Abstract
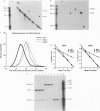
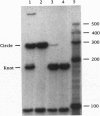
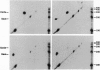
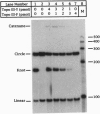
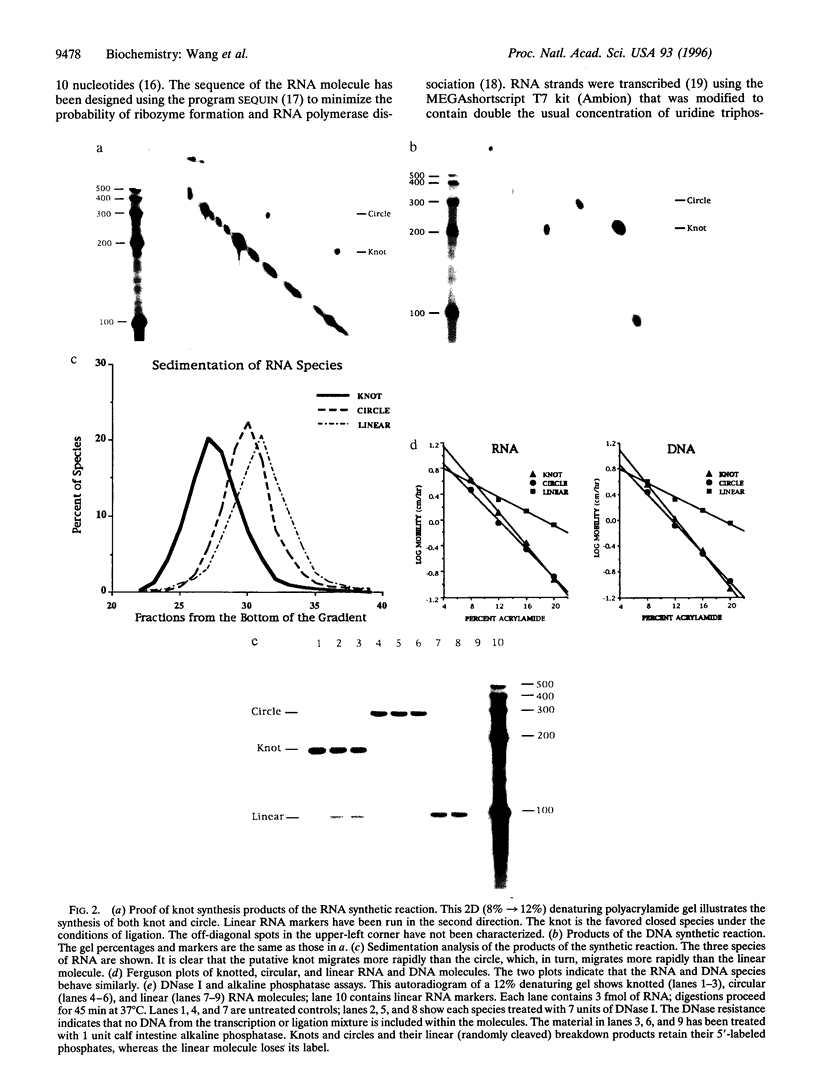
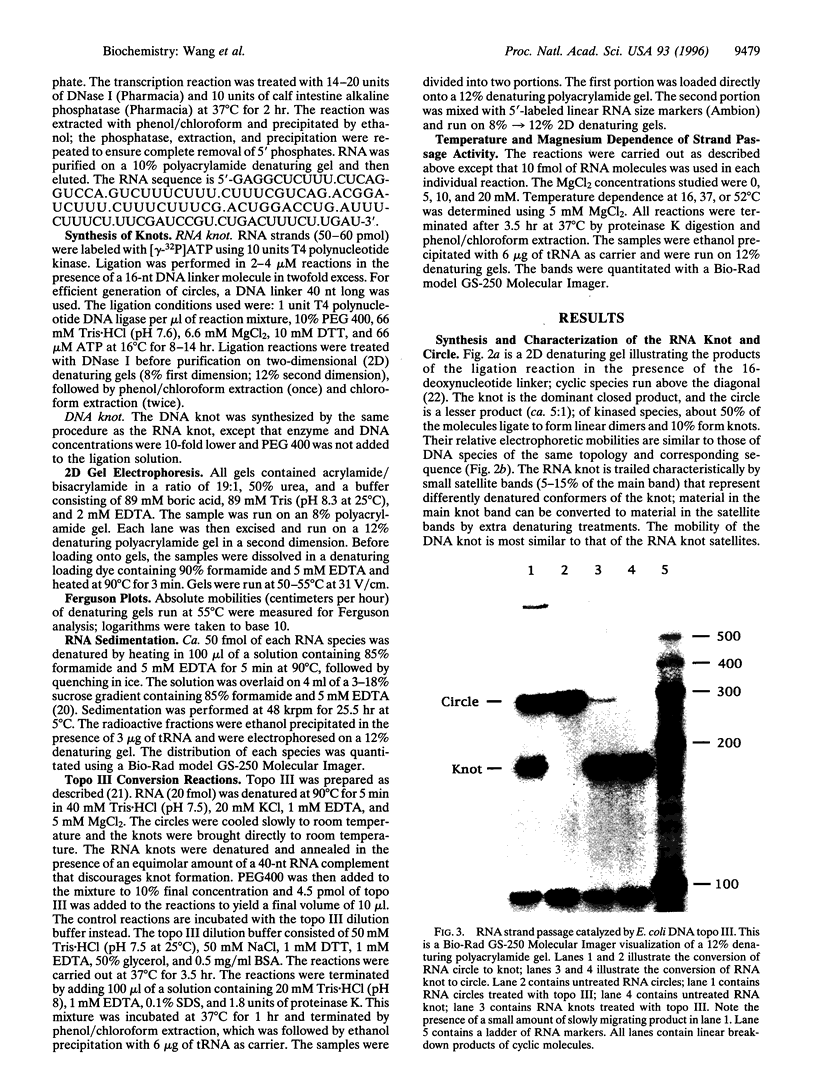
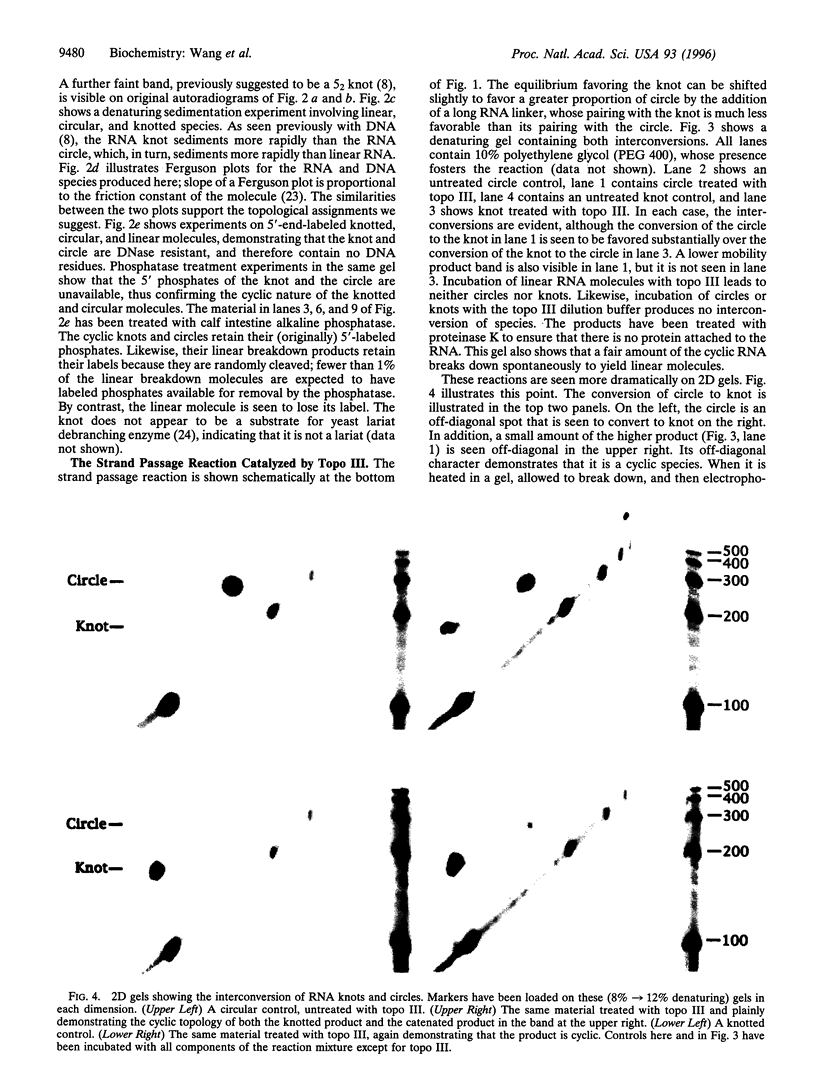
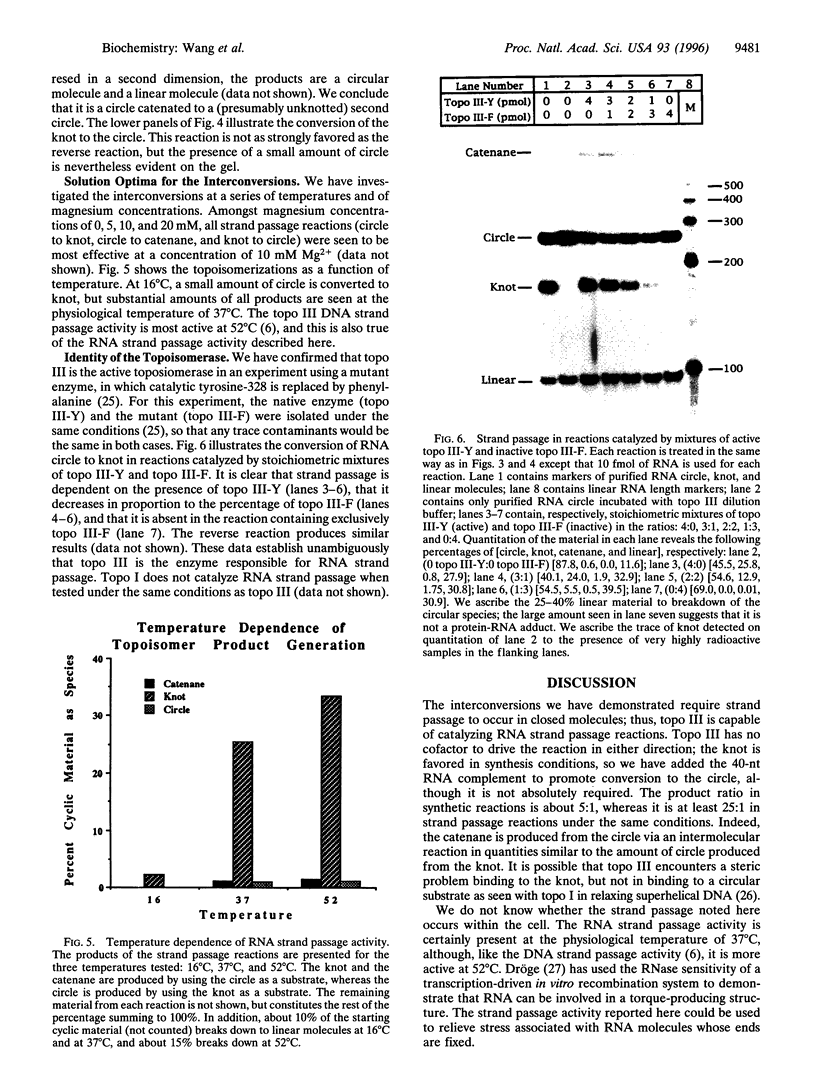
A synthetic strand of RNA has been designed so that it can adopt two different topological states (a circle and a trefoil knot) when ligated into a cyclic molecule. The RNA knot and circle have been characterized by their behavior in gel electrophoresis and sedimentation experiments. This system allows one to assay for the existence of an RNA topoisomerase, because the two RNA molecules can be inter-converted only by a strand passage event. We find that the interconversion of these two species can be catalyzed by Escherichia coli DNA topoisomerase III, indicating that this enzyme can act as an RNA topoisomerase. The conversion of circles to knots is accompanied by a small amount of RNA catenane generation. These findings suggest that strand passage must be considered a potential component of the folding and modification of RNA structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caruthers M. H. Gene synthesis machines: DNA chemistry and its uses. Science. 1985 Oct 18;230(4723):281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- Choi D. J., Marino-Alessandri D. J., Geacintov N. E., Scicchitano D. A. Site-specific benzo[a]pyrene diol epoxide-DNA adducts inhibit transcription elongation by bacteriophage T7 RNA polymerase. Biochemistry. 1994 Jan 25;33(3):780–787. doi: 10.1021/bi00169a020. [DOI] [PubMed] [Google Scholar]
