Abstract
Aims
The aim of the study was to identify and quantify factors that control the plasma concentrations of urate during allopurinol treatment and to predict optimal doses of allopurinol.
Methods
Plasma concentrations of urate and creatinine (112 samples, 46 patients) before and during treatment with various doses of allopurinol (50–600 mg daily) were monitored. Non-linear and multiple linear regression equations were used to examine the relationships between allopurinol dose (D), creatinine clearance (CLcr) and plasma concentrations of urate before (UP) and during treatment with allopurinol (UT).
Results
Plasma concentrations of urate achieved during allopurinol therapy were dependent on the daily dose of allopurinol and the plasma concentration of urate pre-treatment. The non-linear equation: UT = (1 – D/(ID50 + D)) × (UP – UR) + UR, fitted the data well (r2 = 0.74, P < 0.0001). The parameters and their best fit values were: daily dose of allopurinol reducing the inhibitable plasma urate by 50% (ID50 = 226 mg, 95% CI 167, 303 mg), apparent resistant plasma urate (UR = 0.20 mmol l−1, 95 % CI 0.14, 0.25 mmol l−1). Incorporation of CLcr did not significantly improve the fit (P = 0.09).
Conclusions
A high baseline plasma urate concentration requires a high dose of allopurinol to reduce plasma urate below recommended concentrations. This dose is dependent on only the pre-treatment plasma urate concentration and is not influenced by CLcr.
Keywords: allopurinol, creatinine clearance, gout, urate, uric acid
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Despite recommendations for reduced dosages of allopurinol in patients with renal impairment (due to accumulation of the active metabolite, oxypurinol), recent studies indicate allopurinol dosages should be higher than those recommended by creatinine clearance.
The number of patients reaching target concentrations of plasma urate (0.30 to 0.36 mmol l−1) is low.
Low doses of allopurinol are recommended upon initiation of allopurinol therapy to avoid adverse reactions.
WHAT THIS STUDY ADDS
This study confirms that higher doses of allopurinol than those indicated by creatinine clearance are required to reach recommended targets of plasma urate.
The final, maintenance dose of allopurinol required to reach the target urate concentration is dependent on the pre-treatment, (baseline) plasma urate concentration but not on creatinine clearance.
Knowing the final dose is very useful for the physician in educating the patient about dosing with allopurinol.
Introduction
Allopurinol is the most common therapy for the prevention of acute attacks of gout and the treatment of chronic gout. It is largely metabolized to oxypurinol, which inhibits the enzyme xanthine oxidoreductase, thereby decreasing the synthesis of uric acid (urate at physiological pH) from purines and reducing the risk of acute attacks [1]. The development of gout is generally associated with plasma concentrations of urate above 0.42 mmol l−1 (∼7 mg dl−1), the risk increasing with concentration. The aim of dosage with allopurinol is to reduce the plasma concentrations of urate to levels where acute gout will not recur and which allow dissolution of urate deposits. Although allopurinol has been used widely for decades, clear guidance regarding optimal dosage for effectiveness and safety has been slow to emerge. The recommended target plasma concentrations of urate are 0.36 mol l−1 (∼6 mg dl−1; European League Against Rheumatism, EULAR; American College of Rheumatology, ACR) [2, 3] and 0.30 mmol l−1 (∼5 mg dl−1; British Society of Rheumatology, BSR) [4].
Failure to reduce plasma urate below recommended target concentrations is common. This is particularly the case at the widely used dosage of 300 mg daily [5]. It is commonly recommended that the dosage of allopurinol should be reduced in patients with renal impairment. This guidance arose from the influential work of Hande et al. [6] who suggested the uncommon occurrence of allopurinol hypersensitivity (AH) was associated with excessive accumulation of oxypurinol occurring in patients with renal impairment. AH is potentially fatal as it includes Stevens Johnson syndrome and toxic epidermal necrolysis. However, the use of low doses in renal impairment often does not lead to satisfactory reductions in the plasma concentrations of urate in many patients and higher doses of allopurinol are often required [7–10]. Further, AH has also been reported with low doses of allopurinol in the presence and absence of renal impairment [7, 11]. Although clinical trials involving relatively few patients indicate that allopurinol does not necessarily produce AH at higher doses, the concerns about its use in renal impairment remain and are widely held [10].
