INTRODUCTION
Acute exacerbations are the leading cause of morbidity and mortality associated with asthma, frequently leading to hospitalization, and accounting for approximately half of the total health care costs associated with asthma.1 Asthma exacerbations have been linked to progressive loss of lung function,2, 3 which may lead to increased asthma severity later in life. Upper respiratory tract viral infections (URIs) are the most significant risk factor for asthma exacerbations. In the northern hemisphere, there is clear correlation between URI outbreaks in September and concurrent hospitalizations for asthma exacerbations.4 Nearly 85% of acute asthma exacerbations in children and approximately 60% in adults have been linked to the presence of a viral infection through the use of reverse transcription-polymerase chain reaction (RT-PCR),5, 6 with human rhinoviruses (HRVs) as the most common cause.7, 8 While current therapies are effective in controlling day-to-day asthma symptoms, they are less effective at preventing exacerbations,9 suggesting that new strategies are needed for exacerbation prevention.
Airway epithelial cells are recognized a s the primary site of HRV infection and play an important role in the intrinsic antiviral response.10 Interferons (IFNs) are critical to host defense against HRV replication. Increased viral replication, decreased epithelial production of IFN-β and IFN-λ, and reduced induction of apoptosis in response to HRV-16 infection have been reported in asthmatic individuals, suggesting a deficient innate immune response in these individuals that may contribute to the risk of viral exacerbations.11, 12 While not all groups have identified differences in epithelial cell IFN expression between asthmatic subjects and healthy controls,13, 14 it is important to note that deficiencies in peripheral blood IFN-α and -λ1 responses to viruses have been identified in allergic asthmatic children as well.15, 16
Interestingly, treatment with exogenous IFN-β restored induction of apoptosis and inhibited HRV replication in bronchial epithelial cells (BECs) from asthmatic individuals, suggesting potential therapeutic benefit.11, 17 In an animal model of rotavirus infection, treating suckling mice systemically with IFN-λ1 led to decreased replication of rotavirus in the gut and less severe viral illness,18 further supporting the potential utility of exogenous supplementation of IFN as a clinical treatment for viral infections.
Although IFN-α and -β (type I) and IFN-λ (type III) activate similar intracellular signaling pathways and biological activities, type I and type III IFNs use distinct receptor complexes on the cell surface. Unlike the type I high-affinity receptor component, which is broadly expressed, the type III high-affinity receptor component is restricted to dendritic cells and cells of epithelial origin.19 Collectively, these findings suggest that the impact on HRV replication and epithelial antiviral and inflammatory responses may be IFN-type-dependent.
Taken together, these data suggest that IFNs may be useful in treatment of respiratory viral infections and associated asthma exacerbations. In this study, we aimed to determine whether exogenous interferons, at physiologic concentrations, inhibit HRV replication and alter inflammatory responses in primary BECs. Further, we sought to determine whether there were differences in the effects of IFN-α, -β, -λ1, and -λ2. Some of the findings in this manuscript were previously reported in an abstract.20
METHODS
BEC culture
After the protocol was approved by the University of Wisconsin-Madison Health Sciences IRB, human tracheal explants of non-identifiable lung transplant donors were collected using the methods previously described.21 Frozen stocks at passage 0 from six donors were cultured at 37°C in monolayers in 75 cm2 CellBind flasks (Corning Inc., Corning, NY) in 1 mL of bronchial epithelial growth medium (BEGM, Lonza, Walkersville, MD) to 80–90 % confluence. BECs were then passaged into 12-well CellBind plates, grown to 65%–75% confluence and pre-treated for 24 hours with 1 mL of either 0.1 ng/mL, 1 ng/mL or 10 ng/mL doses of IFN-α, IFN-β (Sigma-Aldrich Co. LLC, St. Louis, MO), IFN-λ1 or IFN-λ2 (PeproTech, Rocky Hill, NJ).
Generation of HRV-1A
Due to the fact that minor receptor group viruses utilizing low density lipoprotein (LDL) receptor for cell entry more efficiently infect cultured epithelial cells compared to major group viruses binding to intercellular adhesion molecule 1 (ICAM-1),21 minor group strain HRV-1A was chosen for this study. Purified and concentrated HRV-1A stocks were generated from infected cultures of HeLa cells (Ohio cells) as previously described.22, 23 To minimize any suppressive effects on gene expression, virus was diluted in BEGM with a reduced concentration of hydrocortisone (10−8 M) immediately before infection.
