Abstract
Aim
Apixaban is an oral, direct, factor-Xa inhibitor approved for thromboprophylaxis in patients who have undergone elective hip or knee replacement surgery and for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. This open label, parallel group study investigated effects of extremes of body weight on apixaban pharmacokinetics, pharmacodynamics, safety and tolerability.
Method
Fifty-four healthy subjects were enrolled [18 each into low (≤50 kg), reference (65–85 kg) and high (≥120 kg) body weight groups]. Following administration of a single oral dose of 10 mg apixaban, plasma and urine samples were collected for determination of apixaban pharmacokinetics and anti-factor Xa activity. Adverse events, vital signs and laboratory assessments were monitored.
Results
Compared with the reference body weight group, low body weight had approximately 27% [90% confidence interval (CI): 8–51%] and 20% (90% CI: 11–42%) higher apixaban maximum observed plasma concentration (Cmax) and area under the concentration–time curve extrapolated to infinity (AUC(0,∞)), respectively, and high body weight had approximately 31% (90% CI: 18–41%) and 23% (90% CI: 9–35%) lower apixaban Cmax and AUC(0,∞), respectively. Apixaban renal clearance was similar across the weight groups. Plasma anti-factor Xa activity showed a direct, linear relationship with apixaban plasma concentration, regardless of body weight group. Apixaban was well tolerated in this study.
Conclusion
The modest change in apixaban exposure is unlikely to require dose adjustment for apixaban based on body weight alone. However, caution is warranted in the presence of additional factors (such as severe renal impairment) that could increase apixaban exposure.
Keywords: anticoagulation, apixaban, body weight, factor Xa inhibitor, pharmacodynamics, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Apixaban is an oral, selective, reversible inhibitor of coagulation factor Xa that is approved as a fixed dose in a number of countries for thromboprophylaxis in patients who have undergone elective hip or knee replacement surgery and for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
Apixaban is also under development for treatment of venous thromboembolism.
Single and multiple doses of apixaban in healthy subjects are well tolerated and display a predictable pharmacokinetic/pharmacodynamic profile.
WHAT THIS STUDY ADDS
The present Phase I study investigated the effect of extremes of body weight on apixaban pharmacokinetics, pharmacodynamics, safety and tolerability.
The results demonstrate that extremes of body weight have modest effects on apixaban exposure that are unlikely to be clinically meaningful.
Therefore, no dose adjustment is recommended for apixaban based on body weight alone; however, caution is warranted in the presence of additional factors (such as severe renal impairment) that could increase apixaban exposure.
Introduction
Anticoagulant therapy is an important treatment option for prevention and treatment of venous thromboembolism (VTE) and prevention of stroke in patients with atrial fibrillation, as they remain a worldwide leading cause of morbidity and mortality [1]. It is important to study potential factors that could change the benefit : risk ratio of an anticoagulant, as overdosing of an anticoagulant could result in life-threatening bleeding while underdosing could result in lack of efficacy [2].
Extremes of body weight (obesity or low body weight) may alter the exposure profile of an anticoagulant and its benefit : risk ratio [3–7]. Obesity is associated with an increased risk of recurrent thromboembolism, atrial fibrillation and acute coronary syndrome [8–11]. Obese patients are therefore likely to require anticoagulation therapy as a treatment for these cardiovascular diseases [12]. In addition, patients with low body weight are often associated with an inherently higher risk of bleeding [6, 7]. While traditional anticoagulant therapy often requires pharmacodynamic (PD) monitoring (e.g., international normalized ratio for warfarin dosing) and/or body weight based dosing, or is contraindicated in patients with extremes of body weight [13–19], the new generation of oral anticoagulants including rivaroxaban and dabigatran does not require dose adjustment for body weight [20, 21].
Apixaban is an oral, selective, reversible inhibitor of coagulation factor Xa [22, 23] that is approved as a fixed dose in a number of countries for thromboprophylaxis in patients who have undergone elective hip or knee replacement surgery [24–26] and for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation [[27, 28]. Apixaban is also under development for treatment of VTE [29]. Clinical studies show that apixaban has a predictable pharmacokinetic (PK) profile across a wide range of doses. Apixaban is eliminated by both renal and non-renal pathways. Non-renal elimination pathways include metabolism by CYP enzymes, primarily CYP3A4, as well as biliary and intestinal excretion; renal excretion accounts for approximately 27% of total apixaban clearance [30–34].
The primary objective of the present study was to assess the effects of extremes of body weight on the PK of apixaban relative to subjects with reference body weight. The secondary objectives were to assess the effect of apixaban on anti-factor Xa activity (PD) and to evaluate the safety and tolerability of a single dose of apixaban in each body weight group.
Methods
Study design and subjects
This open label, single dose, parallel group study enrolled healthy subjects aged 18 to 45 years into three groups by body weight: ≤50 kg (low body weight), 65 to 85 kg (reference body weight) and ≥120 kg (high body weight). Subjects in the high body weight groups were also required to have a body mass index (BMI) ≥30 kg m–2 while subjects in the low and reference body weight groups were required to have a BMI ≤30 kg m–2. Female subjects were required to test negative for pregnancy and use an acceptable method of non-hormonal contraception for at least 4 weeks before and after dosing. All subjects were required to give written informed consent. Key exclusion criteria included history of any illness or injury known to be associated with increased bleeding risk, or any gastrointestinal disease or surgery that could interfere with absorption of study drug. Subjects were not permitted to take any prescription drug or over-the-counter acid-controlling agent within 4 weeks of dosing, or any other drug or dietary supplement within 2 weeks of dosing.
