Abstract
Objective
To investigate qualitatively and quantitatively the performance of a programme for managing the child contacts of adult tuberculosis patients in Indonesia.
Methods
A public health evaluation framework was used to assess gaps in a child contact management programme at a lung clinic. Targets for programme performance indicators were derived from established programme indicator targets, the scientific literature and expert opinion. Compliance with tuberculosis screening, the initiation of isoniazid preventive therapy in children younger than 5 years, the accuracy of tuberculosis diagnosis and adherence to preventive therapy were assessed in 755 child contacts in two cohorts. In addition, 22 primary caregivers and 34 clinic staff were interviewed to evaluate knowledge and acceptance of child contact management. The cost to caregivers was recorded. Gaps between observed and target indicator values were quantified.
Findings
The gaps between observed and target performance indicators were: 82% for screening compliance; 64 to 100% for diagnostic accuracy, 50% for the initiation of preventive therapy, 54% for adherence to therapy and 50% for costs. Many staff did not have adequate knowledge of, or an appropriate attitude towards, child contact management, especially regarding isoniazid preventive therapy. Caregivers had good knowledge of screening but not of preventive therapy and had difficulty travelling to the clinic and paying costs.
Conclusion
The study identified widespread gaps in the performance of a child contact management system in Indonesia, all of which appear amenable to intervention. The public health evaluation framework used could be applied in other settings where child contact management is failing.
Résumé
Objectif
Mesurer qualitativement et quantitativement la performance d'un programme de gestion des enfants en contact avec des patients adultes atteints de tuberculose en Indonésie.
Méthodes
Un cadre d'évaluation de la santé publique a été utilisé pour évaluer les lacunes du programme de gestion des enfants en contact avec des patients atteints de tuberculose dans un service hospitalier de pneumologie. Les objectifs des indicateurs de performance du programme ont été déterminés à partir d'indicateurs cibles de programme établis, de la littérature scientifique et de l'opinion d'experts. Le respect du dépistage de la tuberculose, l'initiation du traitement préventif à l'isoniazide chez les enfants âgés de moins de cinq ans, la précision du diagnostic de tuberculose et l'adhésion au traitement préventif ont été évalués chez 755 enfants en contact avec des patients atteints de tuberculose, dans deux cohortes. En outre, 22 aidants familiaux primaires et 34 membres du personnel hospitalier ont été interrogés afin d'évaluer leurs connaissances et acceptation de la gestion des enfants en contact avec des patients atteints de tuberculose. Le coût pour les aidants familiaux a été noté et les écarts entre les valeurs observées et les valeurs des indicateurs cibles ont été quantifiés.
Résultat
Les écarts entre les indicateurs de performance observés et les indicateurs cibles ont été les suivants: 82% pour le respect du dépistage; 64 à 100% pour la précision du diagnostic, 50% pour l'initiation d'un traitement préventif, 54% pour l'adhésion au traitement et 50% pour les coûts. Plusieurs membres du personnel hospitalier ne possédaient pas les connaissances suffisantes ni l'attitude appropriée vis-à-vis de la gestion des enfants en contact avec des patients atteints de tuberculose, en particulier en ce qui concerne le traitement préventif à l'isoniazide. Les aidants familiaux ont montré avoir de bonnes connaissances du dépistage mais pas du traitement préventif et ils ont eu des difficultés pour se rendre à l’hôpital et payer les frais.
Conclusion
L'étude a identifié des lacunes généralisées au niveau de la performance d'un système de gestion des enfants en contact avec des patients atteints de tuberculose en Indonésie, toutes semblant se prêter à une intervention. Le cadre d'évaluation de la santé publique utilisé pourrait être appliqué dans d'autres contextes où la gestion des enfants en contact avec des patients malades a échoué.
Resumen
Objetivo
Investigar cualitativa y cuantitativamente el rendimiento de un programa para gestionar el contacto infantil de pacientes tuberculosos adultos en Indonesia.
Métodos
Se empleó un marco de valoración de la salud pública para evaluar las deficiencias de un programa de gestión del contacto infantil en una clínica de neumología. Los objetivos para los indicadores de rendimiento del programa se determinaron a partir de los objetivos establecidos en el programa de indicadores, la literatura científica y la opinión de especialistas. Se evaluó el cumplimiento de detección de la tuberculosis, la iniciación de la terapia preventiva con isoniazida en niños menores de 5 años, la exactitud de diagnóstico de la tuberculosis y la adherencia a la terapia preventiva en 755 niños en contacto en dos cohortes. Además, se entrevistó a 22 cuidadores principales y 34 trabajadores de la clínica para evaluar el conocimiento y la aceptación de la gestión del contacto infantil, se registró el coste para los cuidadores y se cuantificaron las diferencias entre los valores de los indicadores observados y los objetivos.
Resultados
Las diferencias entre los indicadores de rendimiento observados y objetivos fueron las siguientes: 82 % en el cumplimiento de detección, del 64 al 100 % en la exactitud de diagnóstico, 50 % en la iniciación de la terapia preventiva, 54 % en la adherencia a la terapia y 50 % en costes. Gran parte del personal no disponía de los conocimientos necesarios o la actitud adecuada para gestionar el contacto infantil, especialmente en relación con la terapia preventiva con isoniazida. Los cuidadores contaban con buenos conocimientos sobre detección, pero no sobre la terapia preventiva y tenían dificultades para trasladarse a la clínica y pagar los costes.
Conclusión
El estudio determinó deficiencias generalizadas en el rendimiento de un sistema de gestión del contacto infantil en Indonesia, las cuales parecen susceptibles de intervención. El marco de evaluación de la salud pública utilizado podría aplicarse en otros entornos en los que está fallando la gestión del contacto infantil.
ملخص
الغرض
إجراء تحر نوعي وكمي لأداء أحد البرامج من أجل التدبير العلاجي للبالغين من مخالطي الأطفال مرضى السل في إندونيسيا.
الطريقة
تم استخدام إطار تقييم الصحة العمومية لتقييم الثغرات في برنامج التدبير العلاجي لمخالطي الأطفال في عيادة الرئة. وتم استخلاص أهداف مؤشرات أداء البرنامج من أهداف مؤشرات البرنامج الثابتة والأبحاث العلمية المنشورة وآراء الخبراء. وتم تقييم الامتثال لتحري السل وبدء العلاج الوقائي بالإيزونيازيد لدى الأطفال الأصغر من 5 سنوات، ودقة تشخيص السل والتقيد بالعلاج الوقائي في 755 مخالطاً للأطفال في مجموعتين. بالإضافة إلى ذلك، تم إجراء مقابلات مع 22 مقدم رعاية أولية و34 عاملاً سريرياً لتقييم معرفة التدبير العلاجي لمخالطي الأطفال وقبوله. وتم تسجيل التكلفة التي يتحملها مقدمو الرعاية. وتم تحديد نوعية الثغرات بين قيم المؤشرات الملاحظة والمستهدفة.
