Summary
ARC1172 is a 41-mer DNA aptamer selected to bind the A1 domain of von Willebrand factor (VWF). A derivative of ARC1172 with modifications to increase intravascular survival inhibits carotid artery thrombosis in a Cynomolgus macaque model and inhibits VWF-dependent platelet aggregation in humans, suggesting that such aptamers may be useful to prevent or treat thrombosis. In the crystal structure of a VWF A1-ARC1172 complex, the aptamer adopts a three-stem structure of mainly B-form DNA with three noncanonical base pairs and 9 unpaired residues, 6 of which are stabilized by base-base or base-deoxyribose stacking interactions. The aptamer-protein interface is characterized by cation-π interactions involving Arg, Lys and Gln residues, often stabilized by H-bonds with adjacent bases. The ARC1172 binding site on the A1 domain overlaps with that of botrocetin and clashes with glycoprotein Ibα binding at an adjacent site, which accounts for the antithrombotic activity of ARC1172 and related aptamers.
Introduction
Aptamers are RNA or DNA oligonucleotides that bind specific target molecules, identified by selection in vitro from large random sequence libraries (termed SELEX: systematic evolution of ligands by exponential enrichment) (Ellington and Szostak, 1990; Tuerk and Gold, 1990). Many structures illustrate how proteins have evolved to recognize nucleic acids. However, few structures are available to show how aptamers can adopt complex conformations that enable specific, high affinity binding to proteins that do not normally interact with nucleic acids.
Several properties of aptamers make them an attractive class of therapeutic compounds. For example, aptamers can be isolated that bind many proteins with dissociation constants in the low nanomolar range. Aptamers have little immunogenicity, and simple structural modifications can adjust their bioavailability or pharmacokinetics. In addition, complementary oligonucleotide antidotes can acutely reverse the effects of aptamers, permitting control over their activity in vivo. Aptamers have been developed against proteins involved in infectious diseases, cancer, and cardiovascular diseases (Keefe and Schaub, 2008; Nimjee et al., 2005). One aptamer that targets vascular endothelial growth factor (VEGF) (pegaptinic sodium, Macugen) has been approved for therapy of age-related macular degeneration (Ng et al., 2006).
Von Willebrand factor (VWF) is a giant multimeric plasma protein that plays an essential role in tethering platelets to the injured vessel wall. This process is mediated by interactions between domain A1 of VWF subunits and platelet-receptor glycoprotein Ibα (GPIbα) on the platelet surface (Sadler, 1998), and aptamers have been devised that block this interaction. For example, SELEX applied to a combinatorial library identified a 40-mer RNA that bound to VWF with a Kd <20 nM and also blocked VWF-dependent platelet interactions in vitro (Oney et al., 2007). A similar approach was used to select a modified 41-mer DNA aptamer, ARC1172, that binds with high affinity to the VWF A1 domain and blocks VWF binding to GPIbα (Diener et al., 2009; Gilbert et al., 2007). ARC1779 was derived from ARC1172 (Figure 1A and Figure S1 available online) by adding modifications to prevent digestion by nucleases while preserving the same affinity for the VWF A1 domain. When given intravenously to human volunteers and assayed ex vivo, ARC1779 dose-dependently inhibits VWF activity and prolongs the PFA- 100 closure time with EC90 values of 2-3 μg/ml (Diener et al., 2009; Gilbert et al., 2007). ARC1779 also inhibits thrombus formation on injured porcine arteries ex vivo, and blocks carotid artery thrombosis induced by electrical injury in nonhuman primates (Diener et al., 2009; Keefe and Schaub, 2008). These properties suggest that ARC1779 may be useful to prevent or treat VWF and platelet-dependent thrombosis in acute coronary syndromes, percutaneous coronary intervention procedures, or thrombotic thrombocytopenic purpura (Diener et al., 2009; Gilbert et al., 2007). To characterize this aptamer-protein interaction, we have determined the crystal structure of a complex between recombinant VWF domain A1 and ARC1172. The extensive aptamer-protein interface is characterized by several cation-π interactions and overlaps the GPIbα binding site, which accounts for the antithrombotic activity of ARC1172 and related aptamers.
Figure 1. Structure of aptamer ARC1172 in complex with VWF A1 domain.
(A) Nucleotide sequence of ARC1172. Yellow shading indicates the nucleotides that contact the VWF A1 domain; squares below the sequence identify nucleotides in the consensus sequence that are required for high-affinity binding to VWF; triangles indicate nucleotides that do not tolerate 2′-O-methyl modification (Diener and Lagassé, 2006). (B) Cartoon representation based on the space group P212121 structure. The a-helices (Arabic) and (3-strands (Roman) within the VWF A1 domain are numbered. Nucleotides within 4.2 Å of the VWF A1 domain are colored yellow; nucleotides involved in non-canonical base pairs are labeled and shown by ball and stick. (C) Ball and stick representation of non-canonical base pairs and their composite omit electronic density map at a contour level of 1.0 σ. The dashed lines in cyan indicate hydrogen bonds.
Results
Overall Structure of ARC1172 in Complex with VWF A1
Aptamer ARC1172 was crystallized with the VWF A1 domain (residues Glu497-Thr705, mature VWF subunit numbering) under two different conditions. The crystals belonged to the space groups P212121 (structure I) and P21212 (structure II) and diffracted to a resolution of 2.4 Å and 2.7 Å, respectively (Table 1). The model for structure I was built for VWF A1 domain residues Phe507-Thr705 and for aptamer residues G1-C40 with well defined density except for the T14-C16 loop of the aptamer (see Figure S2 available online). However, this loop was clearly seen in the density map for structure II (Figure S3), which also showed five more residues at the N-terminus of the VWF A1 domain (Pro502-Asp506) and C41 at the 3′ end of the aptamer, but lacked residues Pro703-Thr705 at the C-terminus of the VWF A1 domain. Aside from these differences, which are due to differences in packing between the two crystal environments, both structures are very similar with a RMS deviation of 1.461 Å(normalized RMS deviation (Carugo and Pongor, 2001) of 0.713 Å) for the nucleotides 1-40 (Figure S4). Neither structure had density for the 3′-terminal inverted T, suggesting it is disordered.
