Abstract
Pharmacogenetic (PGx) predictors of response would improve outcomes in antipsychotic treatment. Based on both biological rationale and prior evidence of an impact on Parkinson’s disease, we conducted an association study for 106 SNPs in the synaptic vesicle protein 2C (SV2C) gene using genetic and treatment response data from the Clinical Trial of Antipsychotic Intervention Effectiveness (CATIE). We examined response to the atypical antipsychotics for Caucasian subjects in the blinded phases, Phases 1A, 1B, and 2, of CATIE with sample sizes as follows: olanzapine (N = 134), quetiapine (N = 124), risperidone (N=134), and ziprasidone (N=74). Response was defined as change in the Positive and Negative Syndrome Scale (PANSS) score using a mixed model repeat measures approach. Subjects homozygous for the T allele of rs11960832 displayed significantly worse response to olanzapine treatment, the only finding with study-wide significance (p = 2.94×10−5; false discovery rate = 2.18×10−2). These subjects also displayed worse response to quetiapine with nominal significance (p = 4.56×10−2). While no other SNP achieved study-wide significance, one SNP (rs10214163) influencing Parkinson’s disease displayed nominally significant association with olanzapine and quetiapine response, while the second such SNP (rs30196) showed a statistical trend toward correlating with olanzapine and quetiapine response. Furthermore, both coding SNPs examined (rs31244 and rs2270927) displayed nominally significant correlations with treatment response: one for olanzapine (rs227092), and one for quetiapine (rs31244). The fact that multiple SNPs in SV2C may impact response to atypical antipsychotics suggests that further evaluation of SNPs in this gene as PGx predictors of antipsychotic response is warranted.
Keywords: Pharmacogenomics, CATIE Trial, olanzapine, risperidone, quetiapine, ziprasidone
1. Introduction
Variation in response to antipsychotic treatment complicates the treatment of patients with schizophrenia, and pharmacogenetic (PGx) predictors of efficacy for antipsychotics have proven challenging to find. Several studies have been published on PGx response to antipsychotics, most of which have focused on Genome Wide Association Study (GWAS) approaches using an additive genetic model (Aberg et al., 2012;Adkins et al., 2011;Fijal et al., 2012a;Liu et al., 2012;McClay et al., 2011;Volpi et al., 2009). Though often overlooked, genetic variants might just as well impact treatment response via other modes of inheritance (as tested by dominant and recessive genetic models) (Lavedan et al., 2009;Need et al., 2009;Fijal et al., 2012b).
Recently, the synaptic vesicle protein 2C (SV2C) gene was identified as a potential pharmacogenetic (PGx) modulator of nicotine’s protective effect on Parkinson’s disease incidence (Hill-Burns et al., 2012). Following a genome-wide scan for interaction with smoking and Parkinson’s disease, the authors identified eighteen single nucleotide polymorphisms (SNPs) in the promoter region and early 5’ portion of the gene that moderated the impact of smoking on Parkinson’s susceptibility. Interestingly, rather than experiencing a protective effect from nicotine consumption, subjects homozygous for the minor alleles of rs10214163 and rs30196 experienced an increased incidence of Parkinson’s disease.
The SV2C protein has a number of characteristics that may have relevance to the pathophysiology and pharmacological treatment of affective disorders and psychosis. SV2C is found on the surface of synaptic vesicles, and it has been hypothesized that SV2C plays a role in synaptic function (Dardou et al., 2013). In mice, SV2C has restricted distribution in the brain, being found in specific regions of the striatum, substantia nigra, neostriatum, and pallidum (Dardou et al., 2011). Expression of SV2C is inversely linked to tyrosine hydroxylase expression, with SV2C knockout mice exhibiting large increases in production of tyrosine hydroxylase mRNA (Dardou et al., 2013). As tyrosine hydroxylase is the rate-limiting enzymatic step in the production of dopamine and norepinephrine, SV2C may play a critical role in catecholamine-mediated activities of the basal ganglia (Dardou et al., 2013). Consistently, decreased SV2C expression produces a deficit in the cocaine conditioned place preference procedure, abolishing the preference for cocaine-associated locations (Dardou et al., 2013). Additionally, SV2C appears to preferentially co-localize with Gamma Amino Butyric Acid (GABA) vesicles but not glutamate vesicles (Gronborg et al., 2010). Outside the brain, SV2C is highly expressed in the pancreas, especially in islet cells. It has been linked to glucose-mediated but not Ca++-mediated insulin release (Iezzi et al., 2005).