النتائج
كانت الثغرات بين مؤشرات الأداء الملاحظة والمستهدفة كالتالي: 82 % لامتثال التحري؛ ومن 64 % إلى 100 % للدقة التشخيصية و50 % لبدء العلاج الوقائي و54 % للتقيد بالعلاج و50 % للتكاليف. ولم يتوفر لدى معظم الفريق معرفة كافية بالتدبير العلاجي لمخالطي الأطفال أو اتجاه ملائم صوبه، ولاسيما فيما يتعلق بالعلاج الوقائي بالإيزونيازيد. وتوفر لدى مقدمي الرعاية معرفة جيدة بالتحري دون العلاج الوقائي وعانوا من صعوبة في السفر إلى العيادة ودفع التكاليف.
الاستنتاج
حددت الدراسة ثغرات واسعة الانتشار في أداء نظام التدبير العلاجي لمخالطي الأطفال في إندونيسيا، وتبدو جميعها قابلة للعلاج عن طريق التدخل. ويمكن تطبيق إطار تقييم الصحة العمومية في البيئات الأخرى التي يفشل فيها التدبير العلاجي لمخالطي الأطفال.
摘要
目的
定性和定量调查印尼接触成人肺结核患者的儿童的管理计划的绩效。
方法
使用公共卫生评价框架评估肺病诊疗机构儿童接触管理计划的差距。从已确定的指标目标、科学文献和专家意见中提取计划绩效指标的目标。在两个队列的755 名儿童接触者中进行肺结核筛查符合性、儿童五岁以下开始初始异烟肼预防性治疗的情况、结核诊断准确性以及预防治疗的坚持情况进行了评估。此外,走访了22 名主要监护人和34 名诊疗机构工作人员,评价儿童接触管理的知识和接受度。记录监护人的成本。量化了观测和目标指标值之间的差距。
结果
观测和目标绩效指标的差距:筛查符合性:82%;诊断精确性:64到100%;初始预防性治疗:50%;坚持治疗情况:54%;成本:50%。很多工作人员在儿童接触管理上缺乏足够的知识或正确的态度,在异烟肼预防性治疗方面尤其如此。监护人有很好的筛查知识,但是预防性治疗知识不够,在前往诊疗机构和支付成本方面存在困难。
结论
研究确认了在印度尼西亚儿童接触管理系统绩效上普遍存在的差距,而所有这些看来可通过干预来改变。所使用的公共健康评估框架可在儿童接触管理不成功的其他地方加以应用。
Резюме
Цель
Провести качественное и количественное исследование эффективности программы по управлению контактами ребенка со взрослыми пациентами, больными туберкулезом, в Индонезии.
Методы
Для оценки недостатков программы управления контактами ребенка в легочной клинике была использована схема оценки, применяемая в системе здравоохранения. Целевые значения показателей выполнения программы были получены на основе установленных целевых показателей программы, научной литературы и мнений экспертов. Соблюдение правил скрининга туберкулеза, начало профилактической терапии изониазидом у детей младше 5 лет, точность диагностики туберкулеза и соблюдение правил профилактической терапии оценивалось в отношении 755 контактировавших детей в двух группах. Кроме того, был проведен опрос 22 основных опекунов и 34 сотрудников клиники для оценки знаний и принятия ребенком правил управления контактами. Кроме того, была учтена стоимость расходов опекунов. Также была подсчитана разница между наблюдаемыми и целевыми значениями показателей.
Результаты
Разница между наблюдаемыми и целевыми показателями эффективности составила: 82% для соблюдения правил скрининга; от 64% до 100% для диагностической точности, 50% для начала профилактической терапии, 54% для соблюдения правил терапии и 50% для затрат. Многие сотрудники не обладали достаточными знаниями в вопросах управления контактами детей или не проявляли должного отношения к данным вопросам, особенно в части профилактического лечения изониазидом. Опекуны проявили хорошее знание скрининга, но не превентивной терапии, и им затруднительно было ездить в клинику и оплачивать расходы.
Вывод
Исследование выявило широко распространенные недостатки в работе системы управления контактами детей в Индонезии, все из которых, похоже, поддаются устранению. Использованная схема оценки системы здравоохранения может применяться и в других условиях, когда не удается осуществить управление контактами ребенка.
Introduction
Current efforts to control childhood tuberculosis are failing, with over 100 000 children dying from the disease globally each year.1 Children under 5 years of age who are in contact with a patient with infectious tuberculosis are at an especially high risk of Mycobacterium tuberculosis infection and early progression to tuberculosis disease, which is characterized by the presence of symptoms.2 However, disease progression can be halted using preventive therapy, which has a reported efficacy of up to 93%.3 The World Health Organization (WHO) recommends that children who come into contact with an individual with infectious tuberculosis undergo child contact management, which includes screening for tuberculosis disease and, for those younger than 5 years, 6 months of isoniazid preventive therapy, even if disease is ruled out.4 This strategy can greatly reduce childhood tuberculosis, yet it is rarely practised in endemic settings.
Although earlier research has identified barriers to the success of child contact management programmes, a focus on single barriers has hindered the development of comprehensive programmes.5–9 Previously we presented a public health evaluation framework that involved situational, gap and options analyses and that could be used to identify problem areas and to develop appropriate multi-targeted solutions.10 In this paper we present the findings of the first two stages of a public health evaluation carried out using this framework in Bandung, West Java, Indonesia. Indonesia has the fifth highest tuberculosis case load in the world11 and reports indicate that a substantial proportion of tuberculosis patients in Java (i.e. 11 to 27%) are children.12 We hypothesized that there are widespread gaps between actual and ideal performance across a range of child contact management system parameters.
Methods
The study was conducted between April 2009 and February 2012 at a community lung clinic in Bandung that diagnoses approximately 1500 adults with pulmonary tuberculosis annually. Of these adults, 50% test positive on sputum smear analysis. The clinic has sputum smear and mycobacterial culture facilities, a pharmacy and a paediatric clinic. Screening of household contacts of sputum-smear-positive tuberculosis cases is encouraged but is not subsidized for children.
Data were obtained from administrative records, staff and adult tuberculosis patients and their households. Informed consent was gained from all participants. Ethical approval was given by the Lower South Regional Ethics Committee, New Zealand, and the ethics committee of the Medical Faculty, Padjadjaran University, Bandung.
Performance parameters and indicators
We developed a number of parameters for assessing the performance of the child contact management programme at the clinic, each of which was associated with a performance indicator (Table 1). The targets adopted for each indicator were derived from established programme indicator targets,13,17,18 the scientific literature14,15,19 and expert opinion.
