Abstract
Aim
Cannabinoid receptor type 1 (CB1) antagonists show central side effects, whereas beneficial effects are most likely peripherally mediated. In this study, the peripherally selective CB1 antagonist TM38837 was studied in humans.
Methods
This was a double-blind, randomized, placebo-controlled, crossover study. On occasions 1–4, 24 healthy subjects received 5 × 4 mg THC with TM38837 100 mg, 500 mg or placebo, or placebos only. During occasion 5, subjects received placebo TM38837 + THC with rimonabant 60 mg or placebo in parallel groups. Blood collections and pharmacodynamic (PD) effects were assessed frequently. Pharmacokinetics (PK) and PD were quantified using population PK−PD modelling.
Results
The TM38837 plasma concentration profile was relatively flat compared with rimonabant. TM38837 showed an estimated terminal half-life of 771 h. THC induced effects on VAS feeling high, body sway and heart rate were partly antagonized by rimonabant 60 mg [−26.70% [90% confidence interval (CI) −40.9, −12.6%]; −7.10%, (90%CI −18.1, 5.3%); −7.30%, (90% CI −11.5%, −3.0%) respectively] and TM38837 500 mg [−22.10% (90% CI −34.9, −9.4%); −12.20% (90% CI −21.6%, −1.7%); −8.90% (90% CI −12.8%, −5.1%) respectively]. TM38837 100 mg had no measurable feeling high or body sway effects and limited heart rate effects.
Conclusions
Rimonabant showed larger effects than TM38837, but the heart rate effects were similar. TM38837 100 mg had no impact on CNS effects, suggesting that this dose does not penetrate the brain. This TM38837 dose is predicted to be at least equipotent to rimonabant with regard to metabolic disorders in rodent models. These results provide support for further development of TM38837 as a peripherally selective CB1 antagonist for indications such as metabolic disorders, with a reduced propensity for psychiatric side effects.
Keywords: cannabinoid receptor type 1 antagonist, peripheral CB1 antagonist, pharmacokinetics, PK−PD, rimonabant, TM38837
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The market withdrawal of rimonabant negatively affected cannabinoid antagonist research and most research programmes were stopped. However, preclinical research showed that the beneficial effects of these compounds are peripherally mediated, whereas the unwanted side effects are of central origin. This means that peripherally active cannabinoid antagonists have therapeutic potential.
WHAT THIS STUDY ADDS
This is the first study in which a peripheral cannabinoid antagonist (TM38837) was tested in healthy volunteers. TM38837 showed a larger specificity for peripheral effects than for central effects. This confirms the assumption that cannabinoid type 1 antagonists have the potential of treating several (metabolic) disorders without the central side effects, and should be further developed.
Introduction
Research on the cannabinoid system has largely increased in the last decades, since the discovery of cannabinoid receptors type 1 and 2 (CB1 and CB2) and endogenous cannabinoids from 1988 onwards [1–5]. The endogenous cannabinoid system, or endocannabinoid system (ECS), is located throughout the body. CB1 receptors are present in the central nervous system and at peripheral sites such as the heart, liver, pancreas and adipose tissue [6, 7], whereas CB2 receptors are mainly present in immune cells [3, 8, 9].
Although the exact functions of the endocannabinoid system are unknown, the widespread presence suggests that the system could have a variety of functions, which could be studied for various clinical indications. Obesity and associated diseases are among the major medical conditions for which involvement of the endocannabinoid system is currently studied. Obesity, or severe overweight, is a condition that affects approximately 500 million adults worldwide, and the World Health Organization estimates this number to increase to 700 million adults in 2015 [10].
Rimonabant was the first CB1 receptor antagonist that was registered in 2006 as an adjunct to diet and exercise for the treatment of obese patients, or overweight patients with associated risk factors such as dyslipidaemia, diabetes mellitus type 2 or cardiovascular risk factors [11]. However, 2 years later, rimonabant was withdrawn from the market due to adverse psychiatric effects such as depression [12, 13]. The beneficial effects of rimonabant in patients included decrease of appetite, weight loss and weight loss independent improvement of metabolic parameters such as HDL cholesterol, triglycerides, fasting glucose and insulin concentrations [14] [15, 16].
CB1 receptors are widely distributed throughout the brain, including central nervous system areas that are involved in the regulation of food intake and metabolism (for review, see [17]). Nevertheless, there is considerable evidence to suggest that the beneficial metabolic effects of CB1 antagonists are mediated by CB1 receptors that are present at locations which are specifically associated with metabolic regulation, such as the liver, the pancreas and fat cells [6, 7]. A study in rats demonstrated that centrally administered rimonabant did not affect feeding behaviour, whereas peripheral rimonabant inhibited food intake [18]. Other studies found that peripheral, but not central, CB1 antagonism induced beneficial effects on metabolism and feeding behaviour [19, 20]. A recent study by Tam et al. suggests that peripheral CB1 inverse agonism reduces obesity by reversing obesity-related leptin resistance [21]. This suggests that the beneficial metabolic effects of rimonabant might be regulated by peripheral CB1 receptors, whereas the psychiatric side effects could be regulated by centrally located CB1 receptors.
TM38837 is a new peripherally acting CB1 antagonist that has demonstrated efficacy in pre-clinical studies [22]. TM38837 showed 30 times less potency on centrally induced body temperature effects compared with rimonabant, whereas TM38837 was only 3 to 10 times less potent than rimonabant on gastro-intestinal effects [22]. In a first in human trial, dosages up to 900 mg were well tolerated in healthy subjects, obese patients and liver fibrotic patients [22].
In the current study the central and peripheral effects of TM38837 and rimonabant in healthy subjects were investigated. Since acute administration of CB1 antagonists does not have measurable effects in healthy volunteers, the δ9-tetrahydrocannabinol (THC)-challenge test was used in this study [23, 24]. The THC-challenge test is able to quantify the displacement of the concentration–effect curve of the CB1 agonist THC by different doses of a CB1 antagonist for various pharmacodynamic (PD) parameters. These parameters include measures that are mediated via the central nervous system, such as the subjective effect ‘feeling high’, measures that could be affected by processes at multiple locations, such as postural stability, and heart rate, which is likely to be peripherally mediated [25]. In this way, the central and peripheral characteristics of the effect profile in healthy subjects can be assessed. Rimonabant was used as a positive control for both central and peripheral effects. Quantification of modulation of the concentration–effect curve of THC by CB1 antagonists was done by building a population PK–PD model for THC, TM38837 and rimonabant.
Our hypothesis was that TM38837 would show no effects or small effects on central nervous system parameters, while showing clear effects on biomarkers that are more likely to be peripherally mediated, such as heart rate.
Methods
Study design
This was a double-blind, double dummy, partially randomized, placebo-controlled, crossover, partial parallel study with a washout period of at least 12 days.