The study was conducted at two sites (Advanced Clinical Research Institute, Anaheim, California, and MDS Pharma Services Inc., Lincoln, Nebraska, USA) and performed in accordance with Good Clinical Practice as defined by the International Committee on Harmonization as well as all applicable USA and state laws relating to the protection of clinical trial subjects. In accordance with local laws, the minimum eligibility age was 19 years at the Lincoln study centre and 18 years at the Anaheim site. Institutional Review Boards (Aspire IRB, La Mesa, Arizona and MDS Pharma Services IRB, Lincoln, Nebraska, USA) approved the study protocol and subject consent forms prior to initiation of the study.
Subjects were screened within 21 days prior to the study, were admitted to the clinical facility on the day before dosing and remained at the study centre until completion of study procedures on day 4. On the morning of day 1, subjects received a single 10 mg dose of apixaban administered after an overnight fast. The 10 mg single dose of apixaban was chosen because it represented the high end of the dose range under development in Phase III trials.
Pharmacokinetic assessments
Blood samples for the pharmacokinetic analysis of apixaban were collected prior to dosing and at 0.5, 1, 2, 3, 4, 6, 9, 12, 18, 24, 36, 48, 60 and 72 h post-dose. Cumulative urine samples for determination of renal clearance were collected over time at intervals of 0–12, 12–24, 24–48 and 48–72 h after dosing, in addition to a pre-dose spot sample. Samples were stored at −20°C until analysis by the bioanalytical laboratory [Intertek Pharmaceutical Services (formerly Alta Analytical Laboratory), El Dorado Hills, California, USA].
Apixaban was quantitatively determined in plasma and urine samples using validated high-performance liquid chromatography tandem mass spectrometry methods using 13CD3-labelled apixaban as the internal standard for both assays. Sample extraction for plasma utilized protein precipitation, and for urine, solid-phase extraction was employed. The lower limit of quantification (LLOQ) was 1.0 ng ml–1 for both apixaban plasma and urine assays. The between-run and within-run variability for apixaban in plasma quality control samples, expressed as coefficient of variation, was ≤7.27% and ≤10.0%, respectively, with deviations from nominal concentration of no more than ± 2.54%. The between-run and within-run variability for apixaban in urine quality control samples was ≤4.88% and ≤5.15%, respectively, with deviations from nominal concentration of no more than ± 6.71%. Stability of apixaban was established for at least 589 days in human plasma and for at least 569 days in human urine stored at −20°C, and all samples were analyzed within this period of analyte stability.
Pharmacokinetic parameters were generated by standard non-compartmental methods using the program Kinetica™ (version 4.4.1, Thermo Electron Corporation, Philadelphia, Pennsylvania, USA). The maximum observed plasma concentration of apixaban (Cmax) and the time to reach maximum plasma concentration (tmax) were recorded directly from observed data. The slope (λz) of the terminal phase of the plasma concentration–time profile was determined using no weighting factor (weighting factor of 1) by the method of least squares (log-linear regression of at least three data points, excluding tmax) and the terminal elimination half-life (t1/2) was estimated as ln2/λz. The area under the concentration–time curve extrapolated to infinity (AUC(0,∞)) was determined by summing the areas from zero to the time of last measurable concentration (calculated using conventional trapezoidal and log-trapezoidal methods) and the extrapolated area (determined by dividing the last quantifiable plasma concentration by λz). The dose of apixaban was multiplied by the mean residence time and divided by AUC(0,∞) to obtain apparent plasma volume of distribution (Vss/F) for apixaban. The mean residence time was calculated by dividing the area under the moment curve from time zero to infinity (derived from the plasma apixaban concentration-time versus time plot) with AUC(0,∞). Apparent total body clearance (CLT/F) was calculated by dividing the dose by AUC(0,∞) and renal clearance (CLR) was calculated by dividing the cumulative amount of apixaban excreted in the urine over 72 h post-dose by AUC(0,∞), assuming the renal excretion of apixaban is completed by 72 h after a single dose.
Pharmacodynamic assessments
Blood samples for determination of plasma anti-factor Xa activity were collected prior to dosing, and at 3, 12 and 24 h after dosing. Anti-factor Xa assays were performed at Esoterix Coagulation Laboratory (Englewood, Colorado, USA) using the validated STA Rotachrom® Heparin assay (Diagnostica Stago, Parsippany, New Jersey, USA) as previously described [35]. The results of the assay were reported in activity units of low molecular weight heparin (reportable range: approximately 0.2–18 IU ml–1).
Safety and tolerability assessments
Safety and tolerability were evaluated by assessing subject-reported or directly observed adverse events (AEs), and by investigator review of vital signs and laboratory assessments. Any significant abnormalities in electrocardiogram (ECG) recordings, clinical laboratory or physical evaluations were listed. All recorded AEs were documented and tabulated by system organ class according to the Medical Dictionary for Regulatory Activities database (version 12), preferred term and weight group.