Table 1. Performance of system for managing child contacts of tuberculosis patients, community lung clinic, Bandung, Indonesia, 2009–2012.
| Performance parameter | Indicator | Target performance,a % | Participants, n | Observed performance, a% (95% CI) | Gap between target and observed performance,a % |
|---|---|---|---|---|---|
| Screening compliance | Proportion of child contacts of tuberculosis patients who were eligible for tuberculosis disease screening and who returned to the clinic for screening | > 90b | 437 | 8 (5.5–10.5) | 82 |
| Initiation of isoniazid preventive therapy | Proportion of child contacts younger than 5 years who were eligible for isoniazid preventive therapy and who received therapy | > 90b | 15 | 40 (15.2–61.8) | 50 |
| Accuracy of tuberculosis disease diagnosis | Agreement between attending paediatrician and external paediatric tuberculosis expert on disease diagnosis | kappa >70c | 118 | kappa 6.0 (1.0–11.0) | 64 |
| Proportion of child contacts with a latent tuberculosis infection who were diagnosed with tuberculosis disease using the Indonesian Paediatric Scoring System | 0 | 41 | 100 (NA) | 100 | |
| Adherence to isoniazid preventive therapy | Proportion of children for whom three or more prescriptions were collected over a 6-month period | > 80b | 82 | 26 (16.5–35.5) | 54 |
| Primary caregivers’ knowledge | Proportion of caregivers with adequate knowledge of child tuberculosis disease screening | > 80 | 10 | 100 (NA) | 0 |
| Proportion of caregivers with adequate knowledge of isoniazid preventive therapy | > 80 | 10 | 10 (0–28.6) | 70 | |
| Primary caregivers’ acceptance | Primary caregivers’ acceptance of tuberculosis disease screening | No barriers identified | 10 | 4 barriers identified | 4 barriers |
| Primary caregivers’ acceptance of isoniazid preventive therapy | No barriers identified | 10 | 4 barriers identified | 4 barriers | |
| Medical staff’s knowledge | Proportion of staff who answered correctly at least 75% of questions on knowledge of child contact management | 75d | 34 | 29 (13.8–44.2) | 46 |
| Medical staff’s attitude | Proportion of staff who answered correctly at least 75% of questions on attitudes towards child contact management | 75d | 34 | 44 (27.3–60.7) | 31 |
| Medical staff’s acceptance | Medical staff’s acceptance of tuberculosis disease screening | No barriers identified | 10 | 2 barriers identified | 2 barriers |
| Medical staff’s acceptance of isoniazid preventive therapy | No barriers identified | 10 | 3 barriers identified | 3 barriers | |
| Cost | Proportion of households whose screening costs exceeded 10% of monthly household incomee | 0 | 149 | 50 (41.7–57.7) | 50 |
| Medication availability | Days without a medication supply | 0 daysf | 1 | 0 days | 0 days |
| Medication quality | Supplier of medications compliant with WHO good manufacturing practice | Yes | 1 | Yes | no gap |
CI, confidence interval; NA, not applicable, WHO, World Health Organization.
a All values are percentages except where otherwise noted.
b Targets from the Centers for Disease Control and Prevention, Atlanta, United States of America.13
c Based on concordance between two paediatric expert reviewers.14
d Standard pass rate for medical assessments.
e Based on a definition of catastrophic health shock from the literature.15
f Based on Global Drug Facility and principles of the DOTS strategy.16
Indicators of screening compliance and of the initiation of isoniazid preventive therapy in children (Table 1) were assessed in a cohort of consecutively diagnosed, sputum-smear-positive tuberculosis patients who had been living for the previous 3 months in the same house as at least one child aged 15 years or younger (cohort 1). Participants were informed about child contact management. A paediatric nurse used a standardized form to record each child’s attendance at the paediatric clinic in the 3 months after diagnosis of the index tuberculosis case and these records were cross-checked with the child’s clinical files. Information was also recorded on the outcomes of any diagnostic procedures and on the initiation of isoniazid preventive therapy in children younger than 5 years who were not diagnosed with tuberculosis.
Indicators of the accuracy of tuberculosis disease diagnosis were assessed in a second cohort of consecutively diagnosed, sputum-smear-positive tuberculosis patients (cohort 2). Patients had at least one child contact younger than 10 years. This age limit was selected to enrich the sample of children younger than 5 years. The tuberculosis patients were invited to bring the children for screening and, to encourage attendance, both screening and transportation costs were reimbursed. Screening included symptom evaluation and, in keeping with clinic policy, a tuberculin skin test (2TU PPD RT23, Biofarma, Bandung, Indonesia) that was read after 48 to 72 hours. Children who tested positive (i.e. induration ≥ 10 mm) underwent chest radiography and the radiograph was interpreted by the clinic’s paediatrician. Depending on clinical and radiological findings, antituberculosis medication was prescribed in accordance with the paediatrician’s recommendations. These children were also evaluated using the Indonesian Paediatric Scoring System, which is the clinic’s primary screening and diagnostic tool; it takes into account details of the tuberculosis case contact, symptoms, tuberculin skin test results and chest radiograph findings.16 The children’s chest radiographs were also evaluated by an external paediatric tuberculosis expert and classified according to whether or not they indicated the presence of tuberculosis disease. Two diagnostic accuracy indicators were employed: agreement between the attending paediatrician and the external paediatrician and the proportion of children with a latent tuberculosis infection (i.e. a positive tuberculin skin test result but no clinical or radiological evidence of tuberculosis disease) who were diagnosed with disease using the Indonesian Paediatric Scoring System.
The indicator of adherence to isoniazid preventive therapy was assessed in children younger than 5 years from cohort 2 who were eligible for therapy according to WHO recommendations.4 Prescriptions for therapy were provided exclusively at the clinic and patients had to pay for the medications themselves, as per local practice. The number of prescriptions collected for each child was recorded over a 6-month period. The adherence indicator was the proportion of children for whom three or more prescriptions were collected.
Indicators of primary caregivers’ knowledge and acceptance of tuberculosis disease screening and of isoniazid preventive therapy were assessed by conducting in-depth, unstructured interviews with consenting participants in their homes. Participants were selected to represent primary caregivers with child contacts who complied (n = 5) or did not comply (n = 5) with screening and those whose child contacts did (n = 5) or did not adhere (n = 7) to isoniazid preventive therapy. The proportion of primary caregivers who understood the need for child assessment was used as an indicator of screening knowledge. The absence of barriers to screening and preventive therapy was used an indicator of acceptance.
Indicators of medical staff’s knowledge of and attitude towards child contact management were assessed using a prepiloted, self-administered questionnaire. The questions, which were adapted from an existing health-care worker assessment tool,20 assessed staff’s attitudes and evaluated their knowledge of the provision of information on screening, current child contact management policy, the tuberculosis diagnostic process and treatment options. The proportion of staff who answered at least 75% of questions on knowledge and attitude correctly was used as an indicator of these parameters. Indicators of medical staff’s acceptance of tuberculosis disease screening and of isoniazid preventive therapy were assessed using a semi-structured interview. Staff were selected to represent a cross-section of doctors (n = 5) and nurses (n = 5) at the clinic. The absence of barriers to screening and preventive therapy was used as an indicator of acceptance.
To obtain an indication of the cost of child contact management, we gathered data on the costs associated with routine screening for all child contacts who presented to the clinic. Costs included a consultation fee, a registration fee and payments for the tuberculin skin test and the chest radiograph. For households in cohort 1, we asked index cases about the household’s monthly income and the cost of travelling to the clinic with their children. The total cost of screening was the cost of transport plus the cost of routine screening. It was expressed as a percentage of the household’s monthly income. The cost indicator was the proportion of households whose screening costs exceeded 10% of monthly household income. This measure has been used previously as a threshold for catastrophic expenditure on health.15
Indicators of the availability and quality of medications were evaluated by determining how many days the clinic was without a supply of isoniazid suitable for children in a 3-month period and by examining the credentials of the clinic’s isoniazid supplier.