BEC infection with HRV-1A
24 hours after IFN pre-treatment, BECs were infected with either a “high” (5×106 plaque forming units (pfu)/mL, MOI of 10) or “low” (5×105 pfu/mL, MOI of 1) inoculum of HRV-1A. After a 2 hour attachment period, the monolayers were washed three times with PBS to remove unbound virus and incubated in the presence of the appropriate dose and type of IFN. After 24 hour incubation, supernatants were collected and monolayers were lysed using RLT buffer (Qiagen, Hilden, Germany) and stored at −80° C.
Viral quantification
Total RNA was isolated from cell lysates and purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. This was followed by reverse transcription (TaqMan, Aplied Biosystems) and quantification of viral replication by real-time PCR in duplicate wells using ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). The standard curve was constructed using 10-fold serial dilutions of 107 pfu HRV-1A cDNA. HRV primers and probe were described previously.24
Cytokine and chemokine analysis
Interleukin-1 beta (IL-1β), interferon-inducible protein-10 (IP-10 or CXCL-10), RANTES (CCL-5) and VEGF secretion in the cell supernatant were measured using multiplex cytokine analysis kits (Millipore, Billerica, MA). Plates were run on the Luminex 100 instrument (Luminex Corporation, Austin, TX).
Statistical analyses
Repeated measures ANOVA models were used to assess the effect of exogenous IFN-α, -β, -λ1, and -λ2 on HRV replication and HRV-induced cytokine secretion from airway epithelial cells compared to controls. Logarithmic transformations of the responses were used in order to meet the assumptions of ANOVA. If the P-value of global null hypothesis was significant, then pairwise comparisons were examined (Fisher’s protected LSD). All P-values reported are two-sided. P < 0.05 was the criterion for statistical significance. Analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Effect of IFNs on HRV replication