Subjects
Healthy male volunteers aged 18 to 45 years were included in the study. Subjects had to be cannabis users for at least 1 year with using frequency of no more than once a week, and had to be able to refrain from using cannabinoids from at least 3 weeks prior to the first treatment period up to the end of the study. Previous studies reported that Black subjects have different rimonabant pharmacokinetics (PK) compared with subjects from other races [26, 27]. Therefore, Black people were excluded from the study.
Twenty-four healthy male volunteers were planned to complete five periods. The study was powered as a bio-equivalence study [28]. This was based on the hypothesis that there is no or small difference in central nervous system response between THC alone and THC + TM38837, which could be defined as a lack of effect when comparing TM38837 with THC alone treatment, or bio-equivalent effects according to the bio-equivalence guideline [28]. At the time of study performance, these guidelines included the criteria that the 90% confidence intervals (CI) of the rate ratios for the main effects of the two treatments would lie within the range 0.80–1.25.
Procedure
Subjects gave written informed consent before any study-specific procedure was performed. Eligible subjects were enrolled in the study after a general health screen within 3 weeks before the first study day. Subjects were acquainted with the experimental methods and conditions in a training session including the inhalation procedure using THC vehicle. At all treatment visits, subjects stayed at the clinic for 2 days. Alcohol breath test and urine drug screen had to be negative on each treatment visit. PD and PK measurements were frequently performed on all study days (indicated in Table 1). A follow-up visit was scheduled approximately 14 days after the last study day. The study protocol was approved by the Medical Ethics Review Board of Leiden University Medical Center and complied with the principles of ICH-GCP, the Helsinki declaration and Dutch laws and regulations.
Table 1.
Overview of study day procedures
| Time (h) | Procedures study day |
|---|---|
| −1 h 30 min–0 h 00 min | Arrival, breakfast, vital signs, drug screen, alcohol breath test, PD block* (twice), blood sampling TM38837 or rimonabant |
| 0 h 00 min | TM38837 administration |
| 1 h 30 min | Snack |
| 2 h 00 min | Rimonabant administration |
| 2 h 48 min–4 h 00 min | PD block* (twice), vital signs, lunch |
| 4 h 00 min | 1st THC administration |
| 4 h 05 min–6 h 30 min | Blood sampling TM38837 or rimonabant (4 h 06 min) and THC (4 h 05 min, 4 h 19 min, 5 h 50 min), PD block* (thrice), snack |
| 6 h 30 min | 2nd THC administration |
| 6 h 35 min–9 h 00 min | Blood sampling TM38837 or rimonabant (6 h 36 min) and THC (6 h 35 min, 6 h 49 min, 8 h 20 min), PD block* (thrice), dinner |
| 9 h 00 min | 3rd THC administration |
| 9 h 05 min–24 h 00 min | Blood sampling TM38837 or rimonabant (9 h 06 min) and THC (9 h 05 min, 9 h 19 min, 10 h 50 min), PD block* (four times), vital signs (twice), breakfast |
| 24 h 00 min | 4th THC administration |
| 24 h 05 min–26 h 30 min | Blood sampling TM38837 or rimonabant (24 h 06 min) and THC (24 h 05 min, 24 h 19 min, 24 h 50 min), PD block* (thrice), vital signs, lunch |
| 26 h 30 min | 5th THC administration |
| 26 h 30 min–30 h 00 min | Blood sampling TM38837 or rimonabant (28 h 21 min) and THC (26 h 35 min, 26 h 49 min, 28 h 20 min), PD block* (thrice), vital signs, snack |
PD block consists of body sway measurement, VAS B&L and Bowdle, heart rate measurement. AEs and concomitant medication were recorded continuously.
Treatments
The treatments that were administrated can be found in Table 2. Each CB1 antagonist or placebo administration was followed by five inhaled doses of vaporized THC 4 mg diluted in 400 μl 100% ethanol or THC vehicle, which consisted only of vaporized ethanol. THC was vaporized using a Volcano vaporizer® (Storz & Bickel GmbH & Co. KG, Tuttlingen, Germany). Procedures for vaporizing the solution and inhalation of the vapour were done according to a method previously described by Zuurman et al. [29]. The tmax of TM38837 was expected at approximately 4 h after administration, whereas rimonabant had a tmax of 2 h [22, 26]. Therefore, oral TM38837 was dosed at time point 0 h, oral rimonabant was dosed 2 h later to account for expected differences in tmax, and three subsequent intrapulmonary THC doses were given from t = 4 h with 2.5 h intervals. In this way, the first THC inhalation would be administered at the expected tmax of TM38837 and rimonabant. Two THC doses were administered at 2.5 h intervals 24 h after TM38837 administration. A schematic overview of the administrations and other study day procedures can be found in Table 1.
Table 2.
The study consisted of a four-way cross-over part and a parallel part. Rimonabant or placebo rimonabant were always randomly administered at the fifth occasion. All subjects received all treatments from occasion 1 to 4 and the subjects were split into two groups for occasion 5 with half of the subjects receiving rimonabant 60 mg and the other half receiving placebo rimonabant 60 mg
| Occasion, study design | Study sample | TM38837* | Rimonabant | THC† |
|---|---|---|---|---|
| Occasions 1–4 Crossover | 100% | 100 mg | Placebo | Placebo |
| 500 mg | Placebo | Placebo | ||
| Placebo | Placebo | 5 × 4 mg | ||
| Placebo | Placebo | Placebo | ||
| Occasion 5 Parallel | 50% | Placebo | Placebo | 5 × 4 mg |
| 50% | Placebo | 60 mg | Placebo |
Penn Pharma, Gwent, United Kingdom.
Farmalyse b.v., Zaandam, the Netherlands.
Rimonabant has a terminal half-life of 6–9 days after multiple ascending doses in healthy volunteers [30]. To minimize the risk of long lasting carry-over effects that could complicate the interpretation of the effects of TM38837, each rimonabant treatment arm was always scheduled at the fifth occasion, thereby splitting the study design into a four way crossover part and a parallel part (Table 2).
TM38837 dosages were based on preclinical and clinical studies [22]. The 100 mg dose was selected in order to explore exposure of the anticipated therapeutic concentration. A 500 mg dose in the fed state was expected to give similar exposure to that seen after the highest dose (900 mg) explored in the fasted state, as examined in the first in man single ascending dose study (7TM data on file). This exposure was well tolerated by all subjects. Rimonabant 60 mg dosage was selected in order to obtain plasma concentrations in the clinically effective range. The recommended therapeutic dose of rimonabant was 20 mg. However, as steady-state exposures are 3.3-fold higher than those observed after a single dose [26], a single dose of 60 mg rimonabant per subject was administered in this study in order to achieve a maximum plasma concentration that was comparable with the steady-state concentration with therapeutic dosages. Based on previous cannabinoid challenge studies with CB1 antagonists such as rimonabant, we expected that a dose of 60 mg rimonabant would be sufficient to suppress THC-induced effects [31–33]. THC dosages and dosing schedules were selected in order to obtain and maintain clear, sub-maximal central nervous system effects as predicted by PK−PD models that were based on previous studies [29, 33].