Data analysis
On the basis of previous experience, we estimated that, for cohort 1, 25% (95% confidence interval, CI: 20.8–29.2) of 400 child contacts would attend screening, that 80 (80%; 95% CI: 71.2–88.8) of those attending would be eligible for isoniazid preventive therapy and that 70 (87.5%; 95% CI: 80.2–94.8) would commence treatment. For cohort 2, we estimated that 150 of 300 child contacts would be younger than 5 years, that 120 (80%) of the 150 would be commenced on isoniazid preventive therapy, and that three or more prescriptions would be collected for 90 (75%; 95% CI: 67.3–82.8) of the children receiving isoniazid.
All quantitative data were double-entered into Microsoft Access databases (Microsoft Corporation, Redmond, United States of America) and verified. Summary statistics with 95% CIs were presented where appropriate. Data from all investigations were used to evaluate performance indicators for each parameter. Each indicator was compared against a desired target to derive a value for the gap in the performance of the child contact management system. The gap was expressed as the absolute difference between the result observed for each indicator and its corresponding target. Interobserver agreement on interpretation of the chest radiographs was assessed using the kappa statistic. All quantitative data analyses were conducted using Stata version 11.0 (StataCorp. LP, College Station, USA). All qualitative data were digitally recorded, transcribed and translated to English before being analysed using thematic coding.
Results
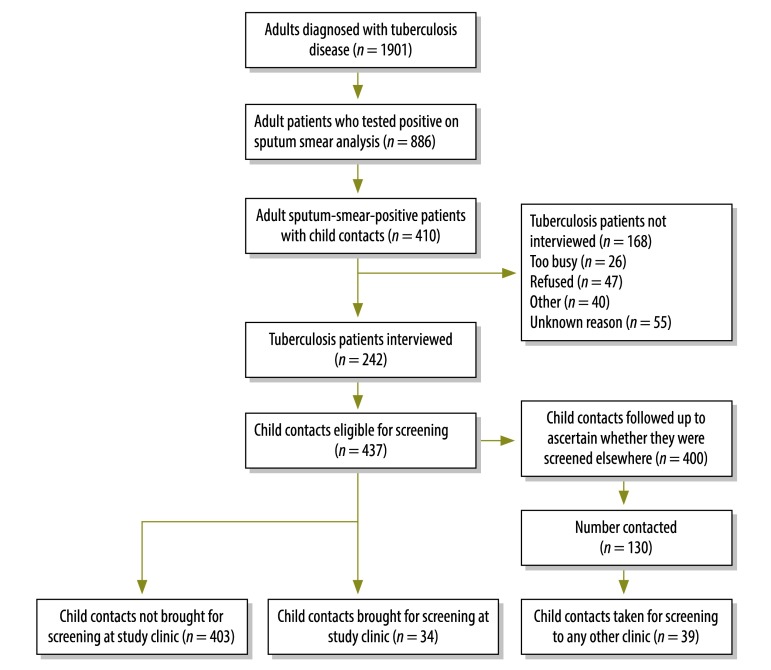
Of 410 eligible tuberculosis patients, 242 were recruited to cohort 1 and interviewed. These patients had 437 child contacts who were eligible for screening (Fig. 1, available at: http://www.who.int/bulletin/volumes/91/12/13-118414). The median age of the tuberculosis patients was 31 years and 52% were male. For child contacts, the median age was 7 years and 53% were male. Overall, 34 of the 437 (7.8%) child contacts returned to the study clinic for screening within 3 months of the adult patient’s diagnosis, which gave a gap of 82% between the observed and target performance (Table 1). Sixteen of the 34 (47%) screened children received antituberculosis medication, while 6 of 15 children (40%) younger than 5 years who were eligible for isoniazid preventive therapy actually received it. This resulted in a gap of 50% between the observed and target performance for the initiation of preventive therapy.
Fig. 1.
Participants recruited to assess disease screening of child contacts of adult tuberculosis patients, community lung clinic, Bandung, Indonesia, 2009–2012
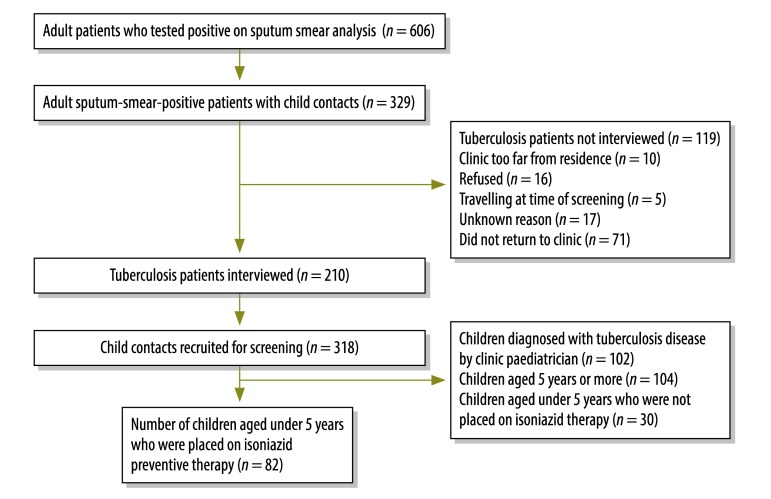
Of 329 eligible tuberculosis patients, 210 were recruited to cohort 2 and interviewed. They had 318 child contacts who were eligible and recruited for screening (Fig. 2, available at: http://www.who.int/bulletin/volumes/91/12/13-118414). The median age of the tuberculosis patients was 31 years and 47% were male. For child contacts, the median age was 5 years and 49% were male. Ninety-two of the 318 (29%) children had symptoms suggestive of tuberculosis disease and 159 (59%) tested positive on the tuberculin skin test. Of these children who tested positive, 102 (64%) had chest radiographs that were interpreted as showing tuberculosis disease by the clinic’s paediatrician. The chest radiographs of 118 child contacts were also evaluated by the external paediatrician: tuberculosis was identified in 59% and 4% of these radiographs by the clinic’s paediatrician and the external paediatrician, respectively. The kappa value for interobserver agreement was 6.0%, which gave a gap of 64% for this indicator (Table 1). The proportion of children with a latent tuberculosis infection who were diagnosed with tuberculosis disease using the Indonesian Paediatric Scoring System was 100% (i.e. 41 of 41 children), which gave a gap of 100% for this indicator.
Fig. 2.
Participants recruited to assess adherence to isoniazid preventive therapy in children younger than 5 years in contact with adult tuberculosis patients, community lung clinic, Bandung, Indonesia, 2009–2012
In total, 82 child contacts younger than 5 years from cohort 2 were prescribed isoniazid preventive therapy. Three or more prescriptions were collected from the clinic for 21 of the 82 (25.6%, 95% CI: 16.5–35.5). This resulted in a gap of 54% between the observed and target performance for adherence to isoniazid preventive therapy.
