR000454 (Atlanta Clinical and Translational Science Institute)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christakos S. DeLuca HF Minireview: Vitamin D: is there a role in extraskeletal health? Endocrinology. 2011;152(8):2930–6. doi: 10.1210/en.2011-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends in endocrinology and metabolism: TEM. 2010;21(6):375–84. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross ACTC, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington D.C.: 2011. [PubMed] [Google Scholar]
- 4.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. The Journal of nutrition. 142(3):498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 5.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. The American journal of clinical nutrition. 2002;76(1):187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 6.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. The Journal of clinical endocrinology and metabolism. 2011;96(10):3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagunova Z, Porojnicu AC, Lindberg F, et al. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer research. 2009;29(9):3713–20. [PubMed] [Google Scholar]
- 8.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. The American journal of clinical nutrition. 2008;88(2):545S–50S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappa HM, Gordon CM, Saslowsky TM, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118(5):1950–61. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoon EJ, Blok BM, Geerling BJ, et al. Bone mineral density in patients with recently diagnosed inflammatory bowel disease. Gastroenterology. 2000;119(5):1203–8. doi: 10.1053/gast.2000.19280. [DOI] [PubMed] [Google Scholar]
- 11.Sentongo TA, Semaeo EJ, Stettler N, et al. Vitamin D status in children, adolescents, and young adults with Crohn disease. The American journal of clinical nutrition. 2002;76(5):1077–81. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 12.Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN. Journal of parenteral and enteral nutrition. 2011;35(3):308–16. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 13.Boot AM, Bouquet J, Krenning EP, et al. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut. 1998;42(2):188–94. doi: 10.1136/gut.42.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflammatory bowel diseases. 2007;13(4):416–23. doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- 15.El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Digestive diseases and sciences. 2011;56(3):825–9. doi: 10.1007/s10620-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 16.Gokhale R, Favus MJ, Karrison T, et al. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology. 1998;114(5):902–11. doi: 10.1016/s0016-5085(98)70309-9. [DOI] [PubMed] [Google Scholar]
- 17.Benchimol EI, Ward LM, Gallagher JC, et al. Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2007;45(5):538–45. doi: 10.1097/MPG.0b013e3180dca0cc. [DOI] [PubMed] [Google Scholar]
- 18.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflammatory bowel diseases. 2011;17(6):1314–21. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital and health statistics. Data from the national health survey 2002246. 2000. pp. 1–190. (Series 11). [PubMed]
- 20.Cluse ZN, Fudge AN, Whiting MJ, et al. Evaluation of 25-hydroxy vitamin D assay on the immunodiagnostic systems iSYS analyser. Annals of clinical biochemistry. 2011 doi: 10.1258/acb.2011.011018. [DOI] [PubMed] [Google Scholar]
- 21.Pappa H, Thayu M, Sylvester F, et al. Skeletal health of children and adolescents with inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2011;53(1):11–25. doi: 10.1097/MPG.0b013e31821988a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. The use of transformation when comparing two means. BMJ. (Clinical research ed) 1996;312(7039):1153. doi: 10.1136/bmj.312.7039.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eidelwein AP, Thompson R, Fiorino K, et al. Disease presentation and clinical course in black and white children with inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2007;44(5):555–60. doi: 10.1097/MPG.0b013e3180335bb3. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. The American journal of gastroenterology. 2006;101(5):1012–23. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Kugathasan S, Loizides A, Babusukumar U, et al. Comparative phenotypic and CARD15 mutational analysis among African American, Hispanic, and White children with Crohn’s disease. Inflammatory bowel diseases. 2005;11(7):631–8. doi: 10.1097/01.mib.0000171279.05471.21. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Pollock N, Stallmann-Jorgensen IS, et al. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125(6):1104–11. doi: 10.1542/peds.2009-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 28.Cole CR, Grant FK, Tangpricha V, et al. 25-hydroxyvitamin D status of healthy, low-income, minority children in Atlanta, Georgia. Pediatrics. 125(4):633–9. doi: 10.1542/peds.2009-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis AF, Hauache OM, Velho G. Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes & metabolism. 2005;31(4 Pt 1):318–25. doi: 10.1016/s1262-3636(07)70200-8. [DOI] [PubMed] [Google Scholar]
- 30.Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 131(1):e152–61. doi: 10.1542/peds.2012-1711. [DOI] [PubMed] [Google Scholar]
- 31.Levis S, Gomez A, Jimenez C, et al. Vitamin d deficiency and seasonal variation in an adult South Florida population. The Journal of clinical endocrinology and metabolism. 2005;90(3):1557–62. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 32.Driscoll RH, Jr., Meredith SC, Sitrin M, et al. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology. 1982;83(6):1252–8. [PubMed] [Google Scholar]
- 33.McCormick DP, Ponder SW, Fawcett HD, et al. Spinal bone mineral density in 335 normal and obese children and adolescents: evidence for ethnic and sex differences. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1991;6(5):507–13. doi: 10.1002/jbmr.5650060513. [DOI] [PubMed] [Google Scholar]
- 34.Bell NH, Shary J, Stevens J, et al. Demonstration that bone mass is greater in black than in white children. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1991;6(7):719–23. doi: 10.1002/jbmr.5650060709. [DOI] [PubMed] [Google Scholar]
- 35.Gilsanz V, Roe TF, Mora S, et al. Changes in vertebral bone density in black girls and white girls during childhood and puberty. The New England journal of medicine. 1991;325(23):1597–600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 36.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflammatory bowel diseases. 2007;13(1):42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 37.Stein EM, Laing EM, Hall DB, et al. Serum 25-hydroxyvitamin D concentrations in girls aged 4-8 y living in the southeastern United States. The American journal of clinical nutrition. 2006;83(1):75–81. doi: 10.1093/ajcn/83.1.75. [DOI] [PubMed] [Google Scholar]
- 38.Rovner AJ, O’Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008;162(6):513–9. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 39.Hill RJ, Brookes DS, Lewindon PJ, et al. Bone health in children with inflammatory bowel disease: adjusting for bone age. Journal of pediatric gastroenterology and nutrition. 2009;48(5):538–43. doi: 10.1097/MPG.0b013e31818cb4b6. [DOI] [PubMed] [Google Scholar]