Abstract
Background: Non Syndromic tooth agenesis is a congenital anomaly with significant medical, psychological and social ramifications. There is sufficient evidence to hypothesize that locus for this condition can be identified by candidate genes. The aim of this study was to test whether MSX1 671 T>C gene variant was involved in etiology of Non Syndromic tooth agenesis in Raichur Patients.
Materials & Methods: Blood samples were collected with informed consent from 50 subjects having Non Syndromic tooth agenesis and 50 controls. Genomic DNA was extracted from the blood samples, Polymerase Chain Reaction was performed (PCR) and Restriction Fragment Length Polymorphism (RFLP) was performed for digestion products that were evaluated.
Results: The Results showed positive correlation between MSX1671 T>C gene variant and Non Syndromic tooth agenesis in Raichur Patients.
Conclusion: MSX1 671 T>C gene variant may be a good screening marker for Non Syndromic tooth agenesis in Raichur Patients .
How to cite this article:Reddy NA, Adusumilli G, Devanna R, Pichai S, Rohra MG, Arjunan S. Msx1 Gene Variant - Its Presence in Tooth Absence - A Case Control Genetic Study. J Int Oral Health 2013; 5(5):20-6.
Key Words: : Case control, Msx1 mutation, tooth agenesis
Introduction
Mammalian teeth develop as ectodermal organs bearing many similarities to other such organs like hair, feathers and mammary glands.1 Basic problems in developmental biology, including cell commitment, reciprocal tissue interactions, pattern formation, positional information and development of complex morphologies may be approached using teeth and dentitions as a model system.2-3
Hypodontia (hypo, greek for ‚less‛) is the most commonly used term even though it may sound like a scientifically sounding simile of ‚missing teeth‛ or may be mixed with reduced size of teeth. Prevalence of tooth agenesis show a rather large variation. Its birth prevalence ranges from 5 to 8 %, except third molars depending on geographical origin. Prevalence is higher among females than among males.4
No general difference in the frequency of agenesis between the left and right sides exists, and for permanent teeth no significant difference between maxilla and mandible has been reported although for individual teeth in different jaws the frequencies differ. For the primary teeth, agenesis is more common in the maxilla.5 Apart from the third molars, many studies among Caucasian populations have scored the lower second premolars most commonly affected, followed by upper lateral incisors and upper second premolars, while some studies have found that upper lateral incisors are most often affected.6-7 Among Asian populations agenesis of lower central incisors is much more common than among Caucasians, this tooth actually being the most often affected, and in the primary dentition.8
The third molars may account for about 75 % of all affected teeth.7 If they are not considered, agenesis of second premolars and upper lateral incisors account for 85 % of all affected teeth among Caucasian populations. The most commonly affected other teeth are, in order, lower incisors, maxillary first premolars, mandibular first premolars, maxillary canines and mandibular second molars. Prevalences of agenesis of all other teeth are less than 0.075 %, corresponding to proportions below 0.7 %, and the most stable teeth are maxillary central incisors (prevalence of agenesis 0.016 %) and mandibular first molars and canines (prevalences of agenesis about 0.03 %).
Bilateral agenesis is observed for most teeth in almost half of the possible occurrences, i.e. when at two teeth are affected.6-7 Bilaterality may be more common for maxillary lateral incisors than for premolars in either jaw.9 The etiology seems complex, but genetics plays a major role.10 MSX1 is located on human chromosome 4p16. The functions of Msx1 in tooth development best understood as regulators of the signaling needed for the formation of the enamel knots.11 Tooth agenesis associated with MSX1 defects was a result of reduced amounts of the MSX1 protein, i.e. haploinsufficiency. The mutations and polymorphisms of MSX1 have been shown to be diverse. One of variation for MSX1 is 671 T>C (resulting in a Leu224Pro substitution).
The advents of molecular biology and advanced genetic techniques have allowed uncovering, characterizing and ultimately manipulating the genes that make up the genome. It is becoming clear that the molecular centre of embryogenic morphology resides at the level of gene.