Although primary caregivers had adequate knowledge of child contact screening, the majority were unaware of the existence of isoniazid preventive therapy (Table 1). Interviews with primary caregivers revealed four barriers to the acceptance of screening: (i) difficulty accessing the clinic for initial patient diagnosis; (ii) problems with health-care workers and their appreciation of the effect of the disease; (iii) inappropriate health behaviour; and (iv) difficulty accessing screening related to transport, cost and the time needed (Table 2). In addition, interviews with caregivers whose children received isoniazid preventive therapy revealed four barriers to the acceptance of therapy: (i) difficulty accessing treatment, such as travel costs and time; (ii) medication issues, such as the ease of administration and experience of side effects; (iii) patients’ experience of the disease and health services, such as waiting times at the clinic; and (iv) patients’ knowledge and beliefs, such as a reluctance to treat healthy children. Details have been reported previously.21
Table 2. Barriers to primary caregivers’ acceptance of disease screening for the child contacts of tuberculosis patients, community lung clinic, Bandung, Indonesia, 2009–2012.
| Barriers | Interviewees | Interview findings | Illustrative quotations |
|---|---|---|---|
| Difficulty accessing the clinic | Compliant with screening (n = 5) | Compliant respondents had few difficulties with general clinic accessibility; those of limited means received government support. | “I couldn't afford the treatment…I have to pay for my children's school…I do not have my own business…luckily I have a GARKIN card,a everything was easier.” (38-year-old male) |
| Not compliant with screening (n = 5) | Noncompliant respondents frequently mentioned the high cost of treatment and transport and a lack of time. | “I had to queue a long time and travel far away from home.” (18-year-old female) | |
| Problems with the disease and its management | Compliant with screening (n = 5) | The majority of compliant respondents experienced symptoms. None reported poor service at the clinic. | “I experienced myself how complicated my disease was…like a bomb waiting to explode…if I did not seek treatment quickly, that would happen to me.” (43-year-old male) |
| Not compliant with screening (n = 5) | Only one noncompliant respondent mentioned their symptoms. The long duration of clinic visits and waiting times was mentioned. | “I had to see nine doctors at my first visit…can you imagine that? I was very ill, I had a headache, I was short of breath.” (30-year-old female) | |
| Inappropriate health behaviour | Compliant with screening (n = 5) | Compliant respondents were clearly aware that tuberculosis is infectious and the majority took measures to protect their family. | “As long as you did not ignore what the doctor said…My children also, they took their medicine every day.” (43-year-old male) |
| Not compliant with screening (n = 5) | Noncompliant respondents were aware that tuberculosis is infectious; only one mentioned taking measures to protect his family. | “Well it was (expensive)…but what could I do? Anything for healthiness, that is my opinion.” (18-year-old female) | |
| Difficulty accessing screening | Compliant with screening (n = 5) | Compliant respondents mentioned difficulties with transport, which led to delays, the cost of screening and the time needed. | “I had no time, I was very ill and could not accompany my family for screening…I did not bring them all at once. I only had my motorbike.” (43-year-old male) |
| Not compliant with screening (n = 5) | Most noncompliant respondents mentioned difficulties with making time available; the cost of screening and transport was also mentioned. | “I was ill…and had financial problems, so I did not have the chance to bring my children…but you still needed money for transport.” (47-year-old male) |
a A GARKIN card entitles the holder to government-sponsored health insurance.
The questionnaire on clinical staff’s knowledge of and attitudes towards child contact management was completed by 22 of 25 (88%) nurses approached and 12 of 15 (80%) doctors. The results are shown in Table 3. On knowledge, 50% of doctors and 18% of nurses answered at least 75% of questions correctly, which gave a gap of 46% between the observed and target performance (Table 1). On attitudes, 50% of doctors and 41% of nurses answered at least 75% of questions correctly, resulting in a gap of 31%. All staff agreed that child contacts should be screened but only 29% agreed that disease-free child contacts younger than 5 years should receive isoniazid preventive therapy. The development of multidrug-resistant tuberculosis due to isoniazid preventive therapy was a major concern.
Table 3. Staff’s questionnaire responses on knowledge of and attitudes towards the management of the child contacts of tuberculosis patients, community lung clinic, Bandung, Indonesia, 2009–2012.
| Questionnaire item | No. (%) of doctors (n = 12) | No. (%) of nurses (n = 22) | Total no. (%) (n = 34) |
|---|---|---|---|
| Knowledge of child contact management | |||
| Inform patients that they should screen household members | 12 (100) | 21 (95) | 33 (97) |
| Inform patients that they should screen child household members | 12 (100) | 21 (95) | 33 (97) |
| Correct understanding of the definition of latent tuberculosis infection | 4 (33) | 12 (55) | 16 (47) |
| Correct method for diagnosing latent tuberculosis infection | 11 (92) | 14 (64) | 25 (74) |
| Correct treatment for disease-free child case contacts younger than 5 years | 6 (50) | 4 (18) | 10 (29) |
| Correct knowledge of National Tuberculosis Programme recommendations on isoniazid preventive therapy | 8 (67) | 3 (14) | 11 (32) |
| Correct knowledge of isoniazid dosing guidelines | 7 (58) | 6 (27) | 13 (38) |
| Correct knowledge of duration of isoniazid preventive therapy | 8 (67) | 16 (23) | 24 (71) |
| Knowledge score > 75% correct responses | 6 (50) | 4 (18) | 10 (29) |
| Has received child contact management traininga | 0 (0) | 0 (0) | 0 (0) |
| Staff’s explanation of current management policy for child contacts younger than 5 yearsa | |||
| Screen children for disease if disease-free and give isoniazid preventive therapy to those with a positive tuberculin skin test result | 1 (8) | 1 (4.5) | 2 (6) |
| Screen children for disease if disease-free and give isoniazid preventive therapy | 2 (17) | 2 (9.1) | 4 (12) |
| Screen children using the Indonesian Paediatric Scoring System and administer full antituberculosis medication if the score is 6 or more | 6 (50) | 14 (64) | 20 (59) |
| Attitudes to child contact management | |||
| Would treat latent tuberculosis infection | 2 (17) | 14 (64) | 16 (47) |
| Agree that child contacts younger than 5 years should be screened | 12 (100) | 22 (100) | 34 (100) |
| Agree that isoniazid preventive therapy protects against tuberculosis disease | 6 (50) | 5 (23) | 11 (32) |
| Agree that isoniazid preventive therapy should be given to disease-free child contacts younger than 5 years | 3 (25) | 7 (32) | 10 (29) |
| Attitude score > 75% correct responses | 6 (50) | 9 (41) | 15 (44) |
| Concerns about child contact management (e.g. the development of multidrug-resistant tuberculosis)a | 5 (42) | 13 (59) | 18 (53) |
| Staff’s view of why caregivers do not comply with screeninga | |||
| Caregivers do not think it is important | 11 (92) | 18 (82) | 29 (85) |
| Caregivers cannot afford to bring their children for screening | 10 (83) | 19 (86) | 29 (85) |
| Caregivers only come if their child is sick | 11 (92) | 18 (82) | 29 (85) |
| Other reasons | 11 (92) | 16 (73) | 27 (79) |
a Not included in knowledge or attitude score.