The incidence of AH is decreased when the starting dose is low and approximately proportional to the clearance of creatinine [12]. For example, it was proposed that the initial dose of allopurinol should be 50 mg every 2 days for an estimated glomerular filtration rate (eGFR) of 16 to 30 ml min−1 1.73 m–2 and 150 mg daily for an eGFR of 91 to 130 ml min−1 1.73 m–2. The initial doses could be even lower in patients with the HLA B*5801 allele, a group at high risk of AH [13, 14]. The ACR recently published guidelines advised that the starting dose of allopurinol should be no greater than 100 mg day−1 for any gouty patient, and 50 mg day−1 in subjects with stage 4 (CLcr 15–29 ml min−1 1.73 m–2) or poorer, chronic kidney disease (CKD) [3]. The low starting dose should be increased gradually (every 2–3 weeks) until a target plasma urate of 0.36 mmol l−1 (6 mg dl−1) is achieved [3].
Our aim was to identify the factors that control the response to allopurinol, response being assessed by the plasma concentration of urate. The factors we examined included the dose of allopurinol, the plasma concentration of urate before dosage, renal function and use of a diuretic. The regression analyses were based on classical dose–response relationships. In the modelling and its examination, four factors were considered.
No matter how high the dose of allopurinol, there is a plasma concentration of urate resistant to further reduction (UR) [1].
Higher baseline concentrations of urate (UP) are associated with a failure to achieve satisfactory plasma concentrations during allopurinol treatment [15].
The renal clearance of oxypurinol is reduced in renal impairment [6].
Diuretics increase the plasma concentrations of urate [16]
Methods
Patients and study design
The present study was conducted in two groups of patients with gout. The first was from a prospective study (n = 10) in which allopurinol dose escalation was a prelude to examining an interaction with probenecid [17] (St Vincent's Hospital Research Ethics Committee approval number: H06/141; Australian and New Zealand Clinical Trials register: ACTRN12607000247471). These patients had their dosage of allopurinol titrated after being on a fixed dose for a period of time based on their CLcr (CLcr > 100 ml min−1, dosage ≥ 7 days, CLcr = 40 to 100 ml min−1; dosage ≥ 14 days). The second group of patients (n = 36, 11 prospective and 25 retrospective) were from private rheumatology practices where the aim was to reduce the plasma urate to below 0.30 mmol l−1 (St Vincent's Hospital Research Ethics Committee approval numbers H06/107, 08/172; Australian and New Zealand Clinical Trials register: ACTRN12611000743965). The 36 patients in private practice had their dose of allopurinol titrated after being on a fixed dose of allopurinol for at least 1 month. All patients gave consent in accordance with the Declaration of Helsinki.
The patients were aged between 17 and 80 years. All except five were male. The doses of allopurinol ranged from 50 to 600 mg daily. Blood was collected at pathology collection centres in Sydney prior to dose titration of allopurinol (Douglass Hanly Moir Pathology Laboratories and SydPath, St Vincent's Hospital, Sydney).
A larger dataset, comprising a total of 125 gout patients [18–22] from five separate published studies, was used as a comparator to the analysis of our data.
Analysis of plasma and urine
Plasma urate and creatinine concentrations were determined using standard methodologies on the Roche/Hitachi Modular P Analyzer Platform, Roche Diagnostics, Australia.
Creatinine clearance
CLcr was used as an estimate of the glomerular filtration rate of the patients and was calculated using the Cockcroft & Gault formula [23] and lean body weight. Lean body weight was calculated using the formula described by Janmahasatian et al. [24]. CLcr was also estimated by the Modification of Diet in Renal Disease (MDRD) formula (eGFR) [25].