Hence, the aim of this study is to derive a association between MSX1 671 T>C gene variant and nonsyndromic tooth agenesis involving mandibular second premolars and maxillary lateral incisors in the Raichur district with use of PCR ( Polymerase Chain Reaction) followed by RFLP ( Restriction fragment length polymorphism) with restriction enzyme Eco31I ( from Escherichia coli) .
Materials and Methods
SOURCE OF DATA
METHOD OF SELECTION OF DATA
SAMPLE SIZE AND DESIGN
METHODOLOGY
Source of Data
This genetic study was conducted in an Institution on the people of Raichur district having non- syndromic tooth agenesis as cases.
Method of Selection of Data:
Inclusion Criteria of Patients:
A Patient having non syndromic tooth agenesis including only mandibular second premolars and maxillary lateral incisors with or without presence of third molars.
A Patient having no abnormalities of the lip, palate, toenails, fingernails, hair, or sweat glands.
No history of extractions within the region of tooth agenesis.
Orthopantomograph and intraoral periapical radiograph demonstrating absence of impaction of tooth considered conginetally absent.
Inclusion Criteria of Controls:
An individual with full complement of teeth with or without presence of third molars.
An individual with no abnormalities of the lip, palate, toenails, fingernails, hair, or sweat glands.
Orthopantomograph and intraoral periapical radiograph demonstrating absence of any impacted or supernumerary teeth or any realted pathology.
Exclusion Criteria of Patients:
Patients having any syndrome related to tooth agenesis with or without third molars.
Patient having abnormalities of the lip, palate, toenails, fingernails, hair, or sweat glands.
Patient having any history of extractions without available records.
Exclusion Criteria of Controls:
An individual without full complement of teeth with or without presence of third molars.
An individual with abnormalities of the lip, palate, toenails, fingernails, hair, or sweat glands.
Orthopantomograph and intraoral periapical radiograph demonstrating presence of any impacted or supernumerary teeth or any realted pathology.
Sample Size:
Group A: Fifty subjects with Non Syndromic tooth agenesis from Raichur district.
Group B: Fifty control subjects from Raichur district.
Methodology
The study was carried out in our institution after approval from institutional ethical committee. Informed written consent was taken from the respective subjects. A study was conducted on 50 Non syndromic tooth agenesis patients and 50 Control subjects. As preoperative evaluations, all patients were screened for the presence of associated anomalies or syndromes by a geneticist, and only those determined to have Non syndromic tooth agenesis were included in the study.
The study was done in four steps:
Collection of Blood Samples: Venous blood samples of 2ml were obtained from both Group A and Group B (Fig. 1).
Genomic DNA Extraction: Genomic DNA was extracted from the blood of the cases and control subjects by using ethanol, chloroform protocol (Fig. 2). Once the isolated genomic DNA precipitate was obtained, it was used as a template for the polymerase chain reaction (PCR) test.
Polymerase Chain Reaction (PCR): Precipitated genomic DNA was amplified using the polymerase chain reaction (PCR) with primers: MSX1ex2.2F: 'GATCGCAGTACCTGTCCATGC' MSX1ex2.2R: 'ACTGTCTCTGCCCTCAGCCCT', giving a PCR product of 448 bases pairs (bp). PCR product purification was performed by ExoSAP-IT® (USB, Cleveland, OH, USA). The PCR product (448 bp) that was obtained was run on 1.5% agarose gel containing ethidium bromide to assess the initial amplified PCR products (Fig. 3).