Statistical analysis
Pharmacokinetic data from 18 subjects in each group (total of 54 subjects) were expected to provide 90% confidence that estimated ratios of apixaban geometric means would be within 19% of its true value for Cmax and 18% for AUC(0,∞), respectively. Accordingly, 18 subjects were enrolled in each body weight group in the study.
Apixaban PK parameters were analyzed in a descriptive manner by summary statistics for each body weight group. Geometric means and coefficients of variation were reported for Cmax, AUC(0,∞), Vss/F, CLT/F and CLR. Values for tmax were reported as medians and ranges, and the mean and SD were provided for t1/2. The log-transformed PK parameters (Cmax and AUC(0,∞)) for apixaban were analyzed using one-way analysis of variance. Point estimates and 90% confidence intervals (CIs) for ratios of Cmax and AUC(0,∞) were calculated for comparisons between low and reference body weight groups and high and reference body weight groups. Log-linear regression analyses were conducted for apixaban Cmax and AUC(0,∞) by body weight and BMI, and plots were constructed. Estimates of intercepts and slopes with associated 90% CIs were obtained, as well as P values for the slopes. The apixaban Cmax and AUC(0,∞) values were normalized by dose and body weight, and were graphically compared for males and females within each body weight group. A scatter plot of anti-factor Xa activity vs. apixaban concentration for individuals across weight groups was constructed. Plasma anti-factor Xa activity was analyzed in a descriptive manner by summary statistics for each of the body weight groups.
Results
Study population
A total of 55 subjects were enrolled, with 18 subjects each enrolled in the low and reference weight groups and 19 in the high body weight group. For the PK and PD analyses, data from 53 subjects were available. Two subjects in the reference body weight group were excluded from all PK and PD analyses: one subject withdrew consent 2 h post-dose resulting in an incomplete PK profile, and one subject had plasma concentrations of apixaban that were all either near or below the LLOQ. The urine apixaban concentrations for this subject and the PD data were consistent with the plasma apixaban concentration findings. The reason is not known for the unexpected findings in this subject.
Demographic characteristics of the subjects in the study are shown in Table 1. Subjects were well matched across the body weight groups with regard to age. The reference body weight group contained approximately equal numbers of males and females (10 females vs. eight males); and as expected, males predominated in the high body weight group and females in the low body weight group (16 males vs. three females) and the low body weight group comprised a higher percentage of females (16 females vs. two males).
Table 1.
Subject demographics
| Characteristic | Body weight group | ||
|---|---|---|---|
| Low (≤50 kg) (n = 18) | Reference (65–85 kg) (n = 18) | High (≥120 kg) (n = 19) | |
| Age (years) | |||
| Mean (SD) | 23 (4) | 28 (7) | 29 (8) |
| Range | 18–31 | 18–43 | 19–41 |
| Gender, n (%) | |||
| Male | 2 (11) | 8 (44) | 16 (84) |
| Female | 16 (89) | 10 (56) | 3 (16) |
| Race, n (%) | |||
| White | 11 (61) | 15 (83) | 17 (89) |
| Black | 1 (6) | 0 | 0 |
| Asian | 4 (22) | 2 (11) | 0 |
| Other* | 2 (11) | 1 (6) | 2 (11) |
| Weight (kg) | |||
| Mean (SD) | 47 (3.6) | 75 (5.5) | 137 (18.3) |
| Range | 38–50 | 67–84 | 120–175 |
| Body mass index (kg m−2) | |||
| Mean (SD) | 18.8 (2) | 26.3 (2) | 42.6 (6) |
| Range | 17–22 | 22–30 | 32–54 |
Other races included Mexican, Hispanic and American Indian/Alaskan Native American. SD, standard deviation.
Pharmacokinetics
The mean plasma concentration–time profiles of apixaban in each body weight group are shown in Figure 1. Apixaban pharmacokinetic parameters are summarized in Table 2. Compared with the reference body weight group, mean apixaban Cmax and AUC(0,∞) were 27% and 20% higher, respectively, in the low body weight group. Conversely, for the high body weight group, mean apixaban Cmax and AUC(0,∞) were 31% and 23% lower, respectively, compared with the reference body weight group. The time to reach maximum apixaban plasma concentration, tmax, was similar in all the weight groups. Apixaban CLT/F was 16% lower in the low body weight group vs. that observed in the reference weight group. In contrast, apixaban CLT/F was 30% higher in the high body weight group. The apixaban CLR did not show any trend in association with body weight. The apparent volume of distribution was 24% higher for the high weight group and 14% lower for the low weight group, as compared with the reference weight group. Apixaban t1/2 was shorter by approximately 3 h in the high body weight group and longer by approximately 4 h in the low body weight group, compared with that observed in the reference weight group. The range of apixaban exposure appeared similar for males and females within each body weight group.
Figure 1.