Non-linear modelling
The classical equation that relates the fractional inhibition to the dose of a drug was modified based on the assumption that allopurinol cannot completely reduce plasma concentrations down to 0 mmol l−1. Hence, the dose–response data was fitted to the non-linear equation:
| (1) |
with UT plasma concentration of urate during treatment with allopurinol, UP plasma concentration of urate pre-treatment, UR resistant plasma urate concentration, D total daily dose of allopurinol and ID50, the dose of allopurinol that has reduced the inhibitable urate (UP − UR) by 50%. Equation 1 is non-linear with respect to D but linear with respect to UP and UR.
Multiple linear regression
The data were also fitted by multiple linear regression using a general function of the form:
| (2) |
The multiple regression included up to three independent variables, x1, x2 and x3, where these variables were UP, D and CLcr. The regression coefficients for the variables were b1, b2 and b3 and a was the common intercept.
Statistical analysis
For non-linear regression, the OFV (objective function value, also known as the residual sum of squares) was obtained using R version 2.15.0 [26]. and the r2 (coefficient of determination) was calculated based on its definition: r2 = 1 − (RSS/TSS), where RSS is the residual sum of squares and TSS is the total sum of squares. Statistical differences between regressions were determined by F tests utilizing the OFV values. A non-parametric bootstrap analysis with 1000 replicates was conducted on the final estimates using R version 2.15.0 [26]. For the multiple linear regression relationships, the r2 and linear regression coefficients (b1, b2, b3) were derived by least squares regression and were obtained using R version 2.15.0 [26].
Results
Urate concentrations and demographics
A total of 112 blood samples was collected from 46 patients. Eight of these patients were maintained on one dose rate, 16 patients were exposed to two different doses, a further 16 patients had three different dose rates and six patients were studied at four different doses, with blood samples taken at each dose. Plasma concentrations of urate, CLcr, doses of allopurinol, age, gender and diuretic treatment were recorded (Supplementary Table S1). Fifteen (33%) patients achieved a target concentration of ≤0.30 mmol l−1, 30 (66%) patients achieved plasma urate concentrations ≤0.36 mmol l−1 and 35 patients (76%) achieved plasma concentrations of urate ≤0.42 mmol l−1. Ten (22%) patients were taking diuretics (thiazides or furosemide) and 17 (40%) had a CLcr ≤ 60 ml min−1.
Non-linear modelling
Overall, the UT was highly dependent upon both UP and D (Table 1). The mean ID50 was 226 mg. The mean value of UR was 0.20 mmol l−1 and represents the apparent plasma urate concentration which cannot be reduced further by allopurinol. There was very close agreement between the plasma urate concentrations predicted by equation 1 and the observed plasma concentrations of urate (Figure 1). A bootstrap analysis indicated a robust fit to equation 1 with no significant change in UR or ID50 (Supplementary Table S2). The dependence of UT on UP is demonstrated at one particular dose (300 mg) in Figure 2.
Table 1.
Best fit parameters to equation 1 (UT = (1 – D/(ID50 + D)) × (UP – UR) + UR) relating the plasma urate concentrations of during treatment (UT) to the plasma concentrations pre-treatment (UP) and the daily dose of allopurinol (D)
| Group (n. patients, n. plasma samples) | UR (95% CI) mmol l−1 | ID50 (95% CI) mg allopurinol daily | Statistical parameters (r2, OFV) |
|---|---|---|---|
| All data (46, 112) | 0.20 (0.14, 0.25) | 226 (170, 303) | 0.738, 0.388 |
| All data* (46, 112) | 0* | 466 (423, 516) | 0.680, 0.474 |
| No diuretics (36, 86) | 0.14 (0.03, 0.22) | 313 (209, 480) | 0.670, 0.218 |
| Diuretics (10, 26) | 0.24 (0.04, 0.36) | 179 (95, 350) | 0.739, 0.159 |
| CLcr > 60 ml min−1 (29, 74) | 0.20 (0.11, 0.26) | 235 (152, 366) | 0.609, 0.190 |
| CLcr ≤ 60 ml min−1 (17, 38) | 0.17 (0.002, 0.27) | 240 (147, 405) | 0.782, 0.195 |
| Literature [18–22] (125, 184) | 0.20 (0.15, 0.23) | 186 (140, 241) | 0.441, 1.00 |
CI, confidence interval; CLcr, creatinine clearance; ID50, dose of allopurinol that has reduced the inhibitable urate (UP – UR) by 50%; OFV, objective function variable (residual sum of squares); r2, coefficient of determination; UR, apparent resistant plasma concentration of urate.