Two main barriers to staff’s acceptance of screening were identified: doubts about the workability of the child contact management programme and doubts about the clinic’s capability (Table 4). Barriers to staff’s acceptance of isoniazid preventive therapy related to: (i) staff’s knowledge of treatment; (ii) compliance with treatment guidelines; and (iii) confusion about who is responsible for prescribing therapy.
Table 4. Barriers to staff’s acceptance of disease screening and isoniazid preventive therapy for the child contacts of tuberculosis patients, community lung clinic, Bandung, Indonesia, 2009–2012.
| Barriers | Interview findingsa | Illustrative quotes |
|---|---|---|
| Screening | ||
| Doubts about the workability of the child contact management programme | Staff expressed doubts and frustration about the practicality of programmes because patients did not participate. They highlighted the need for better education about the importance of child contact screening. | “The challenge is in applying it. For the practitioners, it’s possible to apply it, but for the patients not every patient is willing to follow the procedure.” (nurse) “Mmm…if the parents understand that it’s very important to bring their children for tests, they will.” (doctor) |
| Doubts about the clinic’s capability | The majority of staff felt the clinic had the resources to manage child contacts well. However, several nurses mentioned an increased workload and the risk of personnel shortages. | “From our side, we’re ready. The provision of test equipment is sufficient, human resources are available.” (nurse) “Active case-finding will surely increase the numbers of patients – I don’t think we’re ready for that. To give service to 100 patients a day, we feel very overwhelmed.” (nurse) |
| Isoniazid preventive therapy | ||
| Poor staff knowledge | Staff knowledge of latent tuberculosis infection and of the diagnostic role of the tuberculin skin test was poor. They mentioned that child contacts who tested positive were given full tuberculosis therapy while those who tested negative received isoniazid preventive therapy. | “…we perform tests; if negative, then we assess the score and if it is less than 6, we give them prophylaxis for 3 months. Then, if the Mantoux test is positive and the score is more than 6, we treat them as tuberculosis patients.” (doctor) |
| Lack of compliance with treatment guidelines | Staff members noted that there was a lack of conformity regarding the provision of isoniazid preventive therapy and this negatively affected implementation. | “At the moment, I don’t [prescribe isoniazid], on the assumption that healthy people don’t need medication…” (doctor) |
| Confusion about who is responsible for prescribing | Staff members not directly involved with child care lacked knowledge or interest in child contact management despite the regular rotation of staff through all the clinics. Nurses indicated that they had a limited influence on child contact management. | “But for the practice, because I work in a clinic for adult patients, I don’t know if all children whose parents have tuberculosis are being given prevention.” (doctor) “For us nurses, we don’t have any authority, so it’s all up to the doctors.” (nurse) |
a Barriers to staff’s acceptance of disease screening and of isoniazid preventive therapy were evaluated in interviews with five doctors and five nurses.
The fixed costs of screening were 30 000 Indonesian rupiah (approximately 3 United States dollars, US$) per child for registration and consultation, US$ 3 for the tuberculin skin test and US$ 4 for chest radiography, which was performed if the tuberculin test was positive. The monthly household income was known for 149 of the 242 households in cohort 1 (62%). The median cost of screening was 9.8% (range: 0.8–80) of monthly household income and the cost exceeded 10% of that income for 50% of households.
The pharmaceutical company that supplied isoniazid to the clinic complied with good manufacturing practices and, in the 3 months preceding our evaluation, the pharmacy did not run out of isoniazid suitable for children.
Discussion
To our knowledge, this is the first comprehensive evaluation of a programme for managing the child contacts of tuberculosis patients. Using a public health framework, we identified and quantified gaps between current practice and desired targets. Moreover, our approach illustrated the importance of simultaneously evaluating all barriers. Although we observed considerable gaps in all measures of programme performance, all appear amenable to intervention.
That 92% of eligible children did not come for screening is of concern. Similar poor responses have been found elsewhere. In Malawi, screening compliance rates below 10% have been reported.5,8 In South Africa and Thailand, reported rates were under 3% and 52%, respectively.22,23 In contrast, the compliance rate in India for symptom-based screening conducted at the child’s home was 67%.24 Screening conducted in children’s homes using symptom-based screening, which is recommended by WHO,4 would be expected to improve the performance of child contact management systems.
Since childhood tuberculosis is rarely confirmed bacteriologically, chest radiographs are important for diagnosing disease.25–27 However, their sensitivity and specificity in the diagnosis of tuberculosis are reportedly low.25,27 Furthermore, poor agreement between readers is common: in South Africa, the reported agreement on hilar lymphadenopathy between different observer pairs ranged from 5 to 55%27 and a similar study reported a kappa of 33%.28 In another study from the country, the proportion of children’s chest radiographs judged positive for tuberculosis by three reviewers ranged from 11 to 51%.29 While it is reasonable that the use of chest radiographs for diagnosis in child contact management programmes should be reviewed,25,30 there is, perhaps, as great a need for standardizing the training of readers.
One important finding of our study is that inappropriate use of the Indonesian Paediatric Scoring System resulted in disease being misdiagnosed in children with latent tuberculosis infections. Misdiagnosis was due to the high scores awarded for being a contact of a tuberculosis patient and for a positive result on the tuberculin skin test. Another Indonesian study found that 82% of child contacts with latent tuberculosis infections were diagnosed with tuberculosis disease using the scoring system.31 The performance of other scoring systems is variable: a comparison of nine systems in 1445 children found diagnostic yields ranging from 7 to 89%.29
Rates for the initiation of isoniazid preventive therapy in eligible children ranging from 1.3 to 26% have been reported in settings where tuberculosis is endemic.5,23,32,33 Such low rates are due to poor screening compliance. When the initiation of therapy was evaluated in children who had been screened, the reported rate was 50 to 84%.5,24,32,33 Similarly, in our study, it was 40%. However, as in our study, adherence to therapy was poor in settings where the disease is endemic (range: 15 to 76%).6,7,34–37
In our study, poor staff knowledge of isoniazid preventive therapy and poor compliance with guidelines were found to be barriers to the initiation of therapy. Similar barriers have been reported in other countries: in India, health-care workers reported that unclear guidelines on child contact management inhibited their ability to implement therapy;38 in Thailand, doctors were reluctant to initiate therapy due to concerns about isoniazid toxicity and resistance;22 in Australia, doctors actively advised patients not to use isoniazid preventive therapy;34 in the United States of America, medical graduates did not believe isoniazid preventive therapy was protective against disease progression;39 and, in Malawi, health-care workers felt that screening child contacts by chest radiography was not worthwhile.33
As in other countries, we found that primary caregivers’ acceptance of the child contact management programme was hindered by barriers such as limited knowledge of isoniazid preventive therapy, difficulty accessing screening and treatment and reluctance to treat asymptomatic children.7,22,33,34,40
There is a lack of research on the cost of child contact management to households. However, the cost of tuberculosis disease often exceeds the 10% of monthly household income considered catastrophic41 and a figure as high as 89% of the household’s annual income has been reported.42 We found that the cost of screening child contacts alone exceeded the 10% threshold for many households in Bandung. A symptom-based approach to screening4 could reduce this cost considerably.