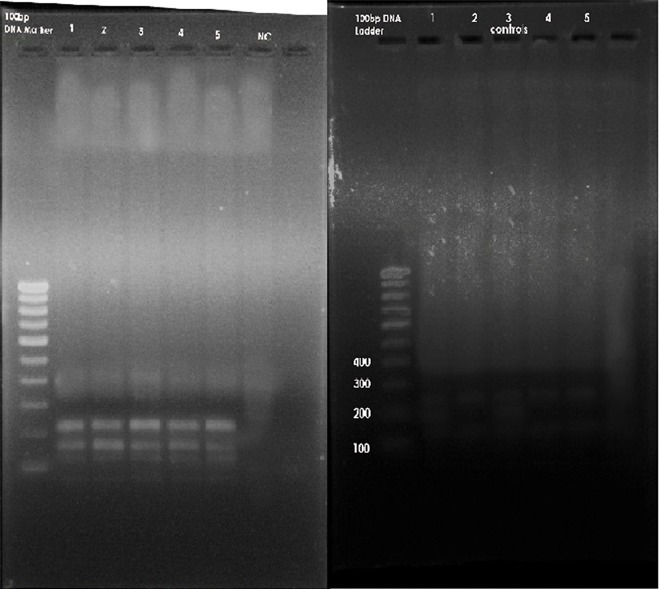
Restriction Fragment Length Polymorphism (RFLP):Genotyping for the MSX1 671 T>C gene variant was carried out by restriction digestion of PCR products with Eco31I (from Escherichia coli ) . The digested PCR products were separated into channels on a 1.5% agarose gel containing ethidium bromide in an electrophoretic chamber (Fig. 4). A U.V.transilluminator was used to see the specific bands of base pairs of the digested PCR products.
Fig 1: Collection of Blood Samples.
Fig 2: A. Genomic DNA extraction of Cases B. Genomic DNA extraction of Controls.
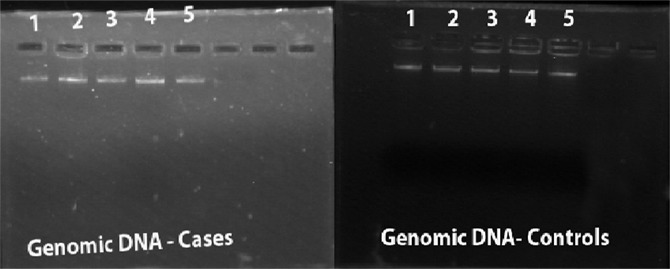
Fig 3: Polymerase Chain Reaction.
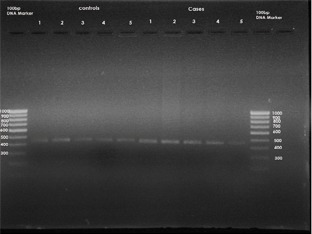
Fig 4: A. Restriction fragment length polymorphism of cases B. Restriction fragment length polymorphism of controls.
Statistical Analysis
The statistics for this study were computed using statistical software (SPSS, version 17.0, Chicago, III). Chi- square test was employed for statistical analysis. The statistical significance level was set at P < 0.001 at 95 % Confidence intervals.
Results
The following results were obtained from the Descriptive data and the Statistical comparisons:
Descriptive data: The initial PCR product of the MSX1 gene was obtained for the fifty subjects .The size of this PCR product was 448 base pairs (Fig.3). After obtaining the initial PCR products of MSX1 gene (448 BP) from groups A and B, samples were then subjected to digestion with the specific restriction enzyme Eco31I ( from Escherichia coli ). After digestion, the 448 bp products were completely digested with two restriction sites and three specific bands at 226, 189 and 139 BP in 46 subjects of Group A (Fig.4) whereas 44 subjects in Group B showed incomplete/no digestion. (Fig.4) So the presence of variant was shown only in 46 subjects of Group A and 6 subjects in group B.
Statistical comparisons: Chi- square test was employed for statistical analysis that gave a p value of <0.001 at 95 % confidence intervals . The odds value was 84.
The Descriptive data and Statistical comparisons demonstrate a significant association between MSX1 671 T>C gene variant and non syndromic tooth agenesis in Raichur patients.
Discussion
Identification of the genes involved in the development of the human craniofacial region can serve as a first step towards developing a better understanding of diagnosis, treatment options and preventions of developmental anomalies of this regions. The genetic background of Non Syndromal tooth agenesis is complex and most causes remain yet to be identified.
The mammalian Msx gene family consists of 3 physically unlinked members, named Msx1, Msx2, and Msx3. But in developing vertebrate embryos, Msx1 and Msx2 are widely expressed at the sites where epithelial mesenchymal interactions take place. The MSX1 homeobox gene is expressed at diverse sites of epithelial–mesenchymal interaction during vertebrate embryogenesis, and has been implicated in signalling processes between tissue layers.