We first investigated the effects of exogenous IFNs (-α2, -β, -λ1 and -λ2) at 0.1 ng/mL, 1 ng/mL or 10 ng/mL on HRV-1A replication in BECs, infected with either a low (5×105 pfu/mL) or a high (5×106 pfu/mL) dose inoculum of HRV-1A. In the samples infected with the low dose inoculum, all IFNs significantly reduced HRV replication in a dose dependent fashion [Figure 1; 0.1 ng/mL (n=5, p<0.003), 1 ng/mL (n=6, p<0.0001) and 10 ng/mL (n=6, p<0.0001)]. Additionally, IFN-α2, -β and -λ1 pretreatment led to greater reductions in HRV replication than IFN-λ2 (p<0.05) at IFN doses of 0.1ng/mL and 1 ng/mL. However, at the 10 ng/mL dose of IFN, only IFN-β pretreatment led to greater reductions in HRV replication than IFN-λ2 (p<0.05) (Figure 1).
Figure 1. Effect of interferons on HRV replication in BECs infected with low-dose HRV.
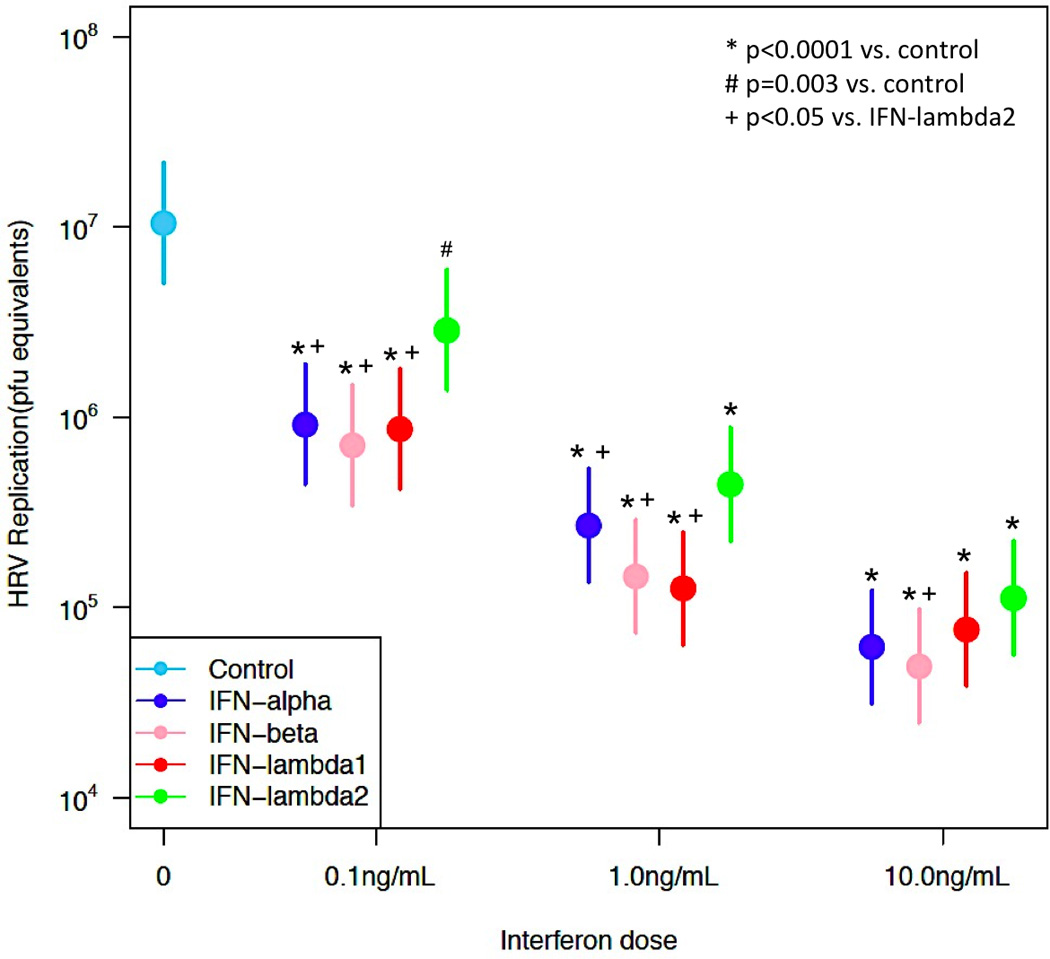
Dose-response comparison of HRV replication (pfu equivalents; mean + standard error of the mean) in BECs infected with a low dose (5×105 pfu/mL) of HRV-1A. Samples were treated with 0 (infected control), 0.1 (n=5), 1 (n=6), or 10 (n=6) ng/mL of exogenous IFN-α2, -β, -λ1 or -λ2 prior to and during infection. *Denotes significance of p<0.0001, # denotes significance of p=0.003, + denotes significance of p<0.05.
Similarly, all IFNs led to a dose dependent reduction in HRV replication in samples infected with the high dose HRV inoculum [Figure 2; 0.1 ng/mL (n=5, p<0.02), 1 ng/mL (n=6, p<0.0001) and 10 ng/mL (n=6, p<0.0001)]. Furthermore, pretreatment with 1 ng/mL of IFN-β and -λ1 led to greater inhibition of HRV replication than IFN-λ2 (p<0.05) (Figure 2).
Figure 2. Effect of interferons on HRV replication in BECs infected with high-dose HRV.
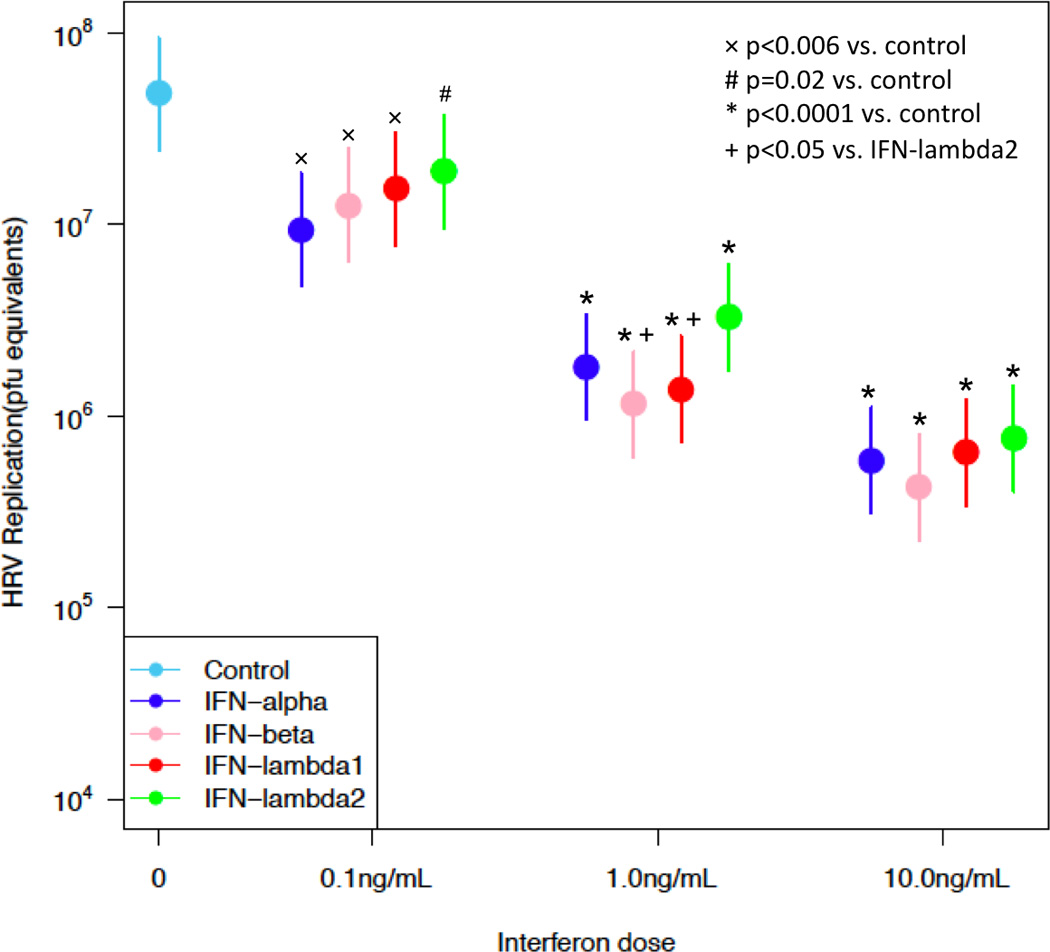
Dose-response comparison of HRV replication (pfu equivalents; mean + standard error of the mean) in BECs infected with a high dose (5×106 pfu/mL) of HRV-1A. Samples were treated with 0 (infected control), 0.1 (n=5), 1 (n=6), or 10 (n=6) ng/mL of exogenous IFN-α2, -β, -λ1 or -λ2 prior to and during infection. *Denotes significance of p<0.0001, # denotes significance of p=0.02, + denotes significance of p<0.05, × denotes significance of p<0.006.
Effect of IFNs on inflammatory responses