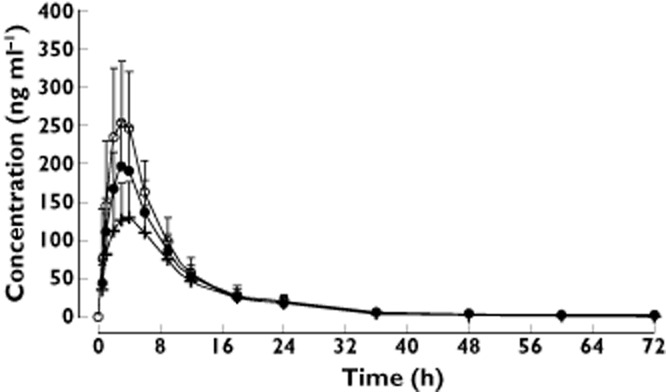
Apixaban mean (+ SD) plasma concentration–time profiles in subjects with low, reference and high body weights. Body weight group:  , low (≤50 kg), n = 18;
, low (≤50 kg), n = 18;  , reference (65–85 kg), n = 16;
, reference (65–85 kg), n = 16;  , high (≥120 kg), n = 19
, high (≥120 kg), n = 19
Table 2.
Summary of pharmacokinetic parameters in subjects with low, normal and high body weight
| Parameter | Body weight group | Geometric mean ratio (90% CI) | |||
|---|---|---|---|---|---|
| Low (≤50 kg) (n = 18) | Reference (65–85 kg) (n = 16) | High (≥120 kg) (n = 19) | Low vs. reference | High vs. reference | |
| Cmax (ng ml−1) | 264 (26) | 207 (24) | 144 (28) | 1.272 (1.075, 1.506) | 0.692 (0.586, 0.818) |
| AUC(0,∞) (ng ml−1 h) | 2424 (26) | 2024 (24) | 1561 (31) | 1.198 (1.011, 1.419) | 0.771 (0.652, 0.912) |
| Median tmax (h) (range) | 3.00 (1.00–6.00) | 3.03 (2.00–6.00) | 3.98 (1.00–6.00) | ||
| Mean t1/2 (h) (SD) | 15.8 (9.8) | 12.0 (5.35) | 8.8 (3.15) | ||
| Vss/F (l) | 52.7 (45) | 61.0 (22) | 75.6 (28) | ||
| CLR (ml min−1) | 14.1 (25) | 12.6 (45) | 17.8 (42) | ||
| CLT/F (ml min−1) | 68.8 (40) | 82.3 (19) | 106.8 (35) | ||
Unless otherwise specified, values are geometric means with percentage coefficient of variation in parentheses. AUC(0,∞), area under the concentration–time curve to infinity; CI, confidence interval; CLR, renal clearance; CLT/F, apparent total body clearance; Cmax, maximum plasma concentration; SD, standard deviation; t1/2, terminal elimination half-life; tmax, time to reach maximum plasma concentration; Vss/F, apparent plasma volume of distribution.
The relationships between apixaban Cmax and AUC(0,∞) and body weight are shown in Figure 2A, B. The log-linear regression analyses showed a significant inverse relationship between apixaban exposure and body weight (P value for slope <0.001 for both Cmax and AUC(0,∞)). As expected, since BMI is derived from body weight as well as height, there were similar relationships between apixaban Cmax and AUC(0,∞) and BMI, as shown in Figure 2C, D (P value for slope <0.001 for both Cmax and AUC(0,∞)). Individual Cmax and AUC(0,∞) values normalized by dose and body weight are plotted by weight group and gender in Figure 3A, B. In spite of the imbalance in gender across the weight groups, the range of apixaban exposure appeared similar for males and females within each body weight group, indicating lack of gender influence in the study.
Figure 2.