Table 1. X-Ray Diffraction Data and Model Refinement Statistics.
| Structure | I (3HXO) | II (3HXQ) |
|---|---|---|
| Data Collection | ||
|
| ||
| Space group | P212121 | P21212 |
| Cell dimensions | ||
| a, b, c (Å) | 52.093, 66.354, 108.749 | 117.781, 48.512, 62.160 |
| Resolution (Å) | 30-2.40 (2.49–2.4)a | 38-2.69 (2.8–2.69) |
| Number of reflections | 14563 | 9819 |
| Completeness (%) | 94.9 (86.3) | 94.8 (83.9) |
| Data redundancy | 6.0 (2.6) | 6.4 (4.0) |
| Rmerge (%) | 6.4 (31.3) | 8.0 (31.6) |
| I/σ | 20.2 (2.6) | 17.2 (2.8) |
|
| ||
| Refinement | ||
|
| ||
| Resolution range (Å) | 25-2.40 (2.59–2.40) | 38-2.69 (3.08–2.69) |
| R-work (%)b | 22.82 (35.90) | 22.06 (29.35) |
| R-free (%)b | 27.37 (37.66) | 27.88 (35.27) |
| No. non-hydrogen atoms | 2464 | 2451 |
| No. water molecules | 177 | 102 |
| Ramachandran plot (% residues)c | ||
| Most favored/additional | 86.1/12.8 | 83.2/16.3 |
| Generous/disallowed | 1.1/0 | 0.5/0 |
| Rms deviations | ||
| Bond lengths (Å) | 0.002 | 0.003 |
| Bond angles (deg) | 0.714 | 0.725 |
| Estimated coordinate error (Å)d | 0.34 | 0.44 |
Numbers in parentheses are for the highest resolution shell;
Phenix;
Procheck (Laskowski et al., 1993);
Maximum-likelihood based error calculated by Phenix.
The VWF A1 domain bound to ARC1172 has essentially the same structure (Figure 1B and Figure S5) as observed previously for the A1 domain in isolation (Emsley et al., 1998) or bound to GPIbα (Dumas et al., 2004; Huizinga et al., 2002), botrocetin (Fukuda et al., 2002), bitiscetin (Maita et al., 2003), or Fab NMC-4 (Celikel et al., 1998), which consists of a central 6-strand β-sheet core (anti-parallel strand β3 and parallel strands β1,2 and 4-6) encompassed by 7 peripheral α-helixes (helix α1-7) (Figure 1B and Figure S5). Therefore, aptamer binding doesn't substantially alter the conformation of the VWF A1 domain.
ARC1172 consists of three helical duplex stems and two hairpin loops (Figures 1B and 2). Nucleotides C32-C41 interact with segments G23-G26 and G1-G6 to form a B-type helix interrupted by a stem-loop comprised of residues C7-T22. Stem I consists of standard Watson-Crick base pairs between the 5′ and 3′ ends of the aptamer. Stem III consists of 4 base pairs between segments G23-G26 and C32-T35, a mismatched base pair (T27 and T31), and a single-residue GCC loop. (Chin and Chou, 2003) Stem II is another B-type helix consisting of 5 base pairs between segments A8-C13 and G17-G21, with T10 exposed as a single residue bulge. The proximal end of Stem II abuts a mismatched internal loop comprised of C7 and T22. In structure I, the distal end of Stem II is capped by a typical pyrimidine-rich tri-loop (T14-C16) in which T15 stacks with C13 and the base of T14 inserts into the minor groove of the adjacent stem to interact with the deoxyribose of C13. (Chou et al., 2003) This tri-loop adopts a different conformation in structure II because of close crystal contacts, so that T14 is some distance away from the minor groove and T15 cannot stack with C13 (Figures S2 and S3).
Figure 2. Diagram showing the base pairing of ARC1172 and interactions with VWF domain A1.
Watson-Crick and non-canonical base pairs are indicated by adjacent labeled boxes. Nucleotides in yellow are within 4.2 Å of VWF A1. Solid black lines show hydrogen bonds (Figure S6 and Table S1); solid red lines indicate salt bridges (Table S2); dashed lines indicate van der Waals interactions.
The aptamer contains just three non-canonical base pairs (Figure 1C). At the proximal end of Stem II, A8 and G21 form a sheared G•A pair (Chou et al., 2003) in which G21 is stabilized by stacking interactions with adjacent C20 and cross-strand stacking with C7, and A8 is stabilized by stacking with the adjacent G9. At the base of Stem III, G23 and T35 form a G•T reverse wobble base pair in which the flipped G23 (syn) locally distorts the B-DNA structure to accommodate the extrusion of Stem II. In addition, the distal GCC loop of Stem III is closed by an unusual sheared-type G(anti)•C(anti) base pair between G28 and C30. A sheared G(anti)•C(syn) base pair can close a single nucleotide GXC loop at the end of a DNA duplex, and the rare syn cytidine conformation is necessary to preserve the geometry of the adjacent Watson-Crick base pair. (Chin and Chou, 2003) In the case of aptamer ARC1172, the sheared G28(anti)•C30(anti) arrangement is possible only because the adjacent nucleotides (T27 and T31) are not paired (Figure 2). Allowing C30 to retain an anti conformation requires T31 to rotate away from the helical axis, where it is stabilized by base stacking with T22. T27 remains in a standard Watson-Crick-like position, stacked between G26 and G28.
Throughout the aptamer all but 9 nucleotides participate in base pairing (Figures 1B and 2), and 6 of the unpaired residues are stabilized by base-base or base-deoxyribose stacking interactions discussed above. Among the remaining three unpaired bases, C29 and T10 make extensive contacts with the A1 domain. Only C16 is fully exposed to solvent, which may allow considerable mobility and explain the poor density for this base in structure ll.