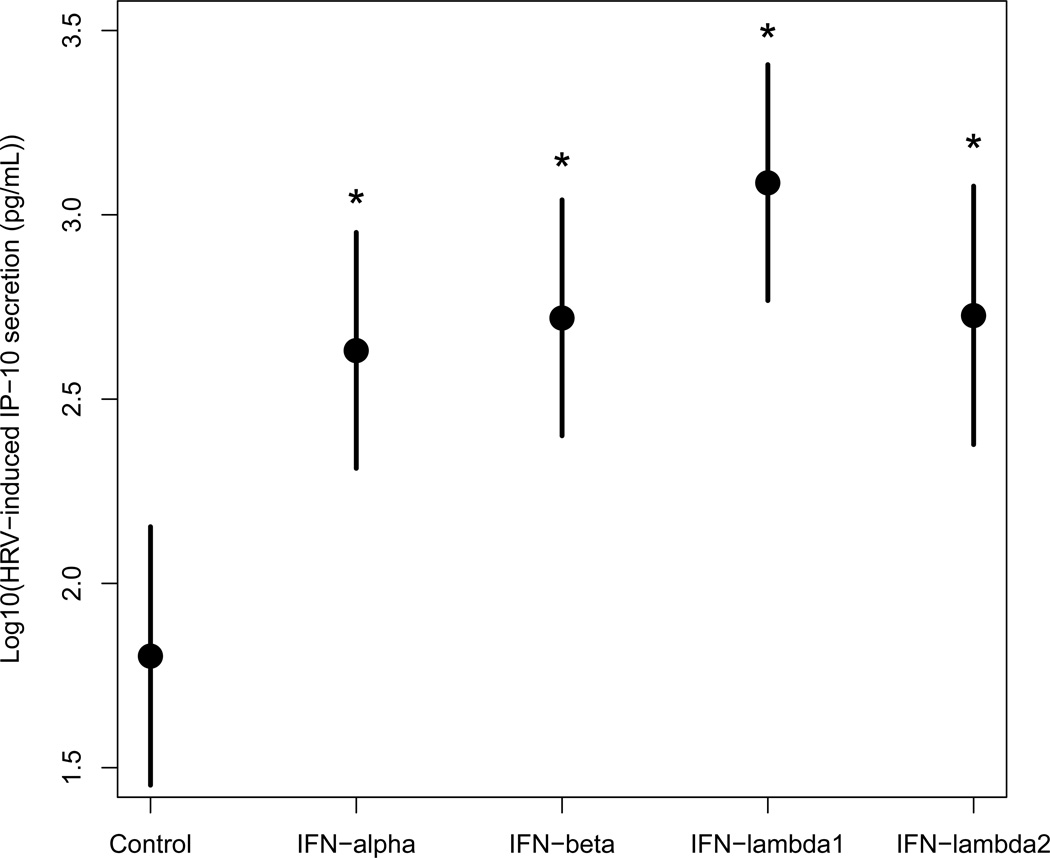
Because virus-induced cytokines, chemokines and growth factors orchestrate airway inflammation and may contribute to clinical symptoms, we also investigated the effects of 1 ng/mL of exogenous IFN-α2, -β, -λ1, or -λ2 on HRV-induced IP-10, RANTES, IL-1B and VEGF protein secretion in BECs from healthy donors after 24 hours (n=6). The addition of exogenous IFNs resulted in significant upregulation of HRV-induced IP-10 secretion compared to infected control (p<0.005) (Figure 3A). The IFN-λ1 treated samples had the highest l evels of HRV-induced IP-10, although this difference was only statistically significant compared to IFN-α2 (p=0.04).
Figure 3. Effect of interferons on cytokine/chemokine secretion in BECs infected with low-dose HRV.
A, Comparison of IP-10 protein secretion (log scale with 95% confidence interval) measured in cell supernatant of BECs from health donors (n=6) treated as infected control or with a 1 ng/mL dose of exogenous IFN-α2, -β, -λ1 or -λ2 prior to and during low dose (5×105 pfu/mL) infection of HRV-1A. Samples treated with IFN-α2, -β, -λ1 or -λ2 showed elevation in IP-10 protein secretion compared to infected control. *Denotes significance of p<0.005. B, Comparison of RANTES protein secretion (log scale with 95% confidence interval) measured in cell supernatant of BECs from health donors (n=6) treated as infected control or with a 1 ng/mL dose of exogenous IFN-α2, -β, -λ1 or -λ2 prior to and during low dose (5×105 pfu/mL) infection of HRV-1A. Samples treated with IFN-λ1 or -λ2 showed significant elevation in RANTES protein secretion compared to infected control. *Denotes significance of p<0.05.
IFN-λ1 and -λ2 also enhanced HRV-induced RANTES secretion, and a similar trend was noted to treatment with IFN-β (Figure 3B). Treatment with interferons did not significantly alter HRV-induced IL-1β or VEGF secretion (data not shown).
DISCUSSION