SV2C has many attributes that make it an interesting candidate PGx gene for antipsychotic response. While the exact mechanism of action for SV2C with regard to vesicle transport of hormones and neurotransmitters remains unclear, its involvement with both GABA and insulin transporters may have implications in the etiology of schizophrenia and its treatment. It is expressed in dopaminergic neurons located in brain areas associated with the pathology of psychosis (Dardou et al., 2013). The entire group of synaptic vesicle family 2 proteins (SV2A, SV2B, and SV2C) provides key components of neurotransmitter vesicles (Gronborg et al., 2010). These proteins interact with many components of the vesicle structure. SV2C appears to be associated preferentially with GABA vesicles and not associated with glutamate vesicles, which instead show enrichment of SV2B protein levels (Gronborg et al., 2010). Several laboratories have shown that down-regulation of mRNAs encoding glutamic acid decarboxylase 67 and reelin decreases the cognate proteins coexpressed in prefrontal cortex GABAergic neurons of postmortem schizophrenic and bipolar brains (Guidotti et al., 2005). GABAergic neurons release reelin that can interact with integrin receptors on cortical pyramidal neurons and regulate mRNA translation. The hypoplasticity of the prefrontal cortex in schizophrenia and bipolar disorder may be associated with such downregulation and may play a role in negative symptomatology.
In the current study, we explored the impact of SNPs in the SV2C gene on response to atypical antipsychotics in Caucasian subjects in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study of antipsychotic response in schizophrenia. This analysis evaluated the three common models of genetic variation (additive, dominant, and recessive) using the mixed model approach developed previously as a measure of pharmacological response (Liu et al., 2012;van den Oord et al., 2009). We report the top results for these analyses and discuss the implications for further evaluation of genetic variation in the SV2C gene as potential PGx markers for antipsychotic response.
2. Materials and methods
2.1 Patients
The patient population and the CATIE data used are described in detail elsewhere (Lieberman et al., 2005;Liu et al., 2012;Stroup et al., 2003;Sullivan et al., 2008). Briefly, this study was limited to self-described Caucasian subjects, diagnosed with schizophrenia and participating in the CATIE trial. All subjects in this study provided informed consent for genetic testing and participated in at least one of the randomized phases of the study, Phases 1A, 1B, and 2 (Lieberman et al., 2005). For each drug, Table 1 presents total number of patients treated, mean and standard deviation for baseline PANSS score and age, and distribution between the sexes. The Center for Collaborative Genomic Studies on Mental Disorders (CCGSMD) provided the genotypes and phenotypes, including drug response data.
Table 1.
Sample Characteristics
| Treatment | Sample Size |
Mean Baseline(SD)* | Mean AGE (SD)** | # of Females** |
# of Males** |
|---|---|---|---|---|---|
| Olanzapine | 134 | 73.5 (18.8) | 40.5 (11.6) | 34 | 100 |
| Quetiapine | 124 | 73.5 (18.0) | 40.5 (11.2) | 29 | 95 |
| Risperidone | 134 | 77.5 (17.2) | 42.2 (11.4) | 29 | 105 |
| Ziprasidone | 74 | 71.3 (16.2) | 40.1 (11.1) | 21 | 53 |
Baseline PANSS was a significant contributor to drug response and therefore was incorporated into the mixed model that provided the response variable used for the genetic analysis;
Both sex and age were considered for inclusion in the response model; however as they did not significantly contribute to response they were not included in the final mixed model.
2.2 Measures of response and drugs used
As described previously, we used data from all time points available from Phases 1, 1A, and 2 in CATIE from Caucasian subjects treated with atypical antipsychotics to develop a treatment response variable. The sample sizes were as follows: olanzapine, 134; perphenazine, 75; quetiapine, 124; risperidone, 134; ziprasidone, 74. We implemented the mixed model report measures (MMRM) approach developed by Van den Oord et al (McClay et al., 2011;van den Oord et al., 2009) as a method of modeling antipsychotic response. This model predicted an overall response as defined as change in Total Score on Positive and Negative Syndrome Scale (PANSS-T). PANSS-T provided a continuous variable for use in genetic analysis.