The Aptamer-Protein Interface
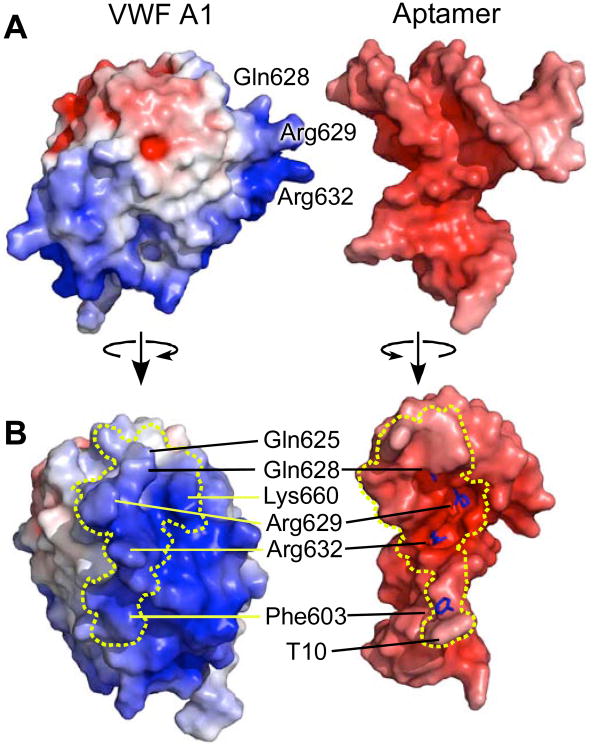
The extensive interface between ARC1172 and the VWF A1 domain buries a solvent accessible surface area of 911 Å2 on the aptamer and 1166 Å2 on the protein (Figure 3). The convex interface on the A1 surface binds to a concave pocket on the aptamer with striking shape and charge complementarity (Figure 3). In structure I, the interface of ARC1172 involves sixteen bases, 14 of which make a total of 24 H-bonds (Figure S6 and Table S1) and 4 salt bridges (Table S2) with the A1 domain. Two unpaired bases that do not participate in base-base stacking interactions are stabilized by contacts with the A1 domain. T10 extends away from Stem II and stacks with the aromatic ring of Phe603 (Baker and Grant, 2007). C29 protrudes from the GCC loop at the end of Stem III and interacts with seven amino acid residues (Gln625, Pro655, His656, Ala657, Asn658, Leu659, and Lys660) (Figure 2).
Figure 3. Interface between ARC1172 and VWF A1 domain.
(A) Surfaces of VWF A1 (left) and aptamer (right), separated for visualization and colored by electrostatic potential red (negative) to blue (positive) that are calculated with APBS (Baker et al., 2001). (B) Contacting surfaces of VWF A1 (left) and the aptamer (right) separated by less than 4.2 Å are boxed by dashed lines. Stick representations (right) show the locations of the side chains of Gln628, Arg629, Arg632, and Phe603, which play major roles within the interface. Compared to the views in panel (A), VWF A1 (left) and ARC1172 (right) were rotated approximately 90° around the vertical axis so that the binding interfaces face the viewer.
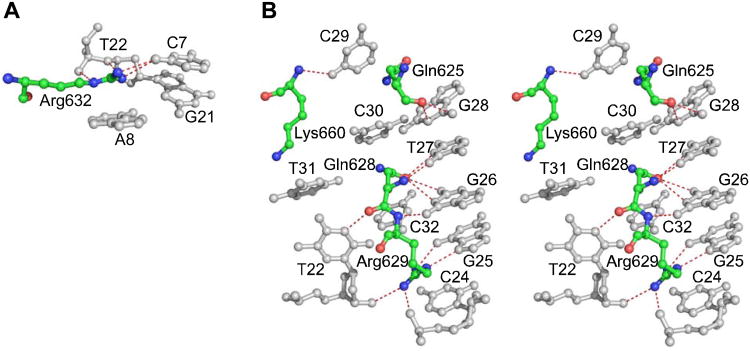
Specific protein-DNA recognition often depends on cation-π interactions between nucleic acid bases and amino acid residues with full or partial positive charge (Arg, Lys, Asn, Gln) (Rooman et al., 2002; Wintjens et al., 2000), as well as π-π interactions for Arg residues (Mao et al., 2003), and the aptamer-A1 interface contains a remarkable cluster of Arg and Gln residues that extend like fingers into deep cavities in the aptamer (Figure 3). The guanidinium group of Arg632 stacks above A8 (Figure 4A), where it is stabilized by H-bonds with C7 at the base of Stem II; this arrangement comprises a typical cation-π/H-bond stair motif (Rooman et al., 2002). Immediately adjacent to this π-stair motif, Stem III displays four additional cation-π interactions (Figure 4B). Arg629 stacks above C24 and H-bonds to G25 in another π-stair motif. Gln628 is sandwiched between C30 and C32 in the position of the normal Watson-Crick partner for T27, to which it makes 2 H-bonds. Gln625 is sandwiched between G28 and C29. In structure I, Lys660 participates in a cation-π interaction with T31 and is also stabilized by a salt bridge with the phosphate of T31; however, crystal packing interactions in structure II displace the side chain of Lys660 so that it forms a salt bridge with a symmetry-related aptamer and does not stack with T31. The amino acid residues involved in these various cation-π interactions account for 65% of the binding interface on the A1 domain, which is consistent with the importance of this extensive network to the strong aptamer-A1 interaction (Kd approximately 2 nM) (Diener et al., 2009).
Figure 4. Cation-π interactions between VWF A1 and ARC1172.
(A) Cation-π/H-bond stair motif formed by Arg632 (red/green/blue) and adjacent nucleotides (gray). (B) Stereo view showing cation-π interactions involving Gln625, Gln628, Arg629, and Lys660 and adjacent nucleotides shown. Atoms of amino acid residues and nucleotides that are within hydrogen bonding distance are joined by dashed lines (red).