Through the use of in vitro culture of primary BECs from healthy individuals, we found that exogenous interferons effectively inhibit HRV replication in a dose dependent manner, while also altering the inflammatory response to the virus. Samples treated with 0.1 ng/mL doses of IFN showed a 0.5-log reduction in HRV replication in comparison to control. Similar results were seen at 1 and 10 ng/mL doses of IFN, where HRV replication was reduced by over 1-log compared to control. Further, both IP-10 and RANTES production were upregulated in response to HRV infection in the presence of IFN.
It has been reported that exogenous IFN-β inhibits HRV replication in cultured BECs.11, 17 However, the effects of exogenous IFN-λ1 and IFN-λ2 on HRV replication have not been previously reported, and are of interest in light of the deficient HRV-induced IFN-λ production that has been described in allergic asthmatic individuals.12, 15 In this study, we demonstrated that IFN-λ1 was as effective as IFN-β in inhibiting HRV replication. This is intriguing based upon the restriction of type III interferon receptors to epithelial and dendritic cells,19 suggesting that exogenous IFN-λ1 could have less systemic side effects than treatment with type I interferons. Indeed, early studies of IFN-λ1 in hepatitis C infection suggest similar efficacy to other interferons with fewer side effects.25
In this study, in addition to their antiviral effects, we found significant effects of the interferons on HRV-induced pro-inflammatory IP-10 and RANTES. RANTES is a known chemoattractant and activator for eosinophils, serving as an inflammatory mediator. IP-10, a chemoattractant for macrophages, dendritic cells and T lymphocytes, promotes adhesion of T lymphocytes to epithelial cells and has gained increasing interest due to its association with HRV infections and asthma exacerbations.26–28 Wark and colleagues28 reported that serum IP-10 levels were elevated in asthmatics with HRV-induced asthma exacerbations, with higher levels associated with more severe bronchial obstruction. Interestingly, in our study, while all interferons enhanced IP-10 production, IFN-λ1 had the most prominent effects on both IP-10 and RANTES secretion after HRV infection of BECs.