2.3 Statistical methods
For each atypical antipsychotic arm, we conducted genetic association analysis in SVS version 7.3.1 software package (Golden Helix Inc. Bozeman, MT) using the additive, dominant, and recessive models with change in PANSS-T as the dependent variable. The genetic association analysis tested the null hypothesis that subjects with varying combinations of minor alleles did not display a differences in mean PANSS-T for a given drug treatment.
When choosing a p-value threshold to report, one must strike a balance between concerns for false positive and disregarding true signals due to sample size issues. Because this study was a candidate gene study with several lines of evidence suggesting that the SV2C gene might have a PGx impact on antipsychotic drug response, we have reported all nominally significant results. We evaluated the impact of tobacco use and alcohol consumption status for all nominally significant findings (using CCGSMD-provided status at study enrollment) by analysis of variance (ANOVA).
To mitigate false positives resulting from the small sample sizes found for some rarer groups comprising homozygous individuals, only those SNPs with homozygosity for the minor allele at a frequency ≥ 0.03 in a given drug arm were used. For SNPs for which there were no cases of homozygosity for the minor alleles, we tested only the dominant model. We analyzed a total of 106 SNPs beginning with rs11960832 in the promoter region and concluding with rs2913257, the 3′ most SNP lying within the transcribed region that was included in the CCGSMD-provided data. Excluding the rare and missing genotype groups resulted in a total of 742 individual tests needed to evaluate the four atypical antipsychotics using the three genetic models. The Benjamini method for False Discovery Rate (FDR) was used to correct for multiple comparisons (Benjamini et al., 2001).
To evaluate if multiple findings for a given drug were due to correlation between the SNPs, we determined the pairwise R2 values for all nominally significant findings. These linkage disequilibrium (LD) values were calculated along with a graphical representation of the results using Haploview software (Version 4.2) (Barrett et al., 2005).
3 Results
3.1 SV2C SNP Association results for response to atypical antipsychotics
Table 2 lists results (p < 0.05) from the SNP association analysis for PGx impact on the atypical antipsychotics in the CATIE trial. Of the 106 SNPs tested, each in three genetic models, a total of only 16 different SNPs produced nominally significant results.
Table 2.
Nominally Significant Association Results for Atypical Antipsychotics in the CATIE Trial
| Rs# | Chr | Base Pair | Minor Allele | P-valuea | FDRb | Responsec | Genetic modeld |
|---|---|---|---|---|---|---|---|
| olanzapine | |||||||
| 11960832e | 5 | 75550742 | T | 2.94E-05 | 2.18E-02 | worse | recessive |
| 3.39E-02 | 9.96E-01 | worse | additive | ||||
| 4580760 | 5 | 75476111 | A | 2.47E-03 | 9.16E-01 | better | additive |
| 3.35E-02 | 9.96E-01 | better | dominant | ||||
| 10214163f | 5 | 75330166 | C | 1.40E-02 | 9.96E-01 | worse | additive |
| 4.00E-02 | 9.96E-01 | worse | dominant | ||||
| 1995381 | 5 | 75606414 | G | 2.01E-02 | 9.96E-01 | worse | dominant |
| 2270927g | 5 | 75627466 | G | 2.47E-02 | 9.96E-01 | worse | dominant |
| 6874435 | 5 | 75610611 | G | 3.22E-02 | 9.96E-01 | worse | dominant |
| 2112865 | 5 | 75465359 | G | 4.18E-02 | 9.96E-01 | better | dominant |
| quetiapine | |||||||
| 10214163f | 5 | 75330166 | C | 4.62E-03 | 9.96E-01 | better | dominant |
| 5.90E-03 | 9.96E-01 | better | additive | ||||
| 2134227 | 5 | 75436223 | C | 7.77E-03 | 9.96E-01 | better | dominant |
| 8.32E-03 | 9.96E-01 | better | additive | ||||
| 31244g | 5 | 75630499 | A | 2.35E-02 | 9.96E-01 | worse | dominant |
| 11960832e | 5 | 75550742 | T | 4.56E-02 | 9.96E-01 | worse | recessive |
| risperidone | |||||||
| 7732173 | 5 | 75513419 | A | 1.76E-02 | 9.96E-01 | worse | recessive |
| 10514062 | 5 | 75513972 | T | 1.76E-02 | 9.96E-01 | worse | recessive |
| 2358531 | 5 | 75515542 | G | 1.76E-02 | 9.96E-01 | worse | recessive |
| 1995380 | 5 | 75548413 | C | 2.35E-02 | 9.96E-01 | better | dominant |
| 6882321 | 5 | 75599586 | C | 3.82E-02 | 9.96E-01 | better | dominant |
| ziprasidone | |||||||
| 12522597 | 5 | 75467455 | A | 1.01E-02 | 9.96E-01 | better | dominant |
| 12655684 | 5 | 75528458 | T | 3.79E-02 | 9.96E-01 | better | dominant |
| 4.95E-02 | 9.96E-01 | better | additive | ||||
The trend correlation p-value (See Material and Methods Section 2.3) For a given drug, SNPs are listed by increasing p-value. Additional nominally significant associations(s) of a SNP using a different genetic model are listed immediately following the most significant result.