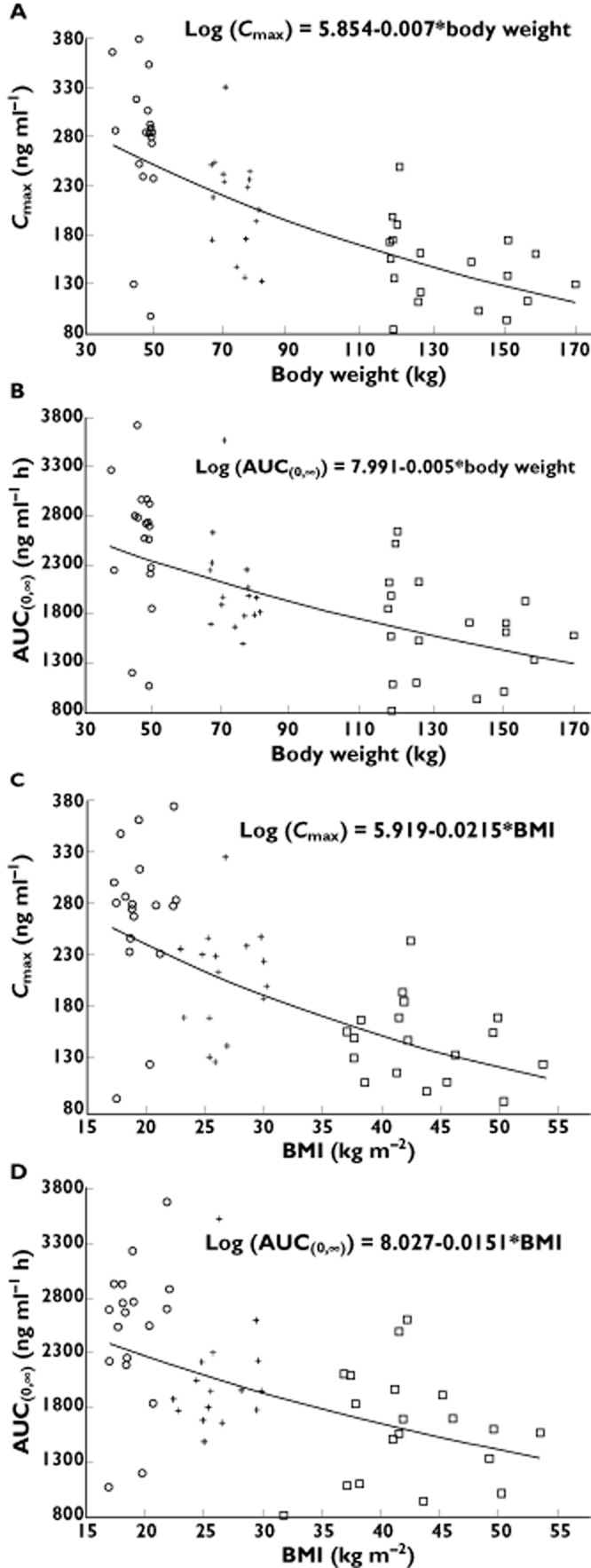
Log–linear regression plots of apixaban exposure vs. body weight for (A) maximum plasma concentration (Cmax) and (B) area under the concentration–time curve to infinity (AUC(0,∞)). Log–linear regression plots of apixaban exposure vs. body mass index (BMI) for (C) Cmax and (D) AUC(0,∞). Individual subject data are plotted with regression lines. Body weight group:  , low (≤50 kg), n = 18;
, low (≤50 kg), n = 18;  , reference (65–85 kg), n = 16;
, reference (65–85 kg), n = 16;  , high (≥120 kg), n = 19
, high (≥120 kg), n = 19
Figure 3.
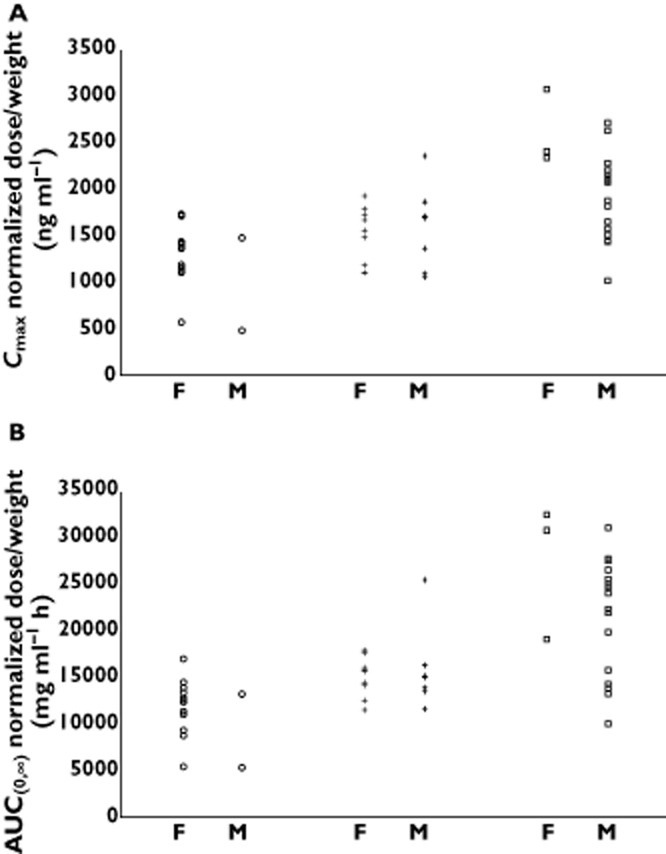
Scatter plot of apixaban exposure normalized to dose and body weight showing male (M) and female (F) subjects in each body weight group for (A) maximum plasma concentration (Cmax) and (B) area under the concentration–time curve to infinity (AUC(0,∞)). Body weight group:  , low (≤50 kg), n = 18;
, low (≤50 kg), n = 18;  , reference (65–85 kg), n = 16;
, reference (65–85 kg), n = 16;  , high (≥120 kg), n = 19
, high (≥120 kg), n = 19
Pharmacodynamics
Anti-factor Xa activity showed a direct linear relationship with apixaban plasma concentration that was consistent across the body weight groups (Figure 4). As observed for apixaban plasma concentration, there was a trend towards lower plasma anti-factor Xa activity with increasing body weight. Mean (SD) anti-factor Xa activity at 3 h post-dose was 3.71 (1.34) IU ml–1 in low body weight subjects, 2.79 (0.85) IU ml–1 in the reference body weight subjects and 1.85 (0.74) IU ml–1 in high body weight subjects. Anti-Xa activity was comparable across the groups at 12 h post-dose with a mean (SD) activity of 0.88 (0.29) IU ml–1 in the low weight group, 0.77 (0.17) IU ml–1 in the reference body weight group and 0.70 (0.29) IU ml–1 in the high body weight group.