The role of type I IFNs in the antiviral community was discovered in 1957, but it was not until 2003 that type III IFNs were discovered.29 As a relatively newly identified antiviral cytokine, much remains to be learned about the role of IFN-λ1 in allergic disease and asthma. A recent clinical study by Miller and colleagues30 found greater levels of IFN-λ1 in nasal secretions of wheezing children with more severe HRV-induced exacerbations, despite replicating prior findings of deficient IFN-λ1 levels at baseline in vivo and in vitro after HRV infection of nasal epithelial cells of wheezing children. These findings, taken together with the effects of IFN-λ1 on HRV replication and inflammatory responses in our study, support further study of this antiviral cytokine in asthma.
It should be noted that the enhanced production of HRV-induced IP-10 and RANTES seen with exogenous interferons in our study was not found in a recent study of effects of IFN-β on HRV replication and inflammatory responses in bronchial epithelial cells.17 Cakebread and colleagues reported that IFN-β treatment diminished HRV-induced IP-10 and RANTES production in BECs from asthmatic individuals. Interestingly, IFN-β also caused a significant increase in IP-10 protein expression above baseline values in the absence of HRV infection. Methodological differences between the two studies include strain of HRV ( HRV-1A vs. HRV-1B), time point of measurement (24 vs. 72 hours), phenotype (asthma vs. no asthma) and source of the airway epithelial cells (bronchial explants vs. brushings). However, we performed a separate experiment and found greater enhancement of IP-10 and RANTES at 72 hours after HRV infection in IFN treated samples, suggesting that duration of HRV infection does not explain the difference between studies (data not shown). Of note, similar to our findings for IFN-β, Korpi-Steiner and colleagues31 previously demonstrated that HRV-induced IP-10 production was upregulated when BECs and monocytes were co-cultured and that these effects could be reduced by blocking type I IFN receptors suggesting that IP-10 release is regulated by type I IFN signaling.
Strengths of our study include the use of primary bronchial epithelial cells, as opposed to cell lines, and the multiple doses of HRV and interferons used for direct comparison of the antiviral and inflammatory effects. In this study we used doses of 0.1, 1.0 and 10 ng/mL of IFN, whereas clinical studies typically administer higher doses intranasally.32, 33 Therapeutic targeting of the lower airway, rather than intranasal delivery, could be important for limiting side effects and controlling asthma exacerbations. In addition, we tested effects of four different interferons within the same study. The sample size of these experiments was modest, but the findings were quite reproducible among cell samples from different donors. Based upon these findings in BECs from healthy donors, further study of the antiviral and inflammatory effects of interferons in cells from asthmatics, with a focus on IFN-λ1 in particular, is warranted.
In conclusion, we have found that exogenous type I and III interferons significantly inhibit HRV replication in primary BECs in a dose-dependent manner. IFN-λ1 is of particular interest base d upon similar efficacy with the potential advantages of a more targeted response and fewer side effects.25 Further studies are warranted to explore the physiologic significance of the enhanced HRV-induced inflammatory responses seen with exogenous IFN-λ1 in our study. Taken together, exogenous administration of IFNs could represent a viable approach for the prevention of asthma exacerbations.
ACKNOWLEGMENTS
We would like to thank James E. Gern, MD for providing the BECs for this study.
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427 and grant UL1RR025011. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- HRV
Human rhinovirus
- IFN
Interferon
- BEC
Bronchial Epithelial Cell
- RT-PCR
Real Time-PCR
- ELISA
Enzyme linked immunosorbent assay
- IP-10
Interferon gamma-induced protein 10
- RANTES
Regulated on activation, Normal T-cell expressed and secreted
- IL-1β
Interleukin-1β
- VEGF
Vascular Endothelial Growth Factor
- URI
Upper respiratory tract viral infection
- LDL
Low density lipoprotein
- ICAM-1
Intercellular adhesion molecule-1
- Pfu
Plaque forming units
- MOI
Multiplicity of infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution:
D.J.J. designed of the study. T.M.B., S.R.D. Y.A.B. and M.K.D. were involved in data generation. T.M.B., V.R. and D.J.J. analyzed and interpreted the data. T.M.B. and D.J.J. prepared the initial manuscript and were involved in critical revision. All authors reviewed and approved the manuscript.