- Coetzee T., Herschlag D., Belfort M. Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev. 1994 Jul 1;8(13):1575–1588. doi: 10.1101/gad.8.13.1575. [DOI] [PubMed] [Google Scholar]
- Dean F., Krasnow M. A., Otter R., Matzuk M. M., Spengler S. J., Cozzarelli N. R. Escherichia coli type-1 topoisomerases: identification, mechanism, and role in recombination. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):769–777. doi: 10.1101/sqb.1983.047.01.088. [DOI] [PubMed] [Google Scholar]
- DiGate R. J., Marians K. J. Escherichia coli topoisomerase III-catalyzed cleavage of RNA. J Biol Chem. 1992 Oct 15;267(29):20532–20535. [PubMed] [Google Scholar]
- Draper D. E. Strategies for RNA folding. Trends Biochem Sci. 1996 Apr;21(4):145–149. [PubMed] [Google Scholar]
- Dröge P. Transcription-driven site-specific DNA recombination in vitro. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2759–2763. doi: 10.1073/pnas.90.7.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S. M., Wang H., Tse-Dinh Y. C., Seeman N. C. Topological transformations of synthetic DNA knots. Biochemistry. 1995 Jan 17;34(2):673–682. doi: 10.1021/bi00002a035. [DOI] [PubMed] [Google Scholar]
- Ford E., Ares M., Jr Synthesis of circular RNA in bacteria and yeast using RNA cyclase ribozymes derived from a group I intron of phage T4. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3117–3121. doi: 10.1073/pnas.91.