It is worth noting that the nucleotides of ARC1172 that contact the A1 domain (C7-T10 and G21-C32) have a significantly lower normalized RMS deviation of 0.497 Å between the two structures compared to 0.991 Å for other nucleotides (Figure S4). This difference suggests that specific interaction with the VWF A1 domain stabilizes the binding pocket of the aptamer.
The nucleotides within the binding interface correspond closely to the consensus nucleic acid sequence required for high-affinity binding to VWF generated from SELEX (Diener and Lagassé, 2006). In addition, the nucleotides in the binding interface do not tolerate replacement by other nucleotides and most do not allow 2′-O-methyl modification without a decrease in binding (Figure 1A).
Structural Basis for the antithrombotic activity of ARC1172
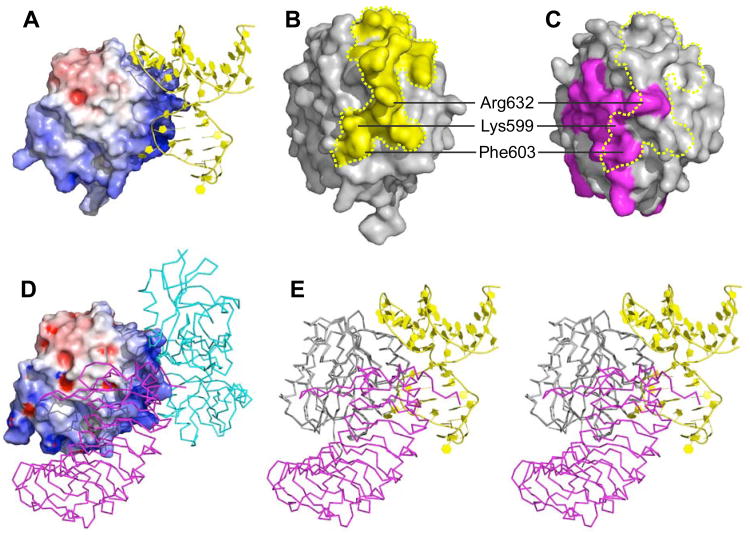
Thrombus formation is initiated by the binding of VWF A1 domain to platelet glycoprotein GPIbα. The concave face of the GPIbα amino-terminal domain wraps around the convex A1 surface (Huizinga et al., 2002), but ARC1172 binding overlaps with the site occupied by GPIbα and sterically excludes it (Figure 5). For example, the thymidine base of T10 projects toward A1 helix α3 to obstruct residues Lys599 and Phe603, and a deep cavity on the aptamer surrounds the side chain of Arg632 (Figure 3B); these amino acid residues contribute to the A1-GPIbα interface (Huizinga et al., 2002). In addition, aptamer residues G9-G11 are predicted to clash with GPIbα residues Asp175, Ser195-Pro198, and Glu225 (Figure 5E and Table S3) (Huizinga et al., 2002). Interference with A1-GPIbα binding accounts for the ability of ARC1172 (or ARC1779) to block VWF-dependent platelet aggregation in vitro and in vivo (Diener et al., 2009; Dienerand Lagassé, 2006).
Figure 5. Structural basis for the antithrombotic activity of ARC1172.
(A) ARC1172-A1 complex (PDB code: 3XHO) with the surface of A1 domain colored according to negative (red) and positive (blue) electrostatic potential. (B) The surface of VWF A1 (Phe507-Thr705) within 4.2 Å of ARC1172 is colored yellow.(C) The surface of VWF A1 (PDB code: 1M10) in contact with GPIα is colored magenta. The yellow dashed line encompasses the surface that is in contact with ARC1172 as in panel B. (D) Surface of VWF A1 domain (Asp498-Thr705) in complex with botrocetin (cyan) and GPIbα (magenta) (PDB ID: 1U0N) showing that botrocetin and ARC1172 occupy similar sites. The surfaces of A1 domain in (A) and (D) are slightly different due to small differences in side chain positions and also to the different length and orientation of the N-terminal residues at the bottom of the structures. (E) Stereo view of VWF A1 complexed with ARC1172 (yellow cartoon) and GPIbα (magenta, PDB ID: 1M10) would clash upon binding to VWF A1 domain. The clashing residues are listed in Table S3. The A1 domain has the same orientation in panels A, D, and E, and is turned approximately 90° around the vertical axis in panels B and C.
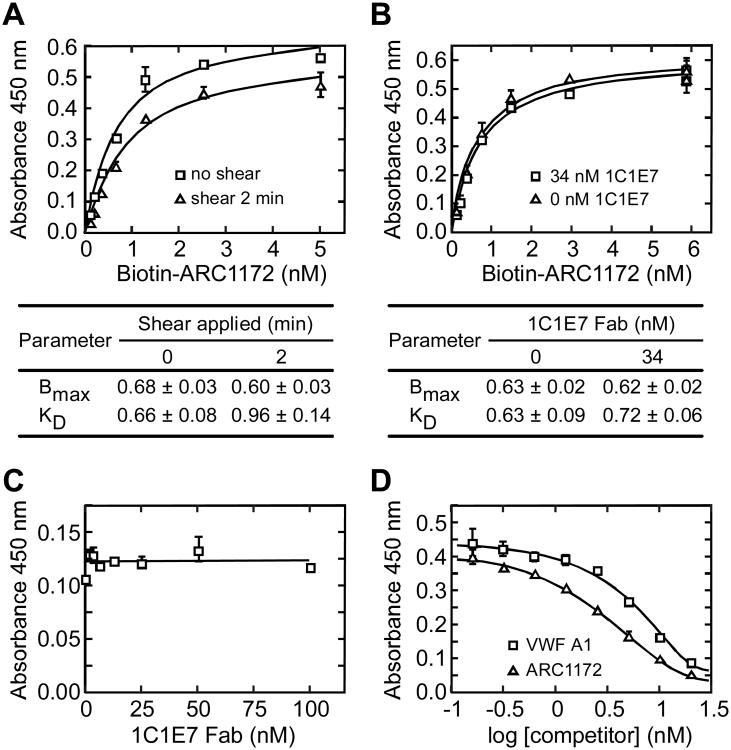
Botrocetin is a snake venom protein that forms a high-affinity ternary complex with VWF domain A1 and platelet GPIbα (Andrews et al., 1989; Fukuda et al., 2005), and thereby promotes VWF-dependent platelet aggregation. ARC1172 and botrocetin occupy a similar binding surface on the VWF A1 domain (Figures 5A and 5D) (Fukuda et al., 2002), which explains the inhibition of botrocetin-induced platelet aggregation by ARC1172 (Diener et al., 2009). Botrocetin binds the VWF A1 domain with a dissociation constant (Kd) of 12 nM (Andrews et al., 1989), and ARC1172 binds with a Kd of approximately 0.6 nM (Figure 6A).