Figure 4.
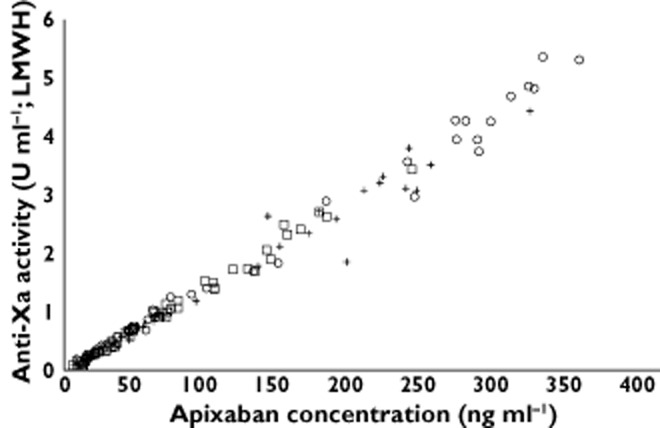
Relationship between apixaban plasma concentration and anti-factor Xa activity. LMWH, low molecular weight heparin. Body weight group:  , low (≤50 kg), n = 18;
, low (≤50 kg), n = 18;  , reference (65–85 kg), n = 16;
, reference (65–85 kg), n = 16;  , high (≥120 kg), n = 19
, high (≥120 kg), n = 19
Safety and tolerability
Apixaban was well tolerated by healthy subjects in the study. Twelve subjects (22%) reported a total of 14 AEs that were uniformly distributed across body weight categories. All AEs were mild or moderate in intensity and resolved without treatment. The most commonly reported AEs were headache (seven subjects) and nausea (two subjects), with no other AEs reported in more than one subject. Of the 14 AEs, seven were considered related to study drug by the investigators (one event of nausea, one of upper abdominal pain and five of headache). No bleeding-related AEs were reported, except for one female subject in the reference body weight group who reported mild epistaxis on day 2 lasting approximately 10 min. The investigator considered this event to be unrelated to study medication. No clinically significant changes were observed in vital signs, laboratory values, clinical examinations or ECGs in any subject.
Discussion
The purpose of this study was to examine the effects of extremes of body weight on the PK and PD of apixaban, and to identify any associated safety or tolerability concerns in these healthy subjects. Compared with the reference weight group in this study, apixaban exposure was approximately 20% to 30% lower and higher in the high and low body weight groups, respectively.
The modest changes in apixaban exposure were in accordance with the changes observed in CLT/F and Vss/F. Apixaban CLT/F increased with increasing body weight with no apparent trend for a relationship between body weight and CLR. Clearance is generally assumed to be positively correlated with body weight and is often expressed using the allometric concept [36]. Body weight is known to explain a portion of the variability in hepatic clearance as liver weight, enzyme content and metabolic rate are correlated with body size [36, 37]. Considering the lack of a trend between CLR and body weight, the observed modest increase in CLT/F may result from increased non-renal elimination pathways of apixaban. The apparent volume of distribution was also higher and lower for apixaban in the high and low body weight groups, respectively, as compared with the reference body weight group. The effect of body weight on Vss/F was modest, consistent with the low volume of distribution of apixaban. Following an intravenous bolus dose, the steady-state volume of distribution for apixaban is ∼21 l, suggesting distribution mainly into the extracellular compartment with limited tissue distribution [31, 33].
The t1/2 range observed across the groups in the study was consistent with the range observed in the previous Phase I studies [38–41]. The differences in the t1/2 of apixaban in the extreme body weight groups relative to the reference group are expected to result in modest (approximately 30%) differences in time to steady state in the low and high body weight groups compared with the reference group and are not expected to be clinically meaningful.
The effect of body weight on apixaban PD was attributed to the differences in plasma concentration as the direct linear relationship between apixaban plasma concentration and anti-factor Xa activity was not affected by body weight. Other clotting time measures were not included in this study as anti-factor Xa activity has been shown to be a more sensitive and precise method for assessing apixaban plasma concentration than prothrombin time, international normalized ratio or activated partial thromboplastin time [35].
Total body weight is not always an accurate assessment of obesity; therefore subjects in the high body weight group were required to have a BMI ≥30 kg m–2 to ensure they were representative of an obese population [42]. The regression results showed a statistically significant inverse relationship between apixaban exposure and both body weight and BMI.
A single dose of apixaban was chosen for this study since single-dose PK is predictive of its multiple-dose PK [38]. An unbalanced ratio of males to females is frequently seen in studies of this kind, due to difficulties in recruiting healthy males with a body weight ≤50 kg [43]. A qualitative comparison of dose- and body weight-normalized Cmax and AUC(0,∞) separated by gender across body weight groups showed no apparent differences between genders. The lack of apparent trend between genders in apixaban exposure is consistent with the results from a study in healthy subjects that demonstrated only a small (15–18%) impact of gender on apixaban exposure [44]. Therefore, the gender imbalance in the study is not surprising and did not appear to influence the study results.