The continuous supply and consistent quality of antituberculosis drugs are key elements of the DOTS strategy.17 At the study clinic, the availability and quality of medications were adequate. In contrast, the quality and, to a lesser extent, the availability of antituberculosis drugs have been reported to be poor in other settings.43,44
The study has a number of limitations. Since it was carried out at a single institution, its external validity is a concern. Moreover, the characteristics of the child contacts recruited to cohort 1 may have differed from those of child contacts recruited to cohort 2, whose costs were reimbursed. It was not possible to document reasons for non-attendance for screening or poor adherence to therapy, although we did collect information to assess risk factors for these outcomes, which will be reported elsewhere. Some of our findings are based on data from few participants (e.g. few staff were interviewed). Also, since we used qualitative methods to identify barriers to child contact management, we could not quantify the effect of these barriers. Our investigation of the diagnostic accuracy of chest radiography in child contacts was hindered by the lack of microbiological confirmation of tuberculosis disease. Another limitation is that the medication audit was conducted retrospectively. Further, we judged medication quality on the basis of the quality of the supplies; however, medications could have been degraded by problems with storage at the clinic, such as lengthy storage.45 The only indirect costs for child contacts’ households we considered were for transportation to screening; a diagnosis of tuberculosis and its treatment would be associated with other indirect costs.46 Although testing for the human immunodeficiency virus was not carried out, infection is rare in the general population and in tuberculosis patients in Indonesia.47–49
In conclusion, we used a public health framework to identify substantial gaps in the performance of a programme for managing the child contacts of tuberculosis patients. The next step is to consider ways of reducing these gaps. The different options available for addressing each gap could be combined using a set of weighted criteria to devise a new child contact management programme. Our public health framework could then be used to evaluate this programme and any further changes made to it. Since problems with child contact management are not unique to our study clinic or to Indonesia, the adoption of a similar evaluation framework could be useful in other settings.
Competing interests:
None declared.
References
- 1.Global tuberculosis control – epidemiology, strategy, financing Geneva: World Health Organization; 2009. Available from: http://www.who.int/tb/publications/global_report/2009/en/index.html [accessed 22 August 2013].
- 2.Marais BJ, Ayles H, Graham SM, Godfrey-Faussett P. Screening and preventive therapy for tuberculosis. Clin Chest Med. 2009;30:827–46. doi: 10.1016/j.ccm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 3.International Union Against Tuberculosis Committee on Prophylaxis Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–64. [PMC free article] [PubMed] [Google Scholar]
- 4.Guidance for national tuberculosis programmes on the management of tuberculosis in children Geneva: World Health Organization; 2006. Available from: whqlibdoc.who.int/hq/2006/WHO_HTM_TB_2006.371_eng.pdf [accessed 22 August 2013]. [PubMed]
- 5.Claessens NJ, Gausi FF, Meijnen S, Weismuller MM, Salaniponi FM, Harries AD. Screening childhood contacts of patients with smear-positive pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2002;6:362–4. [PubMed] [Google Scholar]
- 6.Gomes VF, Wejse C, Oliveira I, Andersen A, Vieira FJ, Carlos LJ, et al. Adherence to isoniazid preventive therapy in children exposed to tuberculosis: a prospective study from Guinea-Bissau. Int J Tuberc Lung Dis. 2011;15:1637–43. doi: 10.5588/ijtld.10.0558. [DOI] [PubMed] [Google Scholar]
- 7.Marais BJ, van Zyl S, Schaaf HS, van Aardt M, Gie RP, Beyers N. Adherence to isoniazid preventive chemotherapy: a prospective community based study. Arch Dis Child. 2006;91:762–5. doi: 10.1136/adc.2006.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyirenda M, Sinfield R, Haves S, Molyneux EM, Graham SM. Poor attendance at a child TB contact clinic in Malawi. Int J Tuberc Lung Dis. 2006;10:585–7. [PubMed] [Google Scholar]
- 9.Sinfield R, Nyirenda M, Haves S, Molyneux EM, Graham SM. Risk factors for TB infection and disease in young childhood contacts in Malawi. Ann Trop Paediatr. 2006;26:205–13. doi: 10.1179/146532806X120291. [DOI] [PubMed] [Google Scholar]
- 10.Hill PC, Rutherford ME, Audas R, van Crevel R, Graham SM. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PLoS Med. 2011;8:e1001105. doi: 10.1371/journal.pmed.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO report 2010: global tuberculosis control Geneva: World Health Organization; 2010. Available from: http://www.doh.state.fl.us/disease_ctrl/tb/Trends-Stats/Fact-Sheets/US-Global/WHO_Report2010_Global_TB_Control.pdf [accessed 27 August 2013].
- 12.Lestari T, Probandari A, Hurtig AK, Utarini A. High caseload of childhood tuberculosis in hospitals on Java Island, Indonesia: a cross sectional study. BMC Public Health. 2011;11:784. doi: 10.1186/1471-2458-11-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National TB program objectives and performance targets for 2015 Atlanta: Centers for Disease Control and Prevention; 2009. Available from: http://www.cdc.gov/tb/programs/evaluation/indicators/default.htm [accessed 29 August 2013].
- 14.Lee EY, Tracy DA, Eisenberg RL, Arellano CMR, Mahmood SA, Cleveland RH, et al. Screening of asymptomatic children for tuberculosis: is a lateral chest radiograph routinely indicated? Acad Radiol. 2011;18:184–90. doi: 10.1016/j.acra.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Pradhan M, Prescott N. Social risk management options for medical care in Indonesia. Health Econ. 2002;11:431–46. doi: 10.1002/hec.689. [DOI] [PubMed] [Google Scholar]
- 16.Triasih R, Rutherford M, Lestari T, Utarini A, Robertson CF, Graham SM. Contact investigation of children exposed to tuberculosis in South East Asia: a systematic review. J Trop Med. 2012;2012:301808. doi: 10.1155/2012/301808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies PD. DOTS plus strategy in resource-poor countries. Int J Tuberc Lung Dis. 1999;3:843–4. [PubMed] [Google Scholar]
- 18.Stob TB Partnership [Internet]. Global drug facility. Geneva: World Health Organization; 2012. Available from: http://www.stoptb.org/gdf/ [accessed 27 August 2013].