Outcome measures
Pharmacokinetic assessments and bio-analyses
Time points of venous blood sampling for PK analyses of TM38837, rimonabant and THC can be found in Table 1.
TM38837 and rimonabant
Venous blood was collected in 4 ml Li-Hep tubes. The blood samples were kept on ice and centrifuged within 30 min of collection at 2000 g at 4°C for 10 min. The supernatant plasma was divided into three or four 2 polypropylene tubes. Samples were stored at −80°C and sent to Quotient Bioresearch (Fordham, UK) for analysis. Measurements of TM38837 and rimonabant concentrations in human plasma samples were performed according to bioanalytical methods that were validated. Concentraions of TM38837 and rimonabant were measured by liquid chromatography with tandem mass spectrometry method with a lower limit of quantification of 0.1 ng ml−1 for TM38837, and 1.0 ng ml−1 for rimonabant. For TM38837 analysis precision was 4.3% and accuracy was −1.3% and for rimonabant precision was 4.5% and accuracy −1.3%.
THC
For determination of the concentration of plasma THC and its metabolites 11-OH-THC and 11-nor-9-carboxy-THC venous blood was collected in 2 ml EDTA tubes. As cannabinoids are photosensitive, samples were protected from light at all times. After blood collection the tubes were put in ice water in aluminium foiled containers, and were centrifuged within 1 h for 10 min at 2000 g at 4°C. The supernatant plasma was divided into two 2 ml brown polypropylane tubes. Plasma samples were stored at a temperature of −20°C and sent to ABL (Assen, the Netherlands) for PK analysis. Plasma THC as well as metabolite concentrations (11-hydroxy-THC and 11-nor-9 carboxy-THC) were determined using tandem mass spectrometry with a lower limit of quantification of 0.1 ng ml−1.
Pharmacodynamic assessments
The choice of the PD end points was based on a previous review and prior studies by Zuurman et al. [29, 33]. PD measurements were performed at time points indicated in Table 1.
Body sway
The body sway meter (André Ibelings, TNO/ICT, Delft, the Netherlands) is an objective assessment of antero-postural sway in mm per 2 min. The antero-postural sway is regulated by different factors, such as attention and motor coordination, involving the central and peripheral nervous system and vestibular processes. Visual feedback was eliminated by closing the eyes. Measurements were performed according to a procedure previously described [29].
Visual analogue scales (VAS)
VAS by Bond & Lader is a 16-item assessment of subjective effect on alertness (composition of items alert/drowsy, strong/feeble, muzzy/clear-headed, well coordinated/clumsy, lethargic/energetic, mentally slow/quick-witted, attentive/dreamy, incompetent/proficient and interested/bored), on mood (composition of items contended/discontended, troubled/tranquil, happy/sad, antagonistic/amicable and withdrawn/gregarious) and calmness (composition of items calm/excited and tense/relaxed) [34]. The adapted version of VAS by Bowdle [35] is a 13-item assessment of subjective effects on item feeling high and on factors of internal and external perception, both compositions of items that are affected differently by THC as previously described [29].
Beck's depression inventory II (BDI)
The BDI is a 21-item self-report questionnaire for measuring the severity of depression with a four-point Likert scale for each question [36]. The questionnaire was included in the study to check for possible mood changes, since previous multiple dose studies with rimonabant reported a larger incidence of subjects suffering from depression [14, 16]. The BDI was performed one time per occasion at 9.5 h after TM38837 or placebo TM38837 administration.
Heart rate and blood pressure
Heart rate and blood pressure were measured using the Nihon-Koden BSM-1101 K monitor (Lifescope EC, Tokyo, Japan) blood pressure apparatus. All heart rate measurements were used for PD analysis.
Adverse events and concomitant medication were recorded from screening until the follow-up period.
Data analysis
For the direct clinical effect, PK and PD comparisons of TM38837 and rimonabant, data were used only from subjects who received rimonabant 60 mg + THC treatment during the fifth study occasion.
Clinical effects
Evaluation of the safety data were based on the review of individual values and descriptive statistics. Analysis of laboratory parameters was performed using screening and end of study assessments. For vital signs (heart rate and blood pressure), raw data and changes from baseline were analyzed by type of measurement and parameter and treatment using descriptive statistics. Heart rate, PR, QRS and QT intervals, corrected QT (QTc) from automatic readings were analyzed as raw parameter value and change from baseline (for HR and QTc only). Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA version 13.0).
Pharmacokinetics
All concentrations and maximal concentration (Cmax), time of maximal concentration (tmax), area under the curve from zero to infinity (AUC(0,∞), and terminal half-life (t1/2) of TM38837, rimonabant, THC, and its metabolites 11-OH-THC and THC-COOH were analyzed using non-compartmental analysis (SAS PROC MIXED 9.1.3).
Pharmacodynamics
To study the effect of repeated doses of THC on the PD measures or other carry-over effects, the fifth occasion of the 12 subjects receiving only THC was compared graphically and statistically with the previous occasion in which subjects received THC only. If no significant period effect could be established, the fifth occasion would be used for a five-way cross-over analysis. For this five-way partial crossover subanalysis, and for the four-way crossover part of the study, the PD variables were analyzed with a mixed model analysis of variance (using SAS PROC MIXED 9.1.3) with treatment, time, and treatment by time as fixed effects, with subject, subject by treatment and subject by time as random effects, and the average baseline value was included as covariate. The parallel part was analyzed with subject as random effect, with treatment, time and treatment by time as fixed effects, and the average baseline value as covariate. A 90% CI around the ratio was used for statistical comparison between TM38837 + THC and placeboTM38837 + THC treatment with α = 0.05 two-sided [28]. Graphs of the Least Squares Means estimates over time by treatment were presented with 90% CI as error bars. Body sway was log transformed before analysis to correct for the log-normal distribution. All PD effects were statistically compared with heart rate effects. We assumed that heart rate primarily represents a peripheral CB1 effect and that beneficial effects mediated by CB1 antagonists are peripherally mediated.
Population PK and PK–PD modelling
Population PK and PK–PD modelling were performed using nonlinear mixed effect modelling (nonmem version 7.1.0, GloboMax LLC, Ellicot City, MD). The pre-dose samples that were taken at an occasion following a study day where TM38837 was administered were also used for the PK model of TM38837. The compartmental population PK analysis was based on the results of previous CHDR studies with multiple THC inhalations, which used a two-compartment model with bolus administration [25]. The empirical Bayes estimates from the THC PK analysis were used to describe the THC profile. Parameter estimation for population PK modelling of THC, rimonabant and TM38837 was performed under ADVAN 5, and the PK–PD modelling of all PD parameters was performed under ADVAN6 TOL 5. First order conditional estimation with interaction was the standard method of estimation, with exception for VAS feeling high, for which LAPLACE was used. Within each model, additive and proportional residual error models were compared.