It has been suggested that the key role of Msx1 is to facilitate the bud to cap stage transition11. Mesenchymal Msx1 expression is initially activated by the epithelial BMP4 signal, and needed for a reciprocal BMP4 signal from the mesenchyme. Bmp4 and Msx1 thus form an autoregulatory loop.12 The BMP4 signal to the epithelium is crucial for the formation of the epithelial signaling center, the enamel knot, and the arrest of the development in Msx1 null mutant teeth can be rescued by external BMP4 or transgenically activated Bmp4 expression.12 The expression of Pax9 is apparently needed to maintain and, by the synergism with Msx1, to enhance this loop. However, as shown by the mice with hypomorphic mutations, Pax9 is also needed later in tooth development.13
The regulation of Msx gene expression is accomplished by diverse mechanisms involving retinoids, antisense 'quenching', growth factor regulation, and complementary/ antagonistic interaction with other transcription factors. This reduced activity of MSX1 due to genetic variation may increase the risk for tooth agenesis.
The present study describes a novel mutation in the MSX1 gene 671 T>C responsible for non syndromic tooth agenesis in raichur patients. This c.T671C transition causes the substitution (Leu224Pro) of a highly conserved residue in the MSX1 protein. The mutated amino acid is located in helix III of the homeodomain (amino acids 207–225), which has been shown to be important not only for DNA binding but also for interactions with other proteins, including members of the DLX family (Zhang H, Hu G, Wang H, Sciavolino P, Iler N, Shen MM, et al., 1997).
A significant association was seen in Group A Subjects with MSX1 gene variant as compared to controls. These findings suggest that these gene variants have a role in the etiology of non syndromal tooth agenesis in our population. In Group B it was observed that there was no significant association with the gene variant. Hence, this suggest that MSX1 671 T>C gene variant is positively associated with non syndromic tooth agenesis in Raichur district.
Functional analysis of the first MSX1 mutation (Arg196Pro) identified as a factor responsible for hypodontia revealed that the mutated protein has perturbed structure and reduced thermostability, and exhibits little or no ability to interact with DNA or other protein factors. Therefore, it has been proposed that the phenotype of affected individuals with tooth agenesis is due to haploinsufficiency.
Interestingly, the recent functional analysis of 5 known MSX1 missense mutations associated with hypodontia (Met61Lys, Ala194Val, Arg196Pro, Ala219Thr and Ala221Glu) showed that none of the studied molecular mechanisms – including disturbances in the interaction with PAX9, deficiencies in protein stability, protein–protein interactions, nuclear translocation or DNA-binding – yielded a satisfactory explanation for the pathogenic effects of these nucleotide variants .
Moreover, a mutation identified in the Polish popu-lation (Ala194Val) showed incomplete penetrance.14 Mutations in the MSX1 gene have also been described in syndromic forms of tooth agenesis, including Witkop syndrome, Wolf-Hirschhorn syndrome, and hypodontia combined with orofacial clefting. It is interesting to note that MSX1 is one of the main candidate genes for non-sydromic cleft lip and palate. It has been proposed that its mutations could contribute to as many as 2% of all cases of this common craniofacial anomaly.15
But there is possibility of involvement of other genes as non syndromal tooth agenesis is of polygenic nature and studies suggest that other genes like PAX9, AXIN2 and EDA genes are involved in the etiology of non syndromal tooth agenesis.
Conclusion
This study indicates that there is strong association between the presence of MSX1 671 T>C gene variant with the incidence of non syndromic tooth agenesis and it may act as a genetic marker for non syndromic tooth agenesis in this population. Studies about such genetic markers of non syndromic tooth agenesis will help us to predict and target at the molecular level in treating such problems. A major challenge is not only a complete cataloguing of all such genes involved in the etiology of non syndromal tooth agenesis but identification of their polygenetic nature to have a thorough understanding of the craniofacial development. The future challenge is to bring this new knowledge from the "level of PCR sequencer into the clinical realms".
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
Contributor Information
Naveen Admala Reddy, Department of Orthodontics & Dento-facial Orthopaedics, AME's Dental College & Hospital, Raichur, Karnataka, India.
Gopinath Adusumilli, Department of Orthodontics & Dento-facial Orthopaedics, AME's Dental College & Hospital, Raichur, Karnataka, India.