- 19.Prescott N. Coping with catastrophic health shocks. Washington: Inter-American Development Bank; 1999. Available from: http://www.docstoc.com/docs/17289818/COPING-WITH-CATASTROPHIC-HEALTH-SHOCKS [accessed 29 August 2013]. [Google Scholar]
- 20.Advocacy, communication and social mobilization for TB control: a guide to developing knowledge, attitude and practice surveys Geneva: World Health Organization; 2008. [Google Scholar]
- 21.Rutherford ME, Ruslami R, Maharani W, Yulita I, Lovell S, Van Crevel R, et al. Adherence to isoniazid preventive therapy in Indonesian children: a quantitative and qualitative investigation. BMC Res Notes. 2012;5:7. doi: 10.1186/1756-0500-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Wyk SS, Reid AJ, Mandalakas AM, Enarson DA, Beyers N, Morrison J, et al. Operational challenges in managing isoniazid preventive therapy in child contacts: a high-burden setting perspective. BMC Public Health. 2011;11:544. doi: 10.1186/1471-2458-11-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tornee S, Kaewkungwal J, Fungladda W, Silachamroon U, Akarasewi P, Sunakorn P. Factors associated with the household contact screening adherence of tuberculosis patients. Southeast Asian J Trop Med Public Health. 2005;36:331–40. [PubMed] [Google Scholar]
- 24.Pothukuchi M, Nagaraja SB, Kelamane S, Satyanarayana S, Shashidhar, Babu S, et al. Tuberculosis contact screening and isoniazid preventive therapy in a South Indian district: operational issues for programmatic consideration. PLoS One. 2011;6:e22500. doi: 10.1371/journal.pone.0022500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Villiers RV, Andronikou S, Van de Westhuizen S. Specificity and sensitivity of chest radiographs in the diagnosis of paediatric pulmonary tuberculosis and the value of additional high-kilovolt radiographs. Australas Radiol. 2004;48:148–53. doi: 10.1111/j.1440-1673.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- 26.Nelson KE, Hiransuthikul N, Hiransuthikul P. Diagnosis and treatment of latent tuberculosis. Int J Tuberc Lung Dis. 2007;11:353. [PubMed] [Google Scholar]
- 27.Swingler GH, du Toit G, Andronikou S, van der Merwe L, Zar HJ. Diagnostic accuracy of chest radiography in detecting mediastinal lymphadenopathy in suspected pulmonary tuberculosis. Arch Dis Child. 2005;90:1153–6. doi: 10.1136/adc.2004.062315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Toit G, Swingler G, Iloni K. Observer variation in detecting lymphadenopathy on chest radiography. Int J Tuberc Lung Dis. 2002;6:814–7. [PubMed] [Google Scholar]
- 29.Hatherill M, Hanslo M, Hawkridge T, Little F, Workman L, Mahomed H, et al. Structured approaches for the screening and diagnosis of childhood tuberculosis in a high prevalence region of South Africa. Bull World Health Organ. 2010;88:312–20. doi: 10.2471/BLT.09.062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George SA, Ko CA, Kirchner HL, Starke JR, Dragga TA, Mandalakas AM. The role of chest radiographs and tuberculin skin tests in tuberculosis screening of internationally adopted children. Pediatr Infect Dis J. 2011;30:387–91. doi: 10.1097/INF.0b013e3182029486. [DOI] [PubMed] [Google Scholar]
- 31.Triasih R, Graham SM. Limitations of the Indonesian Pediatric Tuberculosis Scoring System in the context of child contact investigation. Paediatr Indones. 2011;51:332–7. [Google Scholar]
- 32.Du Preez K, Hesseling AC, Mandalakas AM, Marais BJ, Schaaf HS. Opportunities for chemoprophylaxis in children with culture-confirmed tuberculosis. Ann Trop Paediatr. 2011;31:301–10. doi: 10.1179/1465328111Y.0000000035. [DOI] [PubMed] [Google Scholar]
- 33.Zachariah R, Spielmann MP, Harries AD, Gomani P, Graham SM, Bakali E, et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:1033–9. [PubMed] [Google Scholar]
- 34.Alperstein G, Morgan KR, Mills K, Daniels L. Compliance with anti-tuberculosis preventive therapy among 6-year-old children. Aust N Z J Public Health. 1998;22:210–3. doi: 10.1111/j.1467-842X.1998.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 35.Machado A, Jr, Finkmoore B, Emodi K, Takenami I, Barbosa T, Tavares M, et al. Risk factors for failure to complete a course of latent tuberculosis infection treatment in Salvador, Brazil. Int J Tuberc Lung Dis. 2009;13:719–25. [PubMed] [Google Scholar]
- 36.van Zyl S, Marais BJ, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis. 2006;10:13–8. [PubMed] [Google Scholar]
- 37.Garie KT, Yassin MA, Cuevas LE. Lack of adherence to isoniazid chemoprophylaxis in children in contact with adults with tuberculosis in Southern Ethiopia. PLoS One. 2011;6:e26452. doi: 10.1371/journal.pone.0026452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banu Rekha VV, Jagarajamma K, Wares F, Chandrasekaran V, Swaminathan S. Contact screening and chemoprophylaxis in India’s Revised Tuberculosis Control Programme: a situational analysis. Int J Tuberc Lung Dis. 2009;13:1507–12. [PubMed] [Google Scholar]
- 39.Hirsch-Moverman Y, Tsiouris S, Salazar-Schicchi J, Colson PW, Muttana H, El-Sadr W. Physician attitudes regarding latent tuberculosis infection: international vs. U.S. medical graduates. Int J Tuberc Lung Dis. 2006;10:1178–80. [PubMed] [Google Scholar]
- 40.Lester R, Hamilton R, Charalambous S, Dwadwa T, Chandler C, Churchyard GJ, et al. Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study. AIDS. 2010;24(Suppl 5):S45–8. doi: 10.1097/01.aids.0000391021.18284.12. [DOI] [PubMed] [Google Scholar]
- 41.Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71(Suppl):147–55. [PubMed] [Google Scholar]
- 42.Wyss K, Kilima P, Lorenz N. Costs of tuberculosis for households and health care providers in Dar es Salaam, Tanzania. Trop Med Int Health. 2001;6:60–8. doi: 10.1046/j.1365-3156.2001.00677.x. [DOI] [PubMed] [Google Scholar]
- 43.Bosman MC. Health sector reform and tuberculosis control: the case of Zambia. Int J Tuberc Lung Dis. 2000;4:606–14. [PubMed] [Google Scholar]
- 44.Peloquin CA. Shortages of antimycobacterial drugs. N Engl J Med. 1992;326:714. doi: 10.1056/NEJM199203053261018. [DOI] [PubMed] [Google Scholar]
- 45.Rookkapan K, Chongsuvivatwong V, Kasiwong S, Pariyawatee S, Kasetcharoen Y, Pungrassami P. Deteriorated tuberculosis drugs and management system problems in lower southern Thailand. Int J Tuberc Lung Dis. 2005;9:654–60. [PubMed] [Google Scholar]
- 46.Needham DM, Godfrey-Faussett P, Foster SD. Barriers to tuberculosis control in urban Zambia: the economic impact and burden on patients prior to diagnosis. Int J Tuberc Lung Dis. 1998;2:811–7. [PubMed] [Google Scholar]
- 47.WHO Indonesia. Programme. Tuberculosis 2011. Available from: http://www.ino.searo.who.int/en/Section4/Section21.htm [accessed 22 August 2013].
- 48.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff THM, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 49.Mahendradhata Y, Ahmad RA, Kusuma TA, Boelaert M, Van der Werf MJ, Kimerling ME, et al. Voluntary counselling and testing uptake and HIV prevalence among tuberculosis patients in Jogjakarta, Indonesia. Trans R Soc Trop Med Hyg. 2008;102:1003–10. doi: 10.1016/j.trstmh.2008.04.042. [DOI] [PubMed] [Google Scholar]