False Discovery Rate (See Material and Methods Section 2.3)
Better response is defined as a greater decrease in PANSS-T than the mean for a given arm. Worse response is defined as a smaller decrease (including increases) in PANSS-T than the mean for a given arm.
Describes the type of genetic model used for the association analysis
SNP remained significant after FDR correction in the olanzapine arm and also impacted response in the quetiapine arm
Candidate SNP for nicotine impact on Parkinson’s risk (Hill-Burns et al., 2012)
SNP leads to amino acid coding change
Of these, only the recessive model for rs11960832 and olanzapine response survived correction for multiple-testing. Subjects homozygous for the minor allele of this SNP displayed significantly worse response when treated with olanzapine compared to subjects with at least one copy of the major allele. Interestingly, homozygosity for this same minor allele correlated with poor response in subjects treated with quetiapine as well. In addition, the additive model for this SNP was nominally significant for olanzapine response.
One of the two SNPs previously shown to influence the impact of nicotine on Parkinson’s also appears in Table 2 (Hill-Burns et al., 2012). In the present study, rs10214163 was the third most significant SNP for olanzapine response and the most significant SNP for quetiapine response. Presence of the minor (C) allele of this SNP correlated with poorer response to olanzapine, but better response to quetiapine. While the other SNP implicated in nicotine and Parkinson’s, rs30196, does not appear in Table 2, it barely missed the threshold in the additive model of olanzapine response with a p-value of 0.057.
It is also worth noting that both of the SV2C coding variants included in the genotypes provided by CCGSMD (rs31244 and rs2270927) appear in Table 2. Using a dominant model, the minor allele of rs31244 correlated with poorer response to quetiapine, and the minor allele of rs2270927 correlated with poorer response to olanzapine. Additionally, the dominant model for rs31244 displayed a trend for poorer response to olanzapine (p-value = 0.12), and the dominant model for rs2270927 displayed a trend for poorer response to quetiapine (p-value = 0.18). Neither SNP correlated with response for the other two drugs, risperidone and ziprasidone (p-values > 0.9).
Ziprasidone produced the fewest nominally significant results. One possible explanation for this observation is the limited sample size available for ziprasidone. Ziprasidone was added to the CATIE protocol after initiation of the study. Therefore, ziprasidone was underrepresented in Phase 1A and completely absent in Phase 1B resulting in a lower total number of subjects treated for this drug.
Given the prior evidence of a gene-by-environment interaction between the SV2C gene and nicotine (Hill-Burns et al., 2012), it was of interest to determine if tobacco or alcohol consumption impacted the results in Table 2. Accordingly, we performed ANOVA for all the SNPs and corresponding genetic models shown in Table 2 to test for a gene-by-environment interaction with these environmental factors. Within rounding error for P values, neither variable individually nor combined improved the model fit for any of the findings listed in Table 2.