For PK–PD modelling, an effect compartment was incorporated to account for delay in response, in which the concentration–effect was modelled as a linear and a maximal effect relationship. The drug–effect relations were assumed to cause a horizontal shift on the concentration–effect profile. Therefore the drug–effect relationships were only applied to the parameter describing the concentration at which half the maximum effect is reached (EC50). Internal model selection and validation was performed using minimum objective function value, goodness of fit plots and visual predictive checks (VPCs). For the VPCs, 1000 replications of the model were simulated and the median, 5th and 95th percentiles were calculated for each simulated time point and compared visually with the actual data [37].
The inhibition ratios are defined in percentages and quantify the maximum inhibition of the THC-induced effect (defined as 100%) by either rimonabant, TM38837 100 mg or 500 mg. The median of the inhibition ratios was calculated with their 90% CI by using the PK–PD models, simulated for 1000 individuals.
To minimize the effect of over- and under-dispersion due to the subjectivity of the VAS scale, and to include non-response in the model, the VAS feeling high scale was translated to a binary scale, to accommodate the possibility to construct a probability model for feeling high. The anchor point for this translation was the median of all scores higher than 0.
Results
Subject demographics
Thirty-six healthy young males were randomized and treated, and 24 subjects completed five occasions. Concerning the parallel part of the study, 10 subjects received rimonabant + THC treatment, and 14 subjects received placebo rimonabant + THC treatment. Four subjects dropped out for personal reasons (i.e. time schedule conflict and not liking the study days), two after the first and two after the second occasion. Three subjects dropped out because of adverse events: two during the first occasion (occasion with THC alone treatment) and one during the second (occasion 1 was placebo, occasion 2 THC alone). The adverse events were THC related (i.e. derealization, pre-syncope and anxious feeling). One subject was not compliant and dropped out after the third occasion. Four subjects suffered study schedule delays and could eventually not complete five occasions because of irremediable expiry dates of rimonabant and THC, one subject after occasion 3 and three subjects after occasion 4. All 36 subjects' completed occasions were included in the analyses. Subject demographics were balanced for all dose arms. The average age was 21.2 years (SD 3.8 years), the average BMI was 22.9 kg m−2 (SD 2.1 kg m−2), height was 183.42 cm (SD 6.99 cm) and the average weight was 77.25 kg (SD 10.18 kg).
Adverse effects
Adverse events were of mild to moderate intensity and transitory in nature. No serious adverse events were reported during the study. One subject discontinued during his first occasion with placebo TM38837 + THC treatment due to pre-syncope and anxiety, which occurred 11 min after the first THC inhalation. Another subject discontinued after the first occasion with placebo TM38837 + THC due to anxiety that started 1 h 29 min after placebo TM38837 administration. During the second occasion with placebo TM38837 + THC, one subject decided to discontinue due to derealization that started 14 min after the first THC inhalation.
The total number of subjects who had an adverse event was similar for all treatment groups (90.0%–96.9%), except for the placebo TM38837 + placebo group (63.3%). Most of the adverse events were classified as psychiatric and nervous system disorders, and mainly the psychiatric disorders were considered to be probably THC-related. Euphoric mood (‘feeling high’) was by far the most frequently reported psychiatric effect, especially in the treatment groups that received THC in combination with the 100 mg dose of TM38837, placebo TM38837 or placebo rimonabant (23/32 or 71.9%, 25/34 or 73.5% and 13/14 or 92.9%, respectively). Euphoria was less common when THC followed administration of TM38837 500 mg or rimonabant (16/31 or 51.6%, and 5/10 or 50% respectively). In the nervous system disorder class, the most frequent adverse event was somnolence, which occurred with a similar frequency for TM38837 100 mg (20/32 or 62.5%), 500 mg (20/31 or 64.5%), rimonabant (5/10 or 50.0%) and placebo TM38837 + THC (19/34 or 55.9%) treatment groups, to a larger extent in the placebo rimonabant + THC treatment group (12/14 or 85.7%) and to a lesser extent in the placebo group (10/30 or 33.3%).
Other frequently occurring adverse events in all treatment groups, including placebo, were fatigue (14.3% to 41.2%), dizziness (3.3% to 35.7%), headache (6.7% to 18.8%) and hypersomnia (6.7% to 28.6%). These adverse events were less frequent in the placebo group and of similar frequency in the active treatment groups.
No clinically relevant changes were found for blood pressure, haematology, biochemistry, urinalysis or any of the ECG intervals. Heart rate changes were analyzed as PD parameters.
PK and PK–PD models
The PK of THC, TM38837 and rimonabant were described by a two compartmental PK model with first order elimination. The oral absorption of rimonabant and TM38837 followed a first order process, and the pulmonary absorption of THC was considered as a bolus administration. The increase of the concentration after administration of rimonabant was insufficiently detailed to estimate the first order absorption rate constant with sufficient precision. Therefore this parameter was fixed to the value for the absorption rate constant reported by Martinez et al. [27] at Ka = 1.17 h−1. For all models, the residual error model was proportional and individual empirical Bayes' estimates were employed to describe the concentration profile used in the population PK and PK–PD analyses.
An overview of the PK parameters can be found in Table 3 (non-compartmental analysis) and in Table 4 (compartmental analysis). TM38837 and rimonabant showed different concentration–time profiles (Figure 1). TM38837 had a relatively flat PK profile compared with rimonabant, which was related to the low absorption rate constant and the low clearance. This caused similar exposure of TM38837 during all five THC challenge tests within a study occasion, whereas for rimonabant during the first three THC challenges the exposure levels to the CB1 antagonist were distinctly higher than for the two THC challenges that were given on the second day of a study occasion. tmax of TM38837 (12.55 h to 13.01 h) was longer compared with rimonabant (4.11 h). TM38837 had a long half-life of 771 h, whereas the half-life of rimonabant was 12.7 h as estimated using compartmental analysis.
Table 3.
Pharmacokinetic parameters from non-compartmental analysis with means (SD)
| TM38837 100 mg | TM38837 500 mg | Rimonabant | |
|---|---|---|---|
| Cmax (ng ml−1) | 2860 (2377) | 12 449 (1620) | 620 (113) |
| tmax (h) | 13.01 (8.28) | 12.55 (8.53) | 4.11 (0.03) |
| AUC(0,∞) (ng ml−1 h) | 86 088 (49 862) | 327 907 (190 569) | 6952 (1534) |
Table 4.