Figure 6. Analysis of VWF A1-ARC1172 binding.
Microtiter plates were coated with rabbit anti-VWF (A,B,C) or monoclonal anti-VWF CK 62-12 (D) as described under “Experimental Procedures”. Error bars represent SE. (A) Shear stress does not affect ARC1172-VWF binding. VWF (5 μg/mL) and biotin-ARC1172 (5, 2.5, 1.25, 0.625, 0.3125, 0.155, 0.0775 nM) were mixed and treated without (squares) or with (triangles) shear stress for 2 min (Zhang et al., 2007) before assessment of binding. Values ± SD for maximal binding (Bmax) and KD are tabulated. (B) Binding of biotin-ARC1172 (2.5 nM) to VWF (5 μg/mL) was similar without (triangles) or with (squares) monoclonal antibody 1C1E7 Fab (34 nM), which binds VWF domain D3 and enhances VWF binding to GPIbα with an EC50 of ∼40 nM (Ulrichts et al., 2006). Values ± SD for Bmax and KD are tabulated. (C) 1C1E7 Fab did not inhibit biotin-ARC1172 (2 nM) binding to VWF (0.5 μg/mL). (D) Recombinant VWF A1 (squares) or unbiotinylated ARC1172 (triangles) inhibited the binding of biotin-ARC1172 (1 nM) to VWF (5 μg/mL).
The binding of VWF to platelet GPIbα is potentiated dramatically by fluid shear stress, which appears to shift the VWF A1 domain from a low affinity to a high affinity conformation. Monoclonal antibody 1C1E7 has a similar effect. The epitope of 1C1E7 is within the D′D3 region of VWF, just N-terminal to the A1 domain. 1C1E7 markedly increases the binding of VWF to platelets, apparently by disrupting an inhibitory intramolecular interaction between domains D′D3 and A1 (Ulrichts et al., 2006). In contrast, ARC1172 binds equally well to VWF with or without fluid shear stress (Figure 6A), and with or without antibody 1C1E7 (Figure 6B and C). As expected, ARC1172 binding to VWF is inhibited by soluble recombinant VWF A1 domain or by excess ARC1172 (Figure 6D). These results indicate that the ARC1172 binding site should be fully accessible in circulating plasma VWF.
Discussion
We have little information about how DNA or RNA aptamers adapt structurally to bind proteins that do not physiologically recognize nucleic acids. Only two previous reports describe relevant crystal structures and both involve the same protein, thrombin. In one case, a small palindromic 15-nucleotide DNA aptamer d(GGTTGGTGTGGTTGG), C15-mer (Bock et al., 1992), was bound to thrombin exosite 1, a positively charged surface that is required for the recognition of several natural thrombin substrates. In part because of the extreme local symmetry of this G-quartet arrangement, the precise structure of the thrombin-C15-mer complex was difficult to determine, requiring a combination of NMR and X-ray crystallographic approaches (Padmanabhan and Tulinsky, 1996). The C15-mer aptamer folds into a pair of stacked planar G-quartets connected by two TT loops at the ends and a TGT loop in the center. This arrangement is very different from the predominantly helical structure of ARC1172. Nevertheless, several interactions between C15-mer and thrombin (Figure S7) are similar to the cation-π and aromatic interactions between ARC1172 and VWF A1. For example, thrombin Arg75 stacks with G11 and its guanidinium group makes an H-bond to O4 of T13. Also, thrombin Arg77A stacks with G14 and makes an H-bond to O2 of T13. Both of these interactions constitute cation- π/H-bond stair motifs (Rooman et al., 2002; Wintjens et al., 2000), not remarked previously in descriptions of the thrombin-C15-mer structure. In addition, His71 stacks edgewise with T12, and Tyr76 stacks parallel to T3; both are similar to aromatic interactions in physiological protein-nucleic acid complexes (Baker and Grant, 2007).
Thrombin has another electropositive surface that interacts with heparin, termed exosite 2, and recently a structure was reported for a complex between thrombin and an RNA aptamer, Toggle-25t, selected to bind to exosite 2 (Long et al., 2008). The Toggle-25t-thrombin interface is dominated by cation-π interactions involving two Arg residues: Arg165 stacks with A15, and Arg 233 stacks between A15 and A7. In addition, the guanidinium moiety of Arg233 makes an H-bond to O6 of G16, forming a typical cation-π/H-bond stair motif. These interactions account for the majority of the Toggle-25t-thrombin interacting surface (Long et al., 2008).
Thus, despite the substantial differences in composition and structure of the C15-mer-thrombin, Toggle-25t-thrombin, and ARC1172-VWF A1 aptamer-protein complexes, they all exhibit clusters of cation-π interactions that include π-stair motifs stabilized by H-bonds to adjacent bases. Clustered cation-π and π-stair motifs also are common in naturally occurring protein-nucleic acid complexes (Rooman et al., 2002; Sathyapriya et al., 2008; Sathyapriya and Vishveshwara, 2004; Wintjens et al., 2000), and seem particularly suitable for the construction of highly complementary, interdigitated interfaces that bury relatively large surface areas. Although Gln and Asn residues can participate in cation-π interactions, positively charged Arg and Lys are much more frequent (Rooman et al., 2002; Wintjens et al., 2000). Consequently, aptamers selected in vitro may generally prefer electropositive protein surfaces, especially those with clustered basic residues. A selection method to bias against these interactions may be required to identify aptamers that bind to other sites.