3.2 Linkage disequilibrium (LD) results for significant SNPs
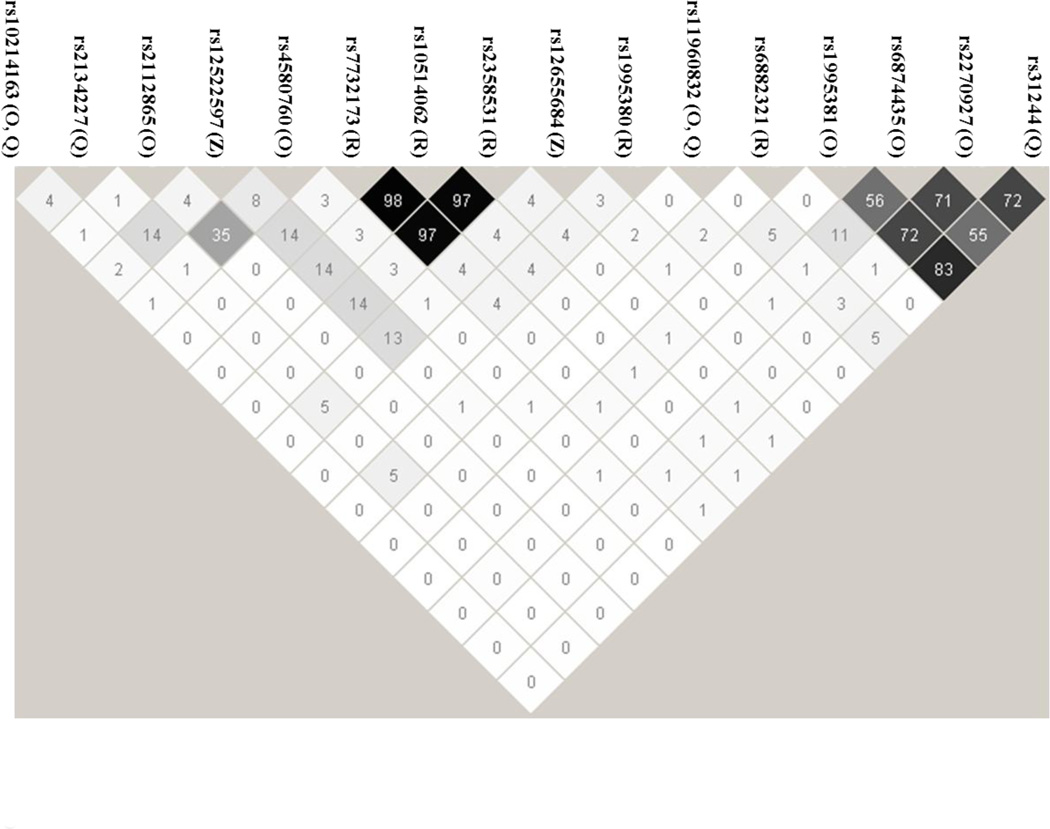
Figure 1 summarizes the LD relationships between the various SNPs listed in Table 2. For the most part the SNPs appear to capture genetically independent loci, with some interesting exceptions.
Figure 1. Linkage Disequilibrium plot of significant SNPs.
The SNPs in Table 2 were used as input for Haploview to calculate linkage disequilibrium parameters for the 16 unique SNPs in Table 2 (See Materials and Methods Section 2.3). The number inside the diamonds represents the r2 value for the given 2-SNP pair. The capital letter(s) following each rs number identifies the drug(s) for which findings are listed in Table 2 as follows: O = olanzapine; Q = quetiapine; R = risperidone; and Z = ziprasidone
In the case of risperidone, the top three SNPs are highly correlated as can be seen by the r2 values which exceed 0.95 for between pairwise comparisons for rs773217, rs10514062, and rs2358531. Thus it is not surprising that the p-values and the best genetic model (recessive) fit for risperidone response are the same for these three SNPs.
Another area of LD for significant SNPS includes the two coding variants. Three SNPs, rs1995381, rs687443, and rs2270927 (the latter being a coding variant) correlate with olanzapine response. Here again the p-values for the three SNPs are similar, and the best fit genetic model (dominant) is the same for olanzapine response for each SNP. The two coding variants, rs2270927 and rs31244 are also in LD with one other. While only rs31244 correlates with quetiapine response at the p ≤ 0.05 level, the minor allele does correlate with poorer response to quetiapine, with a dominant genetic model similar to what is seen for rs2270927 and olanzapine response.
4 Discussion
Out of a total of 106 markers that we examined using three genetic models in four drug arms from CATIE, genotypes of only one SNP, rs11960832 as related to olanzapine response, survived correction for multiple testing. The same SNP also displayed a nominally significant correlation to quetiapine response.