Population PK parameter estimates for TM38837, rimonabant and THC with relative standard error (RSE, %) inter-individual variability as %CV
| Parameter estimate | THC | TM38837 | Rimonabant | ||||
|---|---|---|---|---|---|---|---|
| Estimate (RSE) | IIV | IOV | Estimate (RSE) | IIV | Estimate (RSE) | IIV | |
| Clearance/F (l h−1) | 200 (5.9) | 31.2 | - | 2.20 (9.29) | 66.2 | 9.30 (6.87) | 25.6 |
| Central volume/F (l) | 28.5 (8.91) | 40.8 | 25.1 | 18.7 (16.3) | 132.0 | 39.3 (15.5) | 20.6 |
| Peripheral volume of distribution/F (l) | 107 (14.3) | – | – | 10.8 (42.4) | – | 93.0 (12.8) | – |
| Intercompartmental clearance/F (l h−1) | 106 (6.9) | – | – | 0.00975 (22.0) | – | 17.9 (17.2) | – |
| Absorption rate constant (Ka; h−1) | – | – | – | 0.0789 (9.72) | – | 1.17† | – |
| Terminal half-life (h)* | 1.11 (11.0) | 4.98 | – | 771 (21.8) | – | 12.7 (11.5) | – |
Parameter derived from the model.
Fixed parameter. F, bioavailability; IIV, inter-individual variability (%); IOV, inter-occasion variability (%).
Figure 1.
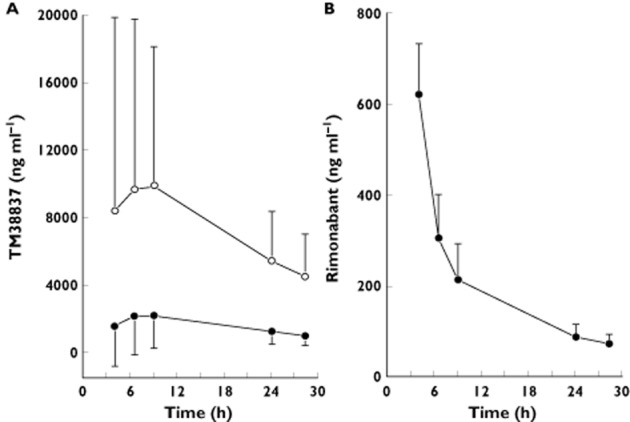
Mean concentration–time profile of TM38837 100 mg (•) and 500 mg (○) (A) and the profile of rimonabant (B). TM38837 treatments were administered at t = 0 h, and rimonabant was administered at t = 2 h. Error bars represent the SD
Pharmacodynamics
THC showed significant increases on body sway, heart rate, feeling high and external perception. For internal perception, almost half of the subjects showed no response (12 non-responders vs. 16 responders) and the average effect was limited. No THC effect was found on VAS mood and only limited effects were found on VAS alertness and calmness (estimate of difference −5.5% and 4.1% respectively). Therefore VAS internal perception, alertness, mood and calmness were not considered relevant efficacy parameters for evaluating inhibition of THC induced effects and were therefore not further described.
THC period effects were found for VAS feeling high (0.101 log mm, P = 0.0004) and heart rate (1.4 beats min−1, P = 0.0338). As these changes were very small compared with the treatment effects, the period effects of VAS feeling high and heart rate parameters were not considered to have had a significant impact on the study results.
A graphical representation of TM38837 and rimonabant effect profiles on THC-induced feeling high can be found in Figure 2. TM38837 antagonizing effects started on day 1 and reached their maximum on day 2, whereas for rimonabant the effects were maximal on the first measurement of day 1 (4 h post-dosing) and diminished during the second day. Overall this seemed to be consistent with the plasma concentration–time profiles and the shorter tmax (≍4 h) and t1/2 (13 h) of rimonabant as compared with TM38837 using non-compartmental PK analysis.
Figure 2.
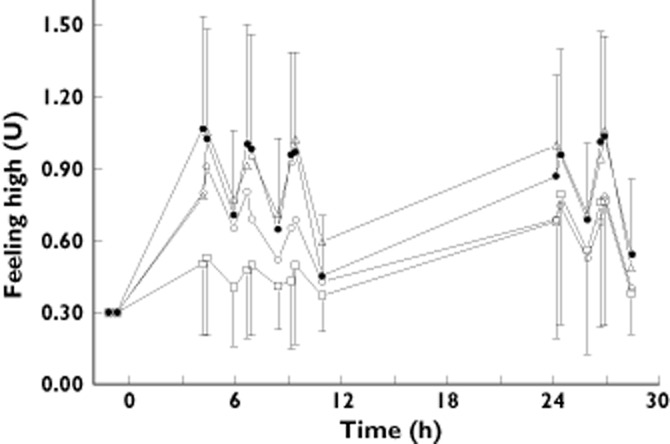
Effect–time profiles of feeling high after TM38837 100 mg (▵), 500 mg (○), rimonabant (□) or placebo antagonist (•) administration
Because of the different time frames of TM38837 and rimonabant time–effect profiles, no proper comparison of peak effects could be made. Instead, the complete effect profiles were compared from the data of the five-way subanalysis. The results of these comparisons are given in Table 5. The results of the four-way crossover analysis including all subjects were very similar to the results from the five-way crossover subanalysis and are therefore not shown. TM38837 100 mg did not significantly inhibit THC effects, except for a small reduction of VAS external perception, which failed to reach significance with the higher dose of TM38837. Both rimonabant 60 mg and TM38837 500 mg inhibited all other THC effects.
Table 5.
Statistical analysis of pharmacodynamic parameters. Estimated difference (90% CI) and P value. Bold numbers are the significant differences (P < 0.05). Data were analyzed for the complete time profile
| VAS feeling high* | VAS External* | Body sway | Heart rate | |
|---|---|---|---|---|
| Rimonabant vs. placebo | – | – | 29.60% (14.3%,47.1%) P = 0.0012 | 4.60% (−0.2%,9.3%) P = 0.1177 |
| TM38837 100 mg vs. THC | 0.20% (−12.5%,12.9%) P = 0.9753 | −10.20% (−18.1%,−2.2%) P = 0.0379 | −1.20% (−11.6%,10.5%) P = 0.8605 | −3.70% (−7.5%,0.2%) P = 0.1137 |
| TM38837 500 mg vs. THC | −22.10% (−34.9%,−9.4%) P = 0.0059 | −7.80% (−15.7%,0.0%) P = 0.1005 | −12.20% (−21.6%,−1.7%) P = 0.0588 | −8.90% (−12.8%,−5.1%) P = 0.0003 |
| Rimonabant vs. THC | −26.70% (−40.9%,−12.6%) P = 0.0030 | −17.80% (−26.5%,−9.1%) P = 0.0016 | −7.10% (−18.1%,5.3%) P = 0.3287 | −7.30% (−11.5%,−3.0%) P = 0.0063 |
| TM38837 100 mg vs. rimonabant | −26.90% (−40.9%,−12.9%) P = 0.0025 | −8.50% (−18.1%,1.1%) P = 0.1440 | −6.00% (−17.0%,6.3%) P = 0.4017 | −3.70% (−8.2%, 0.7%) P = 0.1650 |
| TM38837 500 mg vs. rimonabant | −5.90% (−23.9%,12.1%) P = 0.5815 | −10.80% (−20.2%,−1.5%) P = 0.0586 | 5.70% (−6.6%,19.7%) P = 0.4547 | 1.80% (−2.8%, 6.5%) P = 0.5089 |
Tests were analyzed without placebo results, as these showed no variance. CI, confidence interval; THC, tetrahydrocannabinol.