In the ARC1172-A1 structure, nucleotides interacting directly with protein are confined to Stem III and the proximal segment of Stem II (Figure 2), and most of these nucleotides do not tolerate substitution or modification without loss of binding affinity. In contrast, almost all nucleotides outside these critical regions can be replaced provided that any base pairing is preserved (Diener and Lagassé, 2006). For example, ARC1779 was derived from ARC1172 by introducing 2′-methoxy-ribose modifications at 26 positions, almost all in Stem I and distal Stem II. Additional modifications included deletion of the terminal base pair from Stem I, introduction of a phosphorothioate linkage between G21 and T22, and conjugation of 20 kDa polyethylene glycol to the 5′-G residue (Figure S1). These differences do not directly alter any atoms that contact VWF A1 (Figure 2 and Table S1), and both ARC1779 and ARC1172 bind to VWF A1 with similar affinity (Lagasse et al., 2007).
Currently available antithrombotic agents that inhibit blood clotting (e.g., warfarin, heparin derivatives) or platelet function (e.g., clopidogrel, aspirin, abciximab), also significantly increase the risk of bleeding during treatment. Agents that target the interaction of VWF with GPIbα have not been tested extensively in humans, but may differ in the severity or type of bleeding associated with their use. For example, markedly decreased VWF function, as occurs in von Willebrand disease, is characterized by skin bruising, oral and nasal bleeding, menorrhagia, and sometimes by gastrointestinal bleeding. Bleeding into muscle, brain, or other organs is relatively uncommon (Sadler, 1998). VWF-dependent platelet adhesion is especially important under conditions of high fluid shear stress that occur normally in arterioles, or pathologically in arteries with stenotic lesions caused by atherosclerosis. Therefore, a VWF antagonist might inhibit thrombosis in a high shear subset of the arterial circulation, leaving hemostasis relatively intact in the venous circulation where other mechanisms are dominant. In fact, preclinical animal studies show that several agents able to block VWF-dependent platelet adhesion, by inhibiting VWF or platelet GPIb, can inhibit arterial thrombosis with minimal prolongation of skin bleeding times (De Meyer et al., 2008). Similar results have been reported for ARC1779 in a Cynomolgus macaque model of carotid artery thrombosis (Diener et al., 2009; Keefe and Schaub, 2008).
Whether the apparent selectivity of ARC1779 will have clinical utility in humans remains to be established, but preliminary results seem encouraging. When added to human blood, ARC1779 does not inhibit platelet aggregation induced by ADP, collagen, or arachadonic acid at concentrations (e.g., 10 μg/ml) that completely bock VWF-dependent platelet function (Spiel et al., 2009). A phase 1 trial in healthy volunteers (Gilbert et al., 2007) suggests that ARC1779 is relatively safe for short-term administration and does not cause excessive bleeding at concentrations that block platelet aggregation ex vivo. A recent case report describes one patient with refractory thrombotic thrombocytopenic purpura who received ARC1779 over a period of three weeks, apparently with a reproducible rise in the platelet count (Knobl et al., 2009). Ongoing and planned phase 2 trials should determine whether ARC1779 is safe and efficacious for treating thrombotic thrombocytopenic purpura (NCT00632242, NCT00726544) or for preventing thromboembolism associated with carotid endarterectomy (NCT00742612).
Experimental Procedures
Purification, Crystallization and Data Collection
The VWF A1 domain (VWF amino acid residues Glu497-Thr705, mature subunit numbering) was expressed in E. coli, refolded and purified as described previously (Maita et al., 2003) with minor modifications. Briefly, refolded A1 was adsorbed onto a 5 ml HiTrap-heparin column (GE Healthcare Life Sciences, Piscataway, NJ) in 10 mM Tris-HCl, pH7.4, 150 mM NaCl (TBS) and eluted with a 60 ml linear gradient of 150-500 mM NaCl in TBS. Late eluting fractions containing A1 were pooled and concentrated by ultrafiltration. Aggregates were removed by chromatography on a column of Superdex-200 (16/60, GE Healthcare Life Sciences) equilibrated with TBS. Aptamer ARC1172 was chemically synthesized (IDT DNA Tech., Coralville, USA) and dialyzed against 20 mM HEPES pH7.4, 150 mM NaCl. The A1 protein and aptamer ARC1172 were mixed at a molar ratio of 1: 1.4.
Crystals were obtained with the hanging drop vapor diffusion technique by mixing 1 μL of A1-ARC1172 solution with 1 μL of reservoir solution at 20 degrees. Two different crystal forms were grown in reservoir solution I (25% PEG3350, 0.16 M NaF) and solution II (containing 0.2 M NH4Ac, 0.1 M NaAc pH4.6, 30% PEG4000) and belong to space group P212121 (structure I) and P21212 (structure II), respectively.
The crystals were flash-cooled by plunging into liquid nitrogen after soaking in cryoprotectant of 20% glycerol in corresponding handing drop solution for 1 minute. The diffraction data from a single crystal were then collected at the Advanced Photo Source (APS) using 0.9Å wavelength x-rays collected on an ADSL detector or an in-house diffractometer with a R-AXIS IV imaging plate detector (Rigaku) and processed using HKL2000 software (Otwinowski and Minor, 1997).
Structural calculation and refinement
Molecular replacement was performed with Molrep (Vagin and Teplyakov, 1997) to determine the initial phases using the coordinates of VWF A1 domain at a resolution of 1.8 Å (PDB ID number 1IJB) and generates traceable electronic density for the aptamer in structure I (space group P212121). Structure II (space group P21212) was then solved using the newly generated coordinates for structure I. Models were built using COOT (Emsley and Cowtan, 2004) and refined in Refmac (Murshudov et al., 1997) and Phenix (Zwart et al., 2008) with TLS parameters (Winn et al., 2001). The structures were analyzed using Nucplot (Luscombe et al., 1997), MolProbity (Davis et al., 2007), and CCP4 (1994). A summary of diffraction data and refinement statistics is shown in Table 1. Molecular graphics figures were prepared with PyMOL (DeLano Scientific, Palo Alto, CA).