Consistently, some of our other findings suggest that variation in the SV2C gene may selectively impact response to the benzodiazepine derivatives, olanzapine and quetiapine, relative to risperidone, and ziprasidone. Genotypes for one of the two SNPs previously associated with nicotine’s impact on Parkinson’s disease risk, rs10214163 (Hill-Burns et al., 2012), correlated with response to olanzapine and quetiapine, but not to risperidone and ziprasidone. Furthermore both of the coding variants tested may affect response to olanzapine and/or quetiapine but not to the other two atypical antipsychotics. It is unclear why variation in the SV2C gene may preferentially affect response to olanzapine and quetiapine, but it could potentially relate to the differential receptor binding affinities for the benzodiazepine derivatives.
SV2C gene variation appears to modulate the protective effect of nicotine on Parkinson’s disease risk (Hill-Burns et al., 2012). Given that nicotine enhances dopamine release in the brain and that SV2C is densely expressed in the substantia nigra, Hill-Burns and coworkers (2012) hypothesized that SV2C expression levels may alter neurotransmission in these dopaminergic neurons. The ties to both dopamine and GABA, combined with high expression in areas associated with the pathology of schizophrenia, provide intriguing biological rationale for the involvement of SV2C in the mechanism of action of antipsychotic medications. Moreover, SV2C has been linked to glucose-mediated insulin release in the pancreas (Iezzi et al., 2005). Since the antipsychotics can disrupt this process, the glucose-insulin connection may be worthy of further investigation.
In summary, several lines of evidence suggest that variation in the SV2C gene may differentially influence response to antipsychotics. Genotypes for five of the 106 SNPs evaluated, including the four SNPs with prior evidence of a role in protein structure or interaction with the neuroactive compound nicotine, correlated with olanzapine and quetiapine response. Variation in several other SNPs may impact response to risperidone or ziprasidone. Thus, further investigation of the role of SV2C gene variation in antipsychotic treatment response seems justified.
Acknowledgment
This work was supported in part by NIH grant MH078437 and Kentucky Matching Grant KSTC-184-512-07-007 to MDB. The authors thank all participants as well as the investigators involved in the CATIE Study. The principal investigators of the CATIE trial were Jeffrey A. Lieberman, M.D., T. Scott Stroup, M.D., M.P.H., and Joseph P. McEvoy, M.D. The CATIE trial was funded by a grant from the National Institute of Mental Health (N01 MH900001) along with MH074027 (PI PF Sullivan). Prior for the CATIE study was funded by Eli Lilly and Company. The authors thank Calvin Patrick III for technical support and Janet Miller for proof reading assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors are employees of SureGene, LLC. Dr. Brennan and Timothy Ramsey are equity holders in SureGene, LLC.
Contributors
TLR designed the study, with input from MDB, and wrote the first draft of the manuscript. QL provided statistical model predicted values, performed database queries, provided input into writing the manuscript, and edited the final version. BWM provided insight and wrote text regarding brain anatomy and function. MDB reviewed and approved study design, had oversight of the scientific execution, and assisted with writing and editing the manuscript. All authors have contributed to and approve the final manuscript.
Contributor Information
Timothy L. Ramsey, Email: tim.ramsey@suregene.net.
Qian Liu, Email: qian.liu@suregene.net.
Bill W. Massey, Email: bill.massey@suregene.net.