Raghu Devanna, Department of Orthodontics & Dento-facial Orthopaedics, AME's Dental College & Hospital, Raichur, Karnataka, India.
Saravanan Pichai, Department of Orthodontics & Dento-facial Orthopaedics, AME's Dental College & Hospital, Raichur, Karnataka, India.
Mayur Gobindram Rohra, Department of Orthodontics & Dento-facial Orthopaedics, AME's Dental College & Hospital, Raichur, Karnataka, India.
Sharmila Arjunan, Department of Orthodontics & Dento-facial Orthopaedics, AME's Dental College & Hospital, Raichur, Karnataka, India.
References
- 1.J Pispa, I Thesleff. Mechanisms of ectodermal organogenesis. Dev Biol. 262(2):195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 2.KM Weiss, DW Stock, Z Zhao. Dynamic interactions and the evolutionary genetics of dental patterning. Crit Rev Oral Biol Med. 1998;9(4):369–398. doi: 10.1177/10454411980090040101. [DOI] [PubMed] [Google Scholar]
- 3.I Thesleff, P Nieminen. Tooth induction. Encyclopedia of Life Sciences. New York: Nature Publishing Group, Macmillan Publishers Ltd. Available from: http://http://www.els.net/ [Google Scholar]
- 4.BJ Polder, MA Van't Hof, FP Van der Linden, AM Kuijpers-Jagtman. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004;32(3):217–226. doi: 10.1111/j.1600-0528.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 5.J Daugaard-Jensen, M Nodal, I Kjaer. Pattern of agenesis in the primary dentition: a radiographic study of 193 cases. Int J Paediatr Dent. 2009;19(3):172–175. doi: 10.1111/j.1365-263x.1997.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 6.H Grahnen. Hypodontia in the permanent dentition. Odont Rev. 1956;7:1–100. [Google Scholar]
- 7.K Haavikko. Hypodontia of permanent teeth. Proc Finn Dent Soc. 1971;67:219–225. [Google Scholar]
- 8.T Yonezu, Y Hayashi, J Sasaki, Y Machida. Prevalence of congenital dental anomalies of the deciduous dentition in Japanese children. Bull Tokyo Dent Coll. 1997;38(1):27–32. [PubMed] [Google Scholar]
- 9.GW Thompson, F Popovich. Probability of congenitally missing teeth: results in 1,191 children in the Burlington Growth centre in Toronto. Community Dent Oral Epidemiol. 1974;2(1):26–32. doi: 10.1111/j.1600-0528.1974.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 10.N Burzynski, V Escobar. Classification and genetics of numeric anomalies of dentition. Birth Defects Orig Artic Ser. 1983;19(1):95–106. [PubMed] [Google Scholar]
- 11.H Peters, R Balling. Teeth - where and how to make them. Trends Genet. 1999;15(2):59–65. doi: 10.1016/s0168-9525(98)01662-x. [DOI] [PubMed] [Google Scholar]
- 12.Y Chen, M Bei, I Woo, I Satokata, R Maas. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122(10):3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- 13.R Kist, M Watson, X Wang, P Cairns, C Miles, DJ Reid, H Peters. Reduction of Pax9 gene dosage in an allelic series of mouse mutants causes hypodontia and oligodontia. Hum Mol Genet. 2005;14(23):3605–3617. [Google Scholar]
- 14.A Mostowska, B Biedziak, PP Jagodzinski. Axis inhibition protein 2 (AXIN2) polymorphisms may be a risk factor for selective tooth agenesis. J Hum Genet. 2006;51(3):262–266. doi: 10.1007/s10038-005-0353-6. [DOI] [PubMed] [Google Scholar]
- 15.PA Jezewski, AR Vieira, C Nishimura, B Ludwig, M Johnson, SE O'Brien, S Daack-Hirsch, RE Schultz, A Weber, B Nepomucena, PA Romitti, K Christensen, IM Orioli, EE Castilla, J Machida, N Natsume, JC Murray. Complete sequencing shows a role for MSX1 in nonsyndromic cleft lip and palate. J Med Genet. 2003;40(6):399–407. doi: 10.1136/jmg.40.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]