Another graphical analysis of effects was performed to avoid the differences in time frames between TM38837 and rimonabant. Figure 3 represents a visualization of heart rate effects plotted against body sway and P (feeling high > 12) expressed as inhibition ratios that were estimated using the PK–PD models. Each point estimate represents the effects that were measured after one of the five THC dosages. The estimated values and 90% CI are given in Table 6. When comparing the effects on heart rate to those on P (feeling high > 12) (expressed as inhibition of the THC effect), rimonabant has similar effect magnitudes for both heart rate and P (feeling high > 12) (around 80% inhibition of the THC effect). TM38837 500 mg maximally inhibited THC-induced heart rate increase by 87.8% (90% CI 80.4, 92.5%) and feeling high by 30.4% (90% CI 24.0, 38.6%). TM38837 100 mg shows a 59.3% (90% CI 45.6, 71.3%) heart rate inhibition against a 7.47% (90% CI 5.5, 10.0%) inhibition of feeling high. The relationship between heart rate and body sway is comparable with the association between heart rate and P (feeling high > 12) although slightly less pronounced (see Figure 3 for the relationship between the effects and Table 6 for the estimated values and 90% CI).
Figure 3.
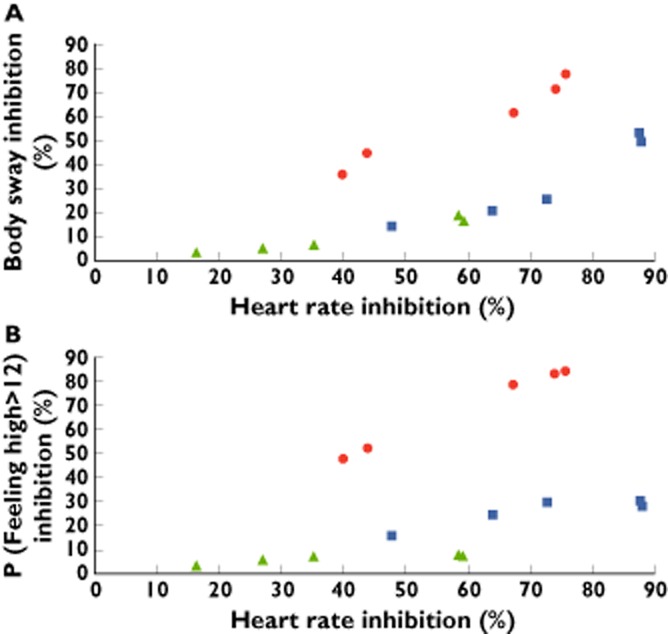
Simulated inhibition ratios (%) of THC-induced effects by TM38837 100 mg (green triangles) and 500 mg (blue squares), and rimonabant 60 mg (red dots) calculated per THC administration. A) shows the relationship between heart rate (assumed to primarily represent a peripheral CB1 effect) and body sway, and B) between heart rate and P (feeling high > 12)
Table 6.
Simulated inhibition ratios (%) with 90% confidence interval (CI) of tetrahydrocannabinol (THC)-induced effects by TM38837 100 mg and 500 mg, and rimonabant 60 mg calculated per THC administration
| Parameter | THC dose | 100 mg TM38837 | 500 mg TM38837 | Rimonabant | |||
|---|---|---|---|---|---|---|---|
| Estimate | 90% CI | Estimate | 90% CI | Estimate | 90% CI | ||
| Body sway | 1 (1st day) | 3.24 | (1.34, 7.85) | 14.1 | (6.33, 29.1) | 77.4 | (58.2, 88.4) |
| 2 (1st day) | 4.89 | (1.94, 11.8) | 20.3 | (8.98, 39.7) | 71.3 | (46.2, 87.9) | |
| 3 (1st day) | 6.42 | (2.47, 15.3) | 25.4 | (11.2, 47.0) | 61.2 | (34.3, 82.7) | |
| 4 (2nd day) | 18.8 | (8.84, 35.9) | 53.2 | (32.2, 73.5) | 44.4 | (22.7, 69.7) | |
| 5 (2nd day) | 16.3 | (7.19, 33.3) | 49.2 | (27.9, 71.1) | 35.7 | (16, 62.2) | |
| Heart rate | 1 (1st day) | 16.4 | (10.2,25.5) | 47.7 | (35.2, 60.4) | 75.6 | (60.6, 85.2) |
| 2 (1st day) | 27 | (17.6, 39.0) | 64 | (51.1, 75.5) | 74 | (54.8, 86.3) | |
| 3 (1st day) | 35.3 | (24.1, 48.5) | 72.6 | (61, 82.1) | 67.2 | (46.0, 82.5) | |
| 4 (2nd day) | 58.4 | (44.8, 70.7) | 87.4 | (79.9, 92.2) | 43.8 | (25.0, 63.6) | |
| 5 (2nd day) | 59.3 | (45.6, 71.3) | 87.8 | (80.4, 92.5) | 39.9 | (22.1, 60.1) | |
| Feeling high | 1 (1st day) | 3.52 | (2.11, 5.77) | 16 | (9.91, 24.5) | 84.6 | (75.7, 89.8) |
| 2 (1st day) | 5.75 | (3.57, 8.73) | 24.6 | (16.1, 34.4) | 83.4 | (66.1, 89.8) | |
| 3 (1st day) | 7.13 | (4.76, 10.6) | 29.7 | (21.0, 40.3) | 78.9 | (56.9, 86.1) | |
| 4 (2nd day) | 7.47 | (5.5, 10.0) | 30.4 | (24.0, 38.6) | 52.2 | (33.4, 63.1) | |
| 5 (2nd day) | 6.85 | (5.09, 9.31) | 28.3 | (22.3, 36.2) | 47.8 | (30.5, 59.1) | |
PK–PD modelling
In Figure 4, schematic overviews of the population PK–PD models of TM38837 and rimonabant are given. All PK–PD models included a baseline level, effect compartments that equilibrated with the plasma concentration and a model to relate the effect compartment concentration to the PD response. The period effects of VAS feeling high and heart rate were included in the THC PK–PD model. Heart rate and body sway were best described by a maximum effect model. For feeling high a probability model was used to quantify the probability for a VAS score > 12 at the study population level. The VAS value of 12 was the central value in the distribution of positive VAS scores in the study, which served as a reference point. All models included the THC challenge effect and the antagonizing effect of rimonabant and TM38837, either by shifting the EC50 (maximum effect model) or decreasing the P (feeling high > 12). An overview of the PK–PD parameters can be found in Table 7. The results of the VPCs can be found in the article's supplement.
Figure 4.