A single 10 mg dose of apixaban was safe and well tolerated in this study. All AEs reported were mild or moderate in intensity and resolved without treatment before study completion. The types of AEs and their frequency were not unusual for short term studies in healthy subjects. The frequency of AEs was well balanced across the weight groups.
Patients with a wide range of body weights were included in the Phase III clinical trials of apixaban for prevention of thromboembolism in knee and hip replacement surgery (28–180 kg) [24–26] and for stroke prevention in patients with atrial fibrillation (29–205 kg) [27, 28]. The available efficacy and safety data in patients from the Phase III trials of apixaban for prevention of thromboembolism following knee and hip replacement surgery and for stroke prevention in atrial fibrillation indicate that dosing adjustment based on body weight alone is not required [24–28, 45]. However, a dose reduction is recommended for those patients with at least two of the following characteristics: age ≥80 years, body weight ≤60 kg or serum creatinine ≥1.5 mg/dl (133 micromole/l) [27, 28].
In summary, the effects of extremes of body weight on apixaban exposure were considered modest and unlikely to be clinically meaningful. The plasma anti-factor Xa activity showed a direct, linear relationship with apixaban plasma concentrations, regardless of body weight groups. Apixaban was well tolerated by all subjects in this study, including those with extremes of body weight as well as those with reference body weight. No dose adjustment is recommended for apixaban based on body weight alone; however, caution is warranted in the presence of additional factors (such as severe renal impairment) that increase apixaban exposure.
Acknowledgments
The authors gratefully acknowledge the contributions of Mr Anh Bui, Discovery Medicine and Clinical Pharmacology, Bristol-Myers Squibb Company, Princeton, New Jersey, USA, Dr Dennis Riff and staff at Advanced Clinical Research Institute, Anaheim, California, USA and Dr Karl S Roth and staff at MDS Pharma Services Inc., Lincoln, Nebraska, USA. Professional medical writing and editing assistance was provided by Andrew Shepherd and Dana Fox at Caudex Medical, Oxford, UK, and funded by Bristol-Myers Squibb Company and Pfizer Inc.
Competing Interests
This study was sponsored by Bristol-Myers Squibb Company and Pfizer Inc. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare VVU, JW, YCB, JP, FPL and CEF had support from Bristol-Myers Squibb (employee/stock/stock options) and WB and RB had support from Pfizer Inc (employee/stock/stock options) for the submitted work.
References
- 1.Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (9th Edition) Chest. 2012;141:e152S–184. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanderink GJ, Le Liboux A, Jariwala N, Harding N, Ozoux ML, Shukla U, Montay G, Boutouyrie B, Miro A. The pharmacokinetics and pharmacodynamics of enoxaparin in obese volunteers. Clin Pharmacol Ther. 2002;72:308–318. doi: 10.1067/mcp.2002.127114. [DOI] [PubMed] [Google Scholar]
- 4.Yee JY, Duffull SB. The effect of body weight on dalteparin pharmacokinetics. A preliminary study. Eur J Clin Pharmacol. 2000;56:293–297. doi: 10.1007/s002280000141. [DOI] [PubMed] [Google Scholar]
- 5.Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol. 2003;56:96–103. doi: 10.1046/j.1365-2125.2003.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Medicines Agency. CHMP Guideline on clinical investigation of medicinal products for prophylaxis of high intra- and post-operative venous thromboembolic risk. Effective 31 May 2008. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003301.pdf (last accessed 17 July 2012)
- 7.European Medicines Agency. Guideline on clinical investigation of medicinal products for prophylaxis of venous thromboembolic risk in non-surgical patients. Effective 30 November 2006. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003317.pdf (last accessed 17 July 2012)
- 8.Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, Heinze G, Kyrle PA. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168:1678–1683. doi: 10.1001/archinte.168.15.1678. [DOI] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 10.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity–results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Wendelboe AM, Hegmann KT, Biggs JJ, Cox CM, Portmann AJ, Gildea JH, Gren LH, Lyon JL. Relationships between body mass indices and surgical replacements of knee and hip joints. Am J Prev Med. 2003;25:290–295. doi: 10.1016/s0749-3797(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 12.Patel JP, Roberts LN, Arya R. Anticoagulating obese patients in the modern era. Br J Haematol. 2011;155:137–149. doi: 10.1111/j.1365-2141.2011.08826.x. [DOI] [PubMed] [Google Scholar]
- 13.Absher RK, Moore ME, Parker MH. Patient-specific factors predictive of warfarin dosage requirements. Ann Pharmacother. 2002;36:1512–1517. doi: 10.1345/aph.1C025. [DOI] [PubMed] [Google Scholar]
- 14.Dos Reis Macedo LG, de Oliveira L, Pintao MC, Garcia AA, Pazin-Filho A. Error in body weight estimation leads to inadequate parenteral anticoagulation. Am J Emerg Med. 2011;29:613–617. doi: 10.1016/j.ajem.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e24S–43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GlaxoSmithKline. Arixtra product information. Revised February 2011. Available at http://us.gsk.com/products/assets/us_arixtra.pdf (last accessed 17 May 2012)