Equation 1 without the resistant urate concentration, i.e. UR is fixed at 0. It is a significantly poorer fit than with the resistant urate concentration (P < 0.001).
Figure 1.
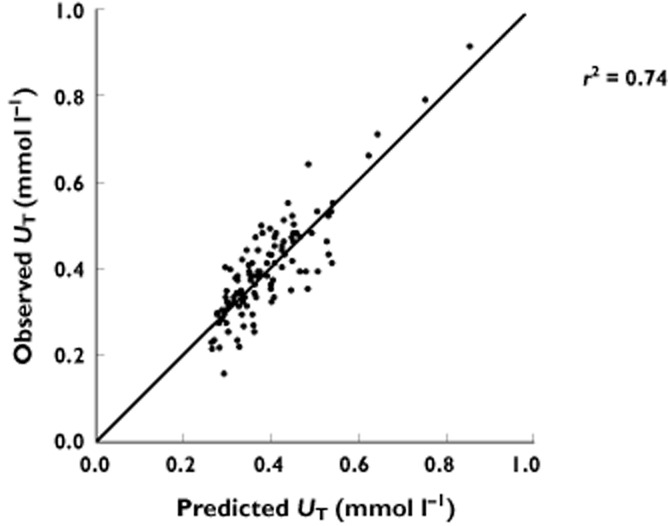
Relationship between observed plasma concentrations of urate during allopurinol treatment (UT) and the concentrations predicted from equation 1 (UT = (1 – D/ID50 + D)) × (UP – UR) + UR. ID50 dose of allopurinol that has reduced the inhibitable urate (UP – UR) by 50%, UP plasma concentration of urate pre-treatment, UR apparent resistant plasma concentration of urate. The line of identity is shown. r2 = 0.74
Figure 2.
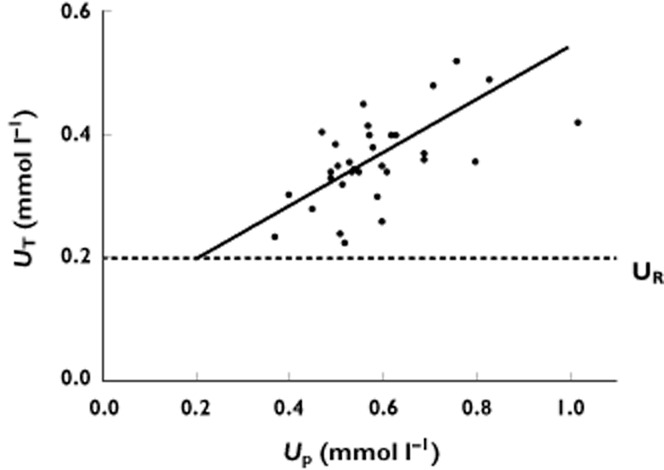
Relationship between plasma concentrations of urate during treatment (UT) and pre-treatment (UP) with 300 mg allopurinol daily. The line represents the predicted UT concentrations derived from a dosing rate of 300 mg daily and the optimal values of ID50 (226 mg day−1) and UR (0.20 mmol l−1) (Table 1). ID50 dose of allopurinol that has reduced the inhibitable urate (UP – UR) by 50%, UR apparent resistant plasma concentration of urate
Setting UR to zero in equation 1 (i.e. removing any resistant urate) made the fit significantly worse (Table 1). When the CLcr was estimated by the Cockcroft & Gault formula, the addition of a term incorporating this clearance (k × CLCR) did not improve the fit (P = 0.09, Supplementary Table S3). The eGFR was also calculated. However, the addition of this estimate of CLcr to equation 1 also did not improve the fit (P = 0.64, Supplementary Table S3).