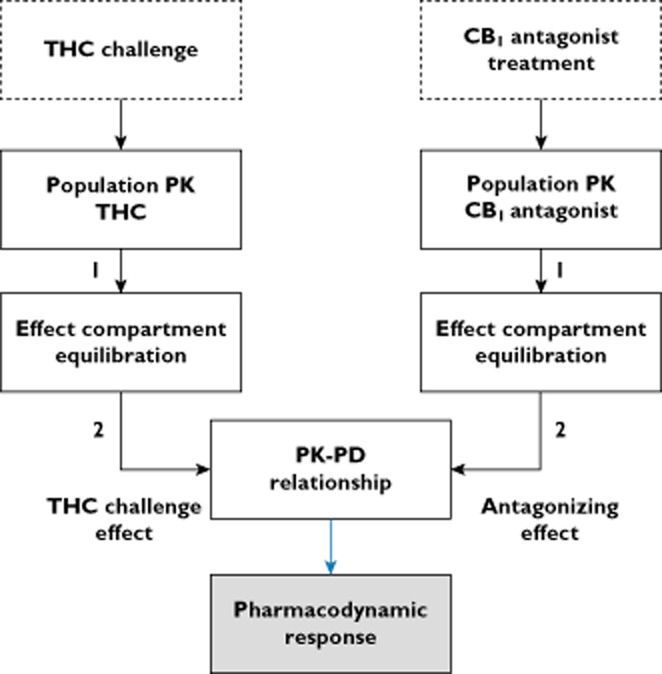
Schematic representation of the PK–PD models of TM38837 and rimonabant. 1: influence of plasma concentration, 2: influence of concentration in effect compartment
Table 7.
Population PK–PD parameter estimates for VAS feeling high, body sway and heart rate with relative standard error (RSE, %)
| Parameter | VAS feeling high P (feeling high > 12) (RSE) | Body sway (mm per 2 min) (RSE) | Heart rate (beats min−1) (RSE) |
|---|---|---|---|
| t1/2, ke0 THC (h) | 1.31 (7.9) | 1.94 (92.6) | 0.217 (19.2) |
| t1/2, ke0 Rimonabant (h) | 1.27 (77.5) | 1.21 (26.2) | 1.27 (32.4) |
| t1/2, ke0 TM38837 (h) | 4.63 (56.4) | 89.4 (24.7) | 85.1 (14.3) |
| Baseline | – | 230 (5.25) | 63.2 (1.8) |
| Emax2 | – | 134 (33.5) | 44.0 (39.0) |
| EC50 THC (ng ml−1) | – | 3.49 (89.2) | 33.1 (54.1) |
| IC50 Rimonabant (ng ml−1) | – | 49.9 (71.2) | 95.6 (55.2) |
| IC50 TM38837 (ng ml−1) | – | 206 (56.3) | 65.4 (33.4) |
| Baseline P (feeling high > 12) | 0.00656 (30.0) | – | – |
| CTHC at P = 0.5 (ng ml−1) | 9.75 (4.4) | – | – |
| CTHC at P = 0.5 during 1st dose (ng ml−1) | 6.21 (7.3) | – | – |
| IC50 Rimonabant (ng ml−1) | 347 (31.9) | – | – |
| IC50 TM38837 (ng ml−1) | 19 500 (27.9) | – | – |
CTHC = concentration of THC; EC50 = concentration at 50% of maximal effect; Emax = maximal effect; IC50 = concentration of antagonist at 50% of maximal inhibition; t1/2 = equilibration half-life; THC, tetrahydrocannabinol.
The population PK–PD models confirmed the expected THC-induced increase of heart rate, body sway and P (feeling high > 12). The equilibration time of THC with the effect compartment was relatively small (0.217 h for heart rate, 1.94 h for body sway and 1.31 h for P (feeling high > 12), indicating a fast onset of the effects. The equilibration half-life of TM38837 was long compared with rimonabant (heart rate 85.5 vs. 1.27 h, body sway 89.4 vs. 1.21 h, feeling high 4.63 vs. 1.27 h). This caused a larger delay in the onset of the effects of TM38837 compared with rimonabant. For heart rate the half maximal inhibitory concentrations (IC50) were similar for TM38837 (65.2 ng ml−1) and rimonabant (95.3 ng ml−1), whereas for body sway and feeling high, the IC50 of rimonabant was four times and 56 times larger, respectively, than for TM38837 (body sway TM38837 49.9 ng ml−1, rimonabant 206 ng ml−1; feeling high TM38837 347 ng ml−1, rimonabant 19 500 ng ml−1).
Similar to the graphical differences in time–effect profiles in the previous sections, the inhibition ratios of heart rate, body sway and P (feeling high > 12) that were estimated using the PK–PD models suggested a maximal inhibition of TM38837 on day 2, whereas rimonabant's inhibition was maximal at the first measurement on day 1, as can be seen in Table 6. These effect profiles are comparable with the PK profiles of the compounds in Figure 1.
Discussion
This study aimed to investigate the central and peripheral effectiveness of TM38837 and rimonabant in healthy subjects. We hypothesized that TM38837 would show no effects or small effects on central nervous system parameters, whereas rimonabant would show large effects on all PD tests. This study suggested that TM38837 is 56 times less potent than rimonabant in antagonizing the THC effect on feeling high when comparing the IC50 values, and four times less potent on body sway in healthy male volunteers. However, the antagonizing effect on heart rate increase had a similar potency for TM38837 and rimonabant. TM38837 100 mg, which is the anticipated effective human therapeutic dose, did not clearly antagonize THC effects when complete time–effect curves were compared, but between 24 to 27 h after administration this dose caused close to 60% inhibition of THC-induced tachycardia. In contrast, a therapeutic concentration of rimonabant showed pronounced maximal inhibition ratios that were about 80% for heart rate, body sway and feeling high. With heart rate increase being suggested to represent a primarily peripheral effect of THC [25], this altogether implies that TM38837 is able to induce clear peripheral effects with much less central activity, whereas rimonabant shows relatively large central effects. These acute outcomes suggest that TM38837 could be effective for peripherally associated clinical indications such as metabolic disorders, with a lower propensity for centrally mediated side effects than rimonabant [14, 16], although this clearly still needs to be demonstrated in prolonged studies with more relevant metabolic end points.
Population PK and PK–PD analysis
The current population PK and PK–PD models could be used for simulations of new study designs. However, the possibilities of TM38837 multiple dose designs could not be explored accurately. Due to the low clearance and the long terminal half-life, an unknown accumulation of the TM38837 plasma concentration and effects could occur after multiple dosing. The low clearance and long terminal half-life could be caused by factors like a slow inter-compartmental clearance, but this could not be examined any further with the current study design. A future multiple dose study, or a study using labelled TM38837 could investigate the influence of the PK parameters on the accumulation of TM38837 in a multiple dose design.
The population PK model of TM38837 calculated a terminal half-life of 771 h, whereas the non-compartmental analysis found a terminal half-life of approximately 12 to 13 h. The reason for this apparent discrepancy is that the population PK model included the pre-dose ‘baseline’ samples, which often contained measurable TM38837 concentrations from previous occasions despite long washout periods. This provided an improved accuracy of terminal half-life estimation. The non-compartmental analysis was not able to include the pre-dose ‘baseline’ because of the sparse and relatively short sampling scheme during the terminal elimination. The terminal half-life that was estimated using the non-compartmental analysis is therefore not a reliable estimation of the terminal half-life, but rather an estimation of a pre-terminal half-life.