Reference List
- Aberg K, Adkins DE, Liu Y, McClay JL, Bukszar J, Jia P, Zhao Z, Perkins D, Stroup TS, Lieberman JA, Sullivan PF, van den Oord EJ. Genome-wide association study of antipsychotic-induced QTc interval prolongation. Pharmacogenomics J. 2012;12:165–172. doi: 10.1038/tpj.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins DE, Aberg K, McClay JL, Bukszar J, Zhao Z, Jia P, Stroup TS, Perkins D, McEvoy JP, Lieberman JA, Sullivan PF, van den Oord EJ. Genomewide pharmacogenomic study of metabolic side effects to antipsychotic drugs. Mol Psychiatry. 2011;16:321–332. doi: 10.1038/mp.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Dardou D, Dassesse D, Cuvelier L, Deprez T, De RM, Schiffmann SN. Distribution of SV2C mRNA and protein expression in the mouse brain with a particular emphasis on the basal ganglia system. Brain Res. 2011;1367:130–145. doi: 10.1016/j.brainres.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Dardou D, Monlezun S, Foerch P, Courade JP, Cuvelier L, De RM, Schiffmann SN. A role for Sv2c in basal ganglia functions. Brain Res. 2013;1507:61–73. doi: 10.1016/j.brainres.2013.02.041. [DOI] [PubMed] [Google Scholar]
- Fijal BA, Stauffer VL, Kinon BJ, Conley RR, Hoffmann VP, Witte MM, Zhao F, Houston JP. Analysis of gene variants previously associated with iloperidone response in patients with schizophrenia who are treated with risperidone. J Clin Psychiatry. 2012a;73:367–371. doi: 10.4088/JCP.10m06507. [DOI] [PubMed] [Google Scholar]
- Fijal BA, Stauffer VL, Kinon BJ, Conley RR, Hoffmann VP, Witte MM, Zhao F, Houston JP. Analysis of gene variants previously associated with iloperidone response in patients with schizophrenia who are treated with risperidone. J Clin Psychiatry. 2012b;73:367–371. doi: 10.4088/JCP.10m06507. [DOI] [PubMed] [Google Scholar]
- Gronborg M, Pavlos NJ, Brunk I, Chua JJ, Munster-Wandowski A, Riedel D, Ahnert-Hilger G, Urlaub H, Jahn R. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci. 2010;30:2–12. doi: 10.1523/JNEUROSCI.4074-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Hill-Burns EM, Singh N, Ganguly P, Hamza TH, Montimurro J, Kay DM, Yearout D, Sheehan P, Frodey K, McLear JA, Feany MB, Hanes SD, Wolfgang WJ, Zabetian CP, Factor SA, Payami H. A genetic basis for the variable effect of smoking/nicotine on Parkinson's disease. Pharmacogenomics J. 2012 doi: 10.1038/tpj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi M, Theander S, Janz R, Loze C, Wollheim CB. SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Sci. 2005;118:5647–5660. doi: 10.1242/jcs.02658. [DOI] [PubMed] [Google Scholar]
- Lavedan C, Licamele L, Volpi S, Hamilton J, Heaton C, Mack K, Lannan R, Thompson A, Wolfgang CD, Polymeropoulos MH. Association of the NPAS3 gene and five other loci with response to the antipsychotic iloperidone identified in a whole genome association study. Mol Psychiatry. 2009;14:804–819. doi: 10.1038/mp.2008.56. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Liu Q, Jamba M, Patrick C, III, Padmanabhan S, Brennan MD. Targeted pharmacogenetic analysis of antipsychotic response in the CATIE study. Pharmacogenomics. 2012;13:1227–1237. doi: 10.2217/pgs.12.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClay JL, Adkins DE, Aberg K, Stroup S, Perkins DO, Vladimirov VI, Lieberman JA, Sullivan PF, van den Oord EJ. Genome-wide pharmacogenomic analysis of response to treatment with antipsychotics. Mol Psychiatry. 2011;16:76–85. doi: 10.1038/mp.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AC, Keefe RS, Ge D, Grossman I, Dickson S, McEvoy JP, Goldstein DB. Pharmacogenetics of antipsychotic response in the CATIE trial: a candidate gene analysis. Eur J Hum Genet. 2009;17:946–957. doi: 10.1038/ejhg.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 2003;29:15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Lin D, Tzeng JY, van den Oord E, Perkins D, Stroup TS, Wagner M, Lee S, Wright FA, Zou F, Liu W, Downing AM, Lieberman J, Close SL. Genomewide association for schizophrenia in the CATIE study: results of stage 1. Mol Psychiatry. 2008;13:570–584. doi: 10.1038/mp.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oord EJ, Adkins DE, McClay J, Lieberman J, Sullivan PF. A systematic method for estimating individual responses to treatment with antipsychotics in CATIE. Schizophr Res. 2009;107:13–21. doi: 10.1016/j.schres.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi S, Heaton C, Mack K, Hamilton JB, Lannan R, Wolfgang CD, Licamele L, Polymeropoulos MH, Lavedan C. Whole genome association study identifies polymorphisms associated with QT prolongation during iloperidone treatment of schizophrenia. Mol Psychiatry. 2009;14:1024–1031. doi: 10.1038/mp.2008.52. [DOI] [PubMed] [Google Scholar]