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D., Khosla M., Tsuchihashi Z., Karpel R. L. An RNA chaperone activity of non-specific RNA binding proteins in hammerhead ribozyme catalysis. EMBO J. 1994 Jun 15;13(12):2913–2924. doi: 10.1002/j.1460-2075.1994.tb06586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa H., DiGate R. J., Marians K. J. Decatenating activity of Escherichia coli DNA gyrase and topoisomerases I and III during oriC and pBR322 DNA replication in vitro. J Biol Chem. 1994 Jan 21;269(3):2093–2099. [PubMed] [Google Scholar]
- Kirkegaard K., Wang J. C. Bacterial DNA topoisomerase I can relax positively supercoiled DNA containing a single-stranded loop. J Mol Biol. 1985 Oct 5;185(3):625–637. doi: 10.1016/0022-2836(85)90075-0. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Uhlenbeck O. C. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Nam K., Hudson R. H., Chapman K. B., Ganeshan K., Damha M. J., Boeke J. D. Yeast lariat debranching enzyme. Substrate and sequence specificity. J Biol Chem. 1994 Aug 12;269(32):20613–20621. [PubMed] [Google Scholar]
- Pley H. W., Flaherty K. M., McKay D. B. Three-dimensional structure of a hammerhead ribozyme. Nature. 1994 Nov 3;372(6501):68–74. doi: 10.1038/372068a0. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- Schofield M. A., Agbunag R., Michaels M. L., Miller J. H. Cloning and sequencing of Escherichia coli mutR shows its identity to topB, encoding topoisomerase III. J Bacteriol. 1992 Aug;174(15):5168–5170. doi: 10.1128/jb.174.15.5168-5170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. G., Finch J. T., Klug A. The crystal structure of an all-RNA hammerhead ribozyme: a proposed mechanism for RNA catalytic cleavage. Cell. 1995 Jun 30;81(7):991–1002. doi: 10.1016/s0092-8674(05)80004-2. [DOI] [PubMed] [Google Scholar]