The current study design did not include measurements between 11 and 24 h after drug administration, which would have been difficult to perform and interpret during the night. This could have influenced the estimation of some of the PD and PK parameters, such as the (time of) maximal concentration and effect, which for TM38837 may well have fallen within this period. In a future study, the PK–PD model should be optimized by the integration of data from another sampling scheme that would compensate for the time points between 11 and 24 h after TM38837 administration. This could result in more accurate estimations of the PK and PD effects. However, the current study found a large variability in the bioavailability of TM38837 that could not be explained completely by the time gap in the sampling scheme. This large inter-individual variability in TM38837 PK could be caused by several factors, such as inter-individual differences in absorption, which could not be explored in the current study.
In conclusion, TM38837 induces relatively strong effects on heart rate compared with the central nervous system effects. At the anticipated therapeutic dose, no clear central nervous system effects were found, in contrast to pronounced heart rate effects. Compared with rimonabant, TM38837 exhibits relatively strong heart rate effects compared with central nervous system effects, which might be an indication of peripheral selectivity. TM38837 doses up to 100 mg can possibly induce beneficial effects in patients suffering from metabolic disorders, without centrally mediated side effects.
Acknowledgments
We thank Jan Freijer for performing the PK–PD modelling, our colleague Justin Hay from the Centre of Human Drug Research for his advice on the study design and our colleague Jasper Stevens for reviewing this article.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from LE Klumpers) and declare 7TM Pharma, manufacturer of the investigational product, financially supported the submitted work; M Fridberg, PB Little, NO Jensen and Christian Elling were employees at 7TM Pharma in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 2.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 5.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez FF. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav. 2010;95:375–382. doi: 10.1016/j.pbb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Bermudez-Silva FJ, Suarez J, Baixeras E, Cobo N, Bautista D, Cuesta-Munoz AL, Fuentes E, Juan-Pico P, Castro MJ, Milman G, Mechoulam R, Nadal A, de Rodriguez FF. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008;51:476–487. doi: 10.1007/s00125-007-0890-y. [DOI] [PubMed] [Google Scholar]
- 8.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le FG, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 9.Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. 2011. Obesity and overweight – Fact sheet. 2-1-2011. Ref Type: Online Source.
- 11.Wathion N. Public statement on Acomplia (rimonabant): withdrawal of the marketing authorisation in the European Union. EMEA/39457/2009. 1-30-2009. Ref Type: Internet Communication.
- 12.The European Medicines Agency (EMEA) The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia. 2008. Doc.Ref.EMEA/CHMP/537777/2008 . 10-23-2008. Ref Type: Internet Communication.
- 13.The European Medicines Agency (EMEA) Questions and answers on the recommendation to suspend the marketing authorisation of Acomplia (rimonabant) 2008. Doc.Ref.EMEA/537153/2008. 10-23-2008. Ref Type: Internet Communication.
- 14.Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J. 2008;29:1761–1771. doi: 10.1093/eurheartj/ehn076. [DOI] [PubMed] [Google Scholar]
- 15.Pan C, Yoo HJ, Ho LT. Perspectives of CB1 antagonist in treatment of obesity: experience of RIO-Asia. J Obes. 2011;2011:957268. doi: 10.1155/2011/957268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 17.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 18.Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del AI, Cippitelli A, Nava F, Piomelli D, de Rodriguez FF. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschop MH. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, Sharkey KA. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, Innis RB, Cheng K, Rice KC, Deschamps JR, Chorvat RJ, McElroy JF, Kunos G. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.7TM Pharma A/S. Investigator's brochure – TM38837, a CB1 receptor antagonist/inverse agonist. 2009. 12-1-2009. Ref Type: Report.
- 23.Zuurman L, Roy C, Schoemaker R, Amatsaleh A, Guimaeres L, Pinquier J, Cohen A, van Gerven J. Inhibition of THC-induced effects on the central nervous system and heart rate by a novel CB1 receptor antagonist AVE1625. J Psychopharmacol. 2010;24:363–371. doi: 10.1177/0269881108096509. [DOI] [PubMed] [Google Scholar]
- 24.Zuurman L, Roy C, Schoemaker RC, Hazekamp A, Den Hartigh J, Bender JC, Verpoorte R, Pinquier JL, Cohen AF, van Gerven JM. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol. 2008;22:707–716. doi: 10.1177/0269881108089581. [DOI] [PubMed] [Google Scholar]
- 25.Strougo A, Zuurman L, Roy C, Pinquier JL, van Gerven JM, Cohen AF, Schoemaker RC. Modelling of the concentration–effect relationship of THC on central nervous system parameters and heart rate – insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J Psychopharmacol. 2008;22:717–726. doi: 10.1177/0269881108089870. [DOI] [PubMed] [Google Scholar]
- 26.Sanofi. Summary of Product Characteristics Rimonabant. 2008. Ref Type: Report.
- 27.Martinez J, Fabre D, Kanamaluru V. Population pharmacokinetics of rimonabant in obesity. PAGE 16. 2007. Ref Type: Abstract.
- 28.Committee for medicinal products for human use (CHMP) Guideline on the investigation of bioequivalence. 1-20-2010. 2010. Ref Type: Report.
- 29.Zuurman L, Roy C, Schoemaker R, Hazekamp A, den Hartigh J, Bender J, Verpoorte R, Pinquier J, Cohen A, van Gerven J. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol. 2008;22:707–716. doi: 10.1177/0269881108089581. [DOI] [PubMed] [Google Scholar]
- 30.Turpault S, Kanamaluru V, Lockwood GF, Bonnet D, Newton J. Rimonabant pharmacokinetics in healthy and obese subjects. Clin Pharmacol Ther. 2006;79:50. Ref Type: Abstract. [Google Scholar]
- 31.Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 32.Huestis MA, Boyd S, Heishman SJ, Preston KL, Bonnet D, Le Fur G, Gorelick DA. Single and multiple doses of rimonabant antagonize acuteeffects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuurman L, Roy C, Schoemaker R, Amatsaleh A, Guimaeres L, Pinquier JL, Cohen AF, van Gerven JMA. Inhibition of THC-induced effects on the central nervous system and heart rate by a novel CB1 receptor antagonist AVE1625. J Psychopharmacol. 2010;24:363–371. doi: 10.1177/0269881108096509. [DOI] [PubMed] [Google Scholar]
- 34.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- 35.Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne RP. Psychedelic effects of ketamine in healthy volunteers. Relationship to steady-state plasma concentrations. Anesthesiology. 1998;88:82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 37.Post TM, Freijer JI, Ploeger BA, Danhof M. Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn. 2008;35:185–202. doi: 10.1007/s10928-007-9081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