- 17.Hurewitz AN, Khan SU, Groth ML, Patrick PA, Brand DA. Dosing of unfractionated heparin in obese patients with venous thromboembolism. J Gen Intern Med. 2011;26:487–491. doi: 10.1007/s11606-010-1551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43:1064–1083. doi: 10.1345/aph.1L194. [DOI] [PubMed] [Google Scholar]
- 19.Sanofi-Aventis U.S. LLC. Lovenox® Prescribing information. Revised April 2011. Available at http://www.lovenox.com (last accessed 3 May 2012)
- 20.Boehringer Ingelheim International GmbH. Pradaxa® (INN – dabigatran etexilate) Summary of Product Characteristics. First authorization March 2008. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf (last accessed 28 November 2011)
- 21.Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47:218–226. doi: 10.1177/0091270006296058. [DOI] [PubMed] [Google Scholar]
- 22.Pinto DJ, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, Wong PC, Rendina AR, Luettgen JM, Knabb RM, He K, Xin B, Wexler RR, Lam PY. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H -pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50:5339–5356. doi: 10.1021/jm070245n. [DOI] [PubMed] [Google Scholar]
- 23.Wong PC, Crain EJ, Xin B, Wexler RR, Lam PY, Pinto DJ, Luettgen JM, Knabb RM. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008;6:820–829. doi: 10.1111/j.1538-7836.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 24.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 25.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 26.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 27.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 28.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek E, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser S, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon J, Pais P, Parkhomenko A, Verheugt F, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 29.Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost. 2008;6:1313–1318. doi: 10.1111/j.1538-7836.2008.03054.x. [DOI] [PubMed] [Google Scholar]
- 30.Bristol-Myers Squibb and Pfizer EEIG. Eliquis® (apixaban tablets) Summary of product characteristics. 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf (last accessed 10 April 2013)
- 31.Frost C, Yu Z, Nepal S, Bragat A, Moore K, Shenker A, Barrett YC, LaCreta F. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation [abstract 148] J Clin Pharmacol. 2008;48:1132. [Google Scholar]
- 32.Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, Zhang D. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81. doi: 10.1124/dmd.108.023143. [DOI] [PubMed] [Google Scholar]
- 33.Vakkalagadda B, Frost C, Wang J, Nepal S, Schuster A, Zhang D, Dias C, Yu Z, Shenker A, LaCreta F. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa [abstract] J Clin Pharmacol. 2009;49:1091–1130. [Google Scholar]
- 34.Wang L, Zhang D, Raghavan N, Yao M, Ma L, Frost CE, Maxwell BD, Chen SY, He K, Goosen TC, Humphreys WG, Grossman SJ. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos. 2010;38:448–458. doi: 10.1124/dmd.109.029694. [DOI] [PubMed] [Google Scholar]
- 35.Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–1271. doi: 10.1160/TH10-05-0328. [DOI] [PubMed] [Google Scholar]
- 36.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 37.Fagerholm U. Prediction of human pharmacokinetics–evaluation of methods for prediction of hepatic metabolic clearance. J Pharm Pharmacol. 2007;59:803–828. doi: 10.1211/jpp.59.6.0007. [DOI] [PubMed] [Google Scholar]
- 38.Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd R, Yu Z, Shenker A, Barrett YC, Mosqueda-Garcia R, LaCreta F. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013 doi: 10.1111/bcp.12106. Mar 14. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, Reeves RA, LaCreta F. Apixaban, an oral, direct factor Xa inhibitor: single-dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2012;75:476–487. doi: 10.1111/j.1365-2125.2012.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamahira N, Imai Y, Wastall P, Liao S, Frost C, Fukase H, Hiraoka M. A placebo-controlled, ascending multiple-dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of apixaban in healthy Japanese subjects. (A637) Can J Clin Pharmacol. 2008;15:e420–781. [Google Scholar]
- 41.Yu Z, Nepal S, Bragat A, Shenker A, Frost C. Single dose apixaban pharmacokinetics and pharmacodynamics in healthy male Japanese and Caucasian subjects (A647) Can J Clin Pharmacol. 2008;15:e420–781. doi: 10.2147/CPAA.S169505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NHLBI Obesity Education Initiative Expert Panel on the Identification Evaluation and Treatment of Obesity in Adults. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Heart, Lung and Blood Institute; 1998. Report No.: 98-4083. [Google Scholar]
- 43.Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24:2757–2765. doi: 10.1185/03007990802361499. [DOI] [PubMed] [Google Scholar]
- 44.Frost C, Nepal S, Barrett Y, LaCreta F. Effects of age and gender on the single-dose pharmacokinetics (PK) and pharmacodynamics (PD) of apixaban. J Thromb Haemost. 2009;7:PP-MO-407. [Google Scholar]
- 45.Pineo GF, Gallus AS, Raskob GE, Chen D, Ramirez LM, Ramacciotti E, Lassen MR, Wang L. Apixaban after hip or knee arthroplasty versus enoxaparin: efficacy and safety in key clinical subgroups. J Thromb Haemost. 2013;11:444–451. doi: 10.1111/jth.12109. [DOI] [PubMed] [Google Scholar]