- Seeman N. C. De novo design of sequences for nucleic acid structural engineering. J Biomol Struct Dyn. 1990 Dec;8(3):573–581. doi: 10.1080/07391102.1990.10507829. [DOI] [PubMed] [Google Scholar]
- Srivenugopal K. S., Lockshon D., Morris D. R. Escherichia coli DNA topoisomerase III: purification and characterization of a new type I enzyme. Biochemistry. 1984 Apr 24;23(9):1899–1906. doi: 10.1021/bi00304a002. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi Z., Khosla M., Herschlag D. Protein enhancement of hammerhead ribozyme catalysis. Science. 1993 Oct 1;262(5130):99–102. doi: 10.1126/science.7692597. [DOI] [PubMed] [Google Scholar]
- Wang H., Du S. M., Seeman N. C. Tight single-stranded DNA knots. J Biomol Struct Dyn. 1993 Apr;10(5):853–863. doi: 10.1080/07391102.1993.10508679. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- Whoriskey S. K., Schofield M. A., Miller J. H. Isolation and characterization of Escherichia coli mutants with altered rates of deletion formation. Genetics. 1991 Jan;127(1):21–30. doi: 10.1093/genetics/127.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. R., Chastain M., Puglisi J. D. Synthesis and purification of large amounts of RNA oligonucleotides. Biotechniques. 1991 Dec;11(6):764–769. [PubMed] [Google Scholar]
- Zhang H. L., DiGate R. J. The carboxyl-terminal residues of Escherichia coli DNA topoisomerase III are involved in substrate binding. J Biol Chem. 1994 Mar 25;269(12):9052–9059. [PubMed] [Google Scholar]