VWF A1-ARC1172 binding assay
Biotin-ARC1172 was synthesized with a biotin moiety at the 5′-end. Microtiter plates were coated with 100 μL of anti-VWF polyclonal antibodies (Dako 082, 3.1 μg/mL) or anti-VWF CK monoclonal antibody (CK62-12, 13 μg/mL) or directly coated with VWF (Haematologic Technologies Inc. Essex junction, VT, USA). Plates were blocked with 200 μL T-PBS (0.1% Tween 20 in phosphate buffered saline) containing 3% bovine serum albumin, and washed three times with T-PBS. Solutions of VWF and biotin-ARC1172 with any other indicated additions were incubated in microtiter wells at room temperature for 30 min. The plates were washed three times with T-PBS and incubated with 100 μL/well of HRP-conjugated streptavidin (1:2500) in T-PBS for 15 or 30 min. Unbound streptavidin was removed by washing three times with T-PBS and the bound HRP-streptavidin signals was detected by incubation with 100 μL/well of freshly made TMB (Pierce). Reactions were stopped with 2 M H2SO4 before reading absorbance at 450 nm.
In some cases, mixtures of VWF and biotin-ARC1172 were subjected to fluid shear stress before addition to microtiter plates coated with anti-VWF. Shear stress was applied to reactions essentially as described (Zhang et al., 2007). Briefly, reactions were incubated at room temperature on a bench-top vortex device (Vortex-Genie 2, Scientific Industries, Inc., Bohemia, NY) at maximal speed (3,200 rpm) for the indicated time.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Health Grants HL72917 (to J.E.S.), AI051426 and GM62414 (to D.H.F.) and by American Heart Association Midwest Affiliate Postdoctoral Fellowship Award 0825817G (to R.-H.H.).
Footnotes
Accession Numbers: Coordinates and structure factors for both crystal forms of VWF A1-ARC1172 have been deposited in the RCSB Protein Data Bank with accession codes 3HXQ and 3HXO.
Supplemental Data: Supplemental Data include seven figures and three tables.
Author contributions: R.-H.H. and J.L.D. contributed to performing experiments; R.-H.H. and D.H.F. performed crystallization and structure determination; R.-H.H., R.G.S. and J.E.S. designed research; R.-H.H. and J.E.S. wrote the manuscript. All authors made comments on the manuscript.
Competing financial interest disclosure: J.L.D. was employed and R.G.S is employed by Archemix Corp. J.E.S. is a consultant for Baxter Innovations and Ablynx, and is a member of clinical advisory boards for Baxter Innovations.
References
- Andrews RK, Gorman JJ, Booth WJ, Corino GL, Castaldi PA, Berndt MC. Cross-linking of a monomeric 39/34-kDa dispase fragment of von Willebrand factor (Leu-480/Val-481-Gly-718) to the N-terminal region of the α - chain of membrane glycoprotein Ib on intact platelets with bis(sulfosuccinimidyl) suberate. Biochemistry. 1989;28:8326, 8336. doi: 10.1021/bi00447a010. [DOI] [PubMed] [Google Scholar]
- Baker CM, Grant GH. Role of aromatic amino acids in protein-nucleic acid recognition. Biopolymers. 2007;85:456–470. doi: 10.1002/bip.20682. [DOI] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992;355:564–566. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- Carugo O, Pongor S. A normalized root-mean-square distance for comparing protein three-dimensional structures. Protein Sci. 2001;10:1470–1473. doi: 10.1110/ps.690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikel R, Varughese KI, Madhusudan, Yoshioka A, Ware J, Ruggeri ZM. Crystal structure of the von Willebrand factor A1 domain in complex with the function blocking NMC-4 Fab. Nat Struct Biol. 1998;5:189–194. doi: 10.1038/nsb0398-189. [DOI] [PubMed] [Google Scholar]
- Chin KH, Chou SH. Sheared-type Ganti•Csyn base-pair: a unique d(GXC) loop closure motif. J Mol Biol. 2003;329:351–361. doi: 10.1016/s0022-2836(03)00440-6. [DOI] [PubMed] [Google Scholar]
- Chou SH, Chin KH, Wang AH. Unusual DNA duplex and hairpin motifs. Nucleic Acids Res. 2003;31:2461–2474. doi: 10.1093/nar/gkg367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, 3rd, Snoeyink J, Richardson JS, Richardson DC. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer SF, Vanhoorelbeke K, Broos K, Salles II, Deckmyn H. Antiplatelet drugs. Br J Haematol. 2008;142:515–528. doi: 10.1111/j.1365-2141.2008.07233.x. [DOI] [PubMed] [Google Scholar]
- Diener J, Daniel Lagasse HA, Duerschmied D, Merhi Y, Tanguay JF, Hutabarat R, Gilbert J, Wagner DD, Schaub R. Inhibition of von Willebrand Factor-mediated platelet activation and thrombosis by Anti-von Willebrand Factor A1-domain aptamer ARC1779. J Thromb Haemost. 2009 doi: 10.1111/j.1538-7836.2009.03459.x. Prepublished April 25, 2009. doi: 2010.1111/j.1538-7836.2009.03459.x. [DOI] [PubMed] [Google Scholar]
- Diener JL, Lagassé DHA. Aptamers to von Wlllebrand factor and their use as thrombotic disease therapeutics. Archemix Corp; Cambridge, MA: 2006. PCT/US2005/032134. [Google Scholar]
- Dumas JJ, Kumar R, McDonagh T, Sullivan F, Stahl ML, Somers WS, Mosyak L. Crystal structure of the wild-type von Willebrand factor A1- glycoprotein Ib α complex reveals conformation differences with a complex bearing von Willebrand disease mutations. J Biol Chem. 2004;279:23327–23334. doi: 10.1074/jbc.M401659200. [DOI] [PubMed] [Google Scholar]