References
- 1.Smith DH, Malone DC, Lawson KA, et al. A national estimate of the economic costs of asthma. Am J Respir Crit Care Med. 1997;156:787–793. doi: 10.1164/ajrccm.156.3.9611072. [DOI] [PubMed] [Google Scholar]
- 2.O'Brian AL, Lemanske RF, Jr, Evans MD, et al. Recurrent severe exacerbations in early life and reduced lung function at school age. J Allergy Clin Immunol. 2012;129:1162–1164. doi: 10.1016/j.jaci.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 4.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. quiz 1188-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traves SL, Proud D. Viral-associated exacerbations of asthma and COPD. Curr Opin Pharmacol. 2007;7:252–258. doi: 10.1016/j.coph.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venarske DL, Busse WW, Griffin MR, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193:1536–1543. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–657. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proud D, Leigh R. Epithelial cells and airway diseases. Immunol Rev. 2011;242:186–204. doi: 10.1111/j.1600-065X.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 11.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Souza N, Favoreto S, Wong H, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390. doi: 10.1016/j.jaci.2009.03.010. e1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bochkov YA, Hanson KM, Keles S, et al. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill MA, Bajwa G, George TA, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cakebread JA, Xu Y, Grainge C, et al. Exogenous IFN-beta has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011;127:1148–1154. doi: 10.1016/j.jaci.2011.01.023. e1149. [DOI] [PubMed] [Google Scholar]
- 18.Pott J, Mahlakoiv T, Mordstein M, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Weerd NA, Nguyen T. The interferons and their receptors--distribution and regulation. Immunol Cell Biol. 2012;90:483–491. doi: 10.1038/icb.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker TM, Durrani SR, Rajamanickam V, Bochkov YA, Jackson DJ. Exogenous Interferons Reduce Rhinovirus Replication in Human Brochial Epithelial Cells. J Allergy Clin Immunol. 2012;129:AB55. [Google Scholar]
- 21.Schroth MK, Grimm E, Frindt P, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 22.Sherry B, Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985;53:137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WM, Monroe SS, Rueckert RR. Role of maturation cleavage in infectivity of picornaviruses: activation of an infectosome. J Virol. 1993;67:2110–2122. doi: 10.1128/jvi.67.4.2110-2122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosser AG, Vrtis R, Burchell L, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 25.Muir AJ, Shiffman ML, Zaman A, et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822–832. doi: 10.1002/hep.23743. [DOI] [PubMed] [Google Scholar]
- 26.Korpi-Steiner NL, Bates ME, Lee WM, Hall DJ, Bertics PJ. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol. 2006;80:1364–1374. doi: 10.1189/jlb.0606412. [DOI] [PubMed] [Google Scholar]
- 27.Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:L85–L95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 28.Wark PA, Bucchieri F, Johnston SL, et al. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120:586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donnelly RP, Kotenko SV. Interferson-lamda: a new addition to an old family. J Interferon Cytokine Res. 2010;30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller EK, Hernandez JZ, Wimmenauer V, et al. A mechanistic role for type III IFN-lambda1 in asthma exacerbations mediated by human rhinoviruses. Am J Respir Crit Care Med. 2012;185:508–516. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korpi-Steiner NL, Valkenaar SM, Bates ME, et al. Human monocytic cells direct the robust release of CXCL10 by bronchial epithelial cells during rhinovirus infection. Clin Exp Allergy. 2010;40:1203–1213. doi: 10.1111/j.1365-2222.2010.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden FG, Gwaltney JM, Jr, Johns ME. Prophylactic efficacy and tolerance of low-dose intranasal interferon-alpha 2 in natural respiratory viral infections. Antiviral Res. 1985;5:111–116. doi: 10.1016/0166-3542(85)90037-3. [DOI] [PubMed] [Google Scholar]
- 33.Hayden FG, Albrecht JK, Kaiser DL, Gwaltney JM., Jr Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon. N Engl J Med. 1986;314:71–75. doi: 10.1056/NEJM198601093140202. [DOI] [PubMed] [Google Scholar]