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Emsley J, Cruz M, Handin R, Liddington R. Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J Biol Chem. 1998;273:10396–10401. doi: 10.1074/jbc.273.17.10396. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Doggett T, Laurenzi IJ, Liddington RC, Diacovo TG. The snake venom protein botrocetin acts as a biological brace to promote dysfunctional platelet aggregation. Nat Struct Mol Biol. 2005;12:152–159. doi: 10.1038/nsmb892. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Doggett TA, Bankston LA, Cruz MA, Diacovo TG, Liddington RC. Structural basis of von Willebrand factor activation by the snake toxin botrocetin. Structure. 2002;10:943–950. doi: 10.1016/s0969-2126(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Gilbert JC, DeFeo-Fraulini T, Hutabarat RM, Horvath CJ, Merlino PG, Marsh HN, Healy JM, Boufakhreddine S, Holohan TV, Schaub RG. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation. 2007;116:2678–2686. doi: 10.1161/CIRCULATIONAHA.107.724864. [DOI] [PubMed] [Google Scholar]
- Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ib α and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- Keefe AD, Schaub RG. Aptamers as candidate therapeutics for cardiovascular indications. Curr Opin Pharmacol. 2008;8:147–152. doi: 10.1016/j.coph.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Knobl P, Jilma B, Gilbert JC, Hutabarat RM, Wagner PG, Jilma-Stohlawetz P. Anti-von Willebrand factor aptamer ARC1779 for refractory thrombotic thrombocytopenic purpura. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02232.x. [DOI] [PubMed] [Google Scholar]
- Lagasse HAD, Merlino PG, Marsh HN, Makim A, Lewis SD, Diener JL. Discovery and characterization of a potent neutralizing anti-VWF A1 domain specific aptamer. J Thromb Haemost. 2007;5:P-S-665. [Google Scholar]
- Laskowski RA, Macarthur MW, Moss DS, Thornton JM. Procheck - a Program to Check the Stereochemical Quality of Protein Structures. J Appl Crystallog. 1993;26:283–291. [Google Scholar]
- Long SB, Long MB, White RR, Sullenger BA. Crystal structure of an RNA aptamer bound to thrombin. RNA. 2008;14:2504–2512. doi: 10.1261/rna.1239308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe NM, Laskowski RA, Thornton JM. NUCPLOT: a program to generate schematic diagrams of protein-nucleic acid interactions. Nucleic Acids Res. 1997;25:4940–4945. doi: 10.1093/nar/25.24.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maita N, Nishio K, Nishimoto E, Matsui T, Shikamoto Y, Morita T, Sadler JE, Mizuno H. Crystal structure of von Willebrand factor A1 domain complexed with snake venom, bitiscetin: insight into glycoprotein Ib α binding mechanism induced by snake venom proteins. J Biol Chem. 2003;278:37777–37781. doi: 10.1074/jbc.M305566200. [DOI] [PubMed] [Google Scholar]
- Mao L, Wang Y, Liu Y, Hu X. Multiple intermolecular interaction modes of positively charged residues with adenine in ATP-binding proteins. J Am Chem Soc. 2003;125:14216–14217. doi: 10.1021/ja036096p. [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- Oney S, Nimjee SM, Layzer J, Que-Gewirth N, Ginsburg D, Becker RC, Arepally G, Sullenger BA. Antidote-controlled platelet inhibition targeting von Willebrand factor with aptamers. Oligonucleotides. 2007;17:265–274. doi: 10.1089/oli.2007.0089. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K, Tulinsky A. An ambiguous structure of a DNA 15-mer thrombin complex. Acta Crystallogr D Biol Crystallogr. 1996;52:272–282. doi: 10.1107/S0907444995013977. [DOI] [PubMed] [Google Scholar]
- Rooman M, Lievin J, Buisine E, Wintjens R. Cation- π/H-bond stair motifs at protein-DNA interfaces. J Mol Biol. 2002;319:67–76. doi: 10.1016/s0022-2836(02)00263-2. [DOI] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Sathyapriya R, Vijayabaskar MS, Vishveshwara S. Insights into protein-DNA interactions through structure network analysis. PLoS Comput Biol. 2008;4:e1000170. doi: 10.1371/journal.pcbi.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyapriya R, Vishveshwara S. Interaction of DNA with clusters of amino acids in proteins. Nucleic Acids Res. 2004;32:4109–4118. doi: 10.1093/nar/gkh733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiel AO, Mayr FB, Ladani N, Wagner PG, Schaub RG, Gilbert JC, Jilma B. The aptamer ARC1779 is a potent and specific inhibitor of von Willebrand Factor mediated ex vivo platelet function in acute myocardial infarction. Platelets. 2009;20:334–340. doi: 10.1080/09537100903085927. [DOI] [PubMed] [Google Scholar]
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Ulrichts H, Udvardy M, Lenting PJ, Pareyn I, Vandeputte N, Vanhoorelbeke K, Deckmyn H. Shielding of the A1 domain by the D′D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J Biol Chem. 2006;281:4699–4707. doi: 10.1074/jbc.M513314200. [DOI] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A. MOLREP: an Automated Program for Molecular Replacement. J Appl Crystallog. 1997;30:1022–1025. [Google Scholar]
- Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr D Biol Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- Wintjens R, Lievin J, Rooman M, Buisine E. Contribution of cation-π interactions to the stability of protein-DNA complexes. J Mol Biol. 2000;302:395–410. doi: 10.1006/jmbi.2000.4040. [DOI] [PubMed] [Google Scholar]
- Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL. The cooperative activity between the carboxyl-terminal TSP1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood. 2007;110:1887–1894. doi: 10.1182/blood-2007-04-083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart PH, Afonine PV, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, McKee E, Moriarty NW, Read RJ, Sacchettini JC, et al. Automated structure solution with the PHENIX suite. Methods Mol Biol. 2008;426:419–435. doi: 10.1007/978-1-60327-058-8_28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.