The ID50 and UR values in patients with CLcr > 60 ml min−1 were not significantly different from the corresponding values in patients with CLcr < 60 ml min−1. In addition, use of diuretics did not significantly change the ‘best-fit’ values of ID50 or UR (Table 1).
Analysis of the literature data (Supplementary Table S4) yielded a highly significant fit to the dose–response equation although the fit was poorer than with our data (Table 1).
Rearrangement of the best fit equation to the form:
| (3) |
allows the estimation of the daily dose required to lower plasma urate to the desired concentrations (Table 2). Equation 3 predicts that higher doses of allopurinol were required with higher concentrations of UP or lower target plasma concentrations of urate.
Table 2.
Predicted daily doses (D) of allopurinol to produce target plasma concentrations of urate (from substitution in equation 3*
| Pre-treatment plasma urate (UP, mmol l−1) | Predicted allopurinol dose (mg day−1) to achieve EULAR target (UT = 0.36 mmol l−1) | Predicted allopurinol dose (mg day−1) to achieve BSR target (UT = 0.30 mmol l−1) |
|---|---|---|
| 0.65 | 405 | 775 |
| 0.6 | 335 | 665 |
| 0.55 | 265 | 554 |
| 0.5 | 195 | 443 |
| 0.45 | 126 | 332 |
Equation 3: D = ID50 × (UP – UT)/(UT – UR).
BSR, British Society of Rheumatology; EULAR, European League Against Rheumatism; ID50, dose of allopurinol that has reduced the inhibitable urate (UP – UR) by 50%; UR, apparent resistant plasma concentration of urate; UT, plasma concentration of urate during treatment with allopurinol.
Optimized values for UR and ID50 (from Table 1) are 0.20 mmol l−1 and 226 mg, respectively.
Multiple linear regression
All simple and multiple linear regressions are reported in Supplementary Table S5. The strongest correlation was the multiple linear regression of D and UP against UT (i.e. UT = a + b1 × D + b2 × UP). It was highly significant (P < 0.0001) with r2 = 0.66 and estimates (95% CI): a,0.21 (0.15, 0.27), b1,−0.45 × 104 (−0.00054, −0.00035) and b2,0.52 (0.42, 0.61). However, the fit to the non-linear dose–response relationship (equation 1) as assessed by the objective function value (OFV) was superior (OFV = 0.388) to the multiple linear regression (OFV = 0.493). As was the case with the dose–response relationship, the addition of a linear term incorporating CLcr (b3 × CLcr) to the multiple linear regression did not improve the fit significantly (Supplementary Table S5).
Discussion
This study has demonstrated for the first time that the dose (D) of allopurinol required to achieve a target urate concentration (UT) was strongly determined by the baseline concentration of plasma urate (UP). This result was seen both in the present study and in data from the literature. The fit of the literature data to the theoretical equation 1 was more scattered but this is not surprising given the use of variable assays for urate analysis in those studies [18–22]. This result should have substantial clinical significance in the management of gout. It gives reassurance to both the physician and patient as to the final goal and can be used as part of the education of the patient and the effort to improving adherence that is known to be very poor in gout patients [27–31].
It may seem self-evident that for an individual, a larger dose of allopurinol would be needed the higher the baseline plasma urate concentrations but we are not aware that this notion has been emphasized previously. We suspect that this is because studies of the response to drugs such as allopurinol are often reported as percentage of subjects achieving target plasma urate concentrations or percentage reductions from baseline values. Also, in many studies of the treatment of gout, individual pre-treatment concentrations of urate are often not published.
A notable and unexpected result was that the addition of CLcr did not significantly change the ID50 or UR parameters in the dose–response equation (equation 1). However, this can be understood by considering the major mode of elimination of urate and oxypurinol, the major and active metabolite of allopurinol. Decreased renal function leads to decreased clearance and thus to higher plasma concentrations of oxypurinol unless the dosage of allopurinol is reduced [32]. These higher plasma concentrations of oxypurinol should lead to a greater inhibition of urate synthesis. However, this effect is offset by the similarly decreased renal clearance of urate with reduced CLcr [33]. Hence, greater inhibition of urate synthesis is required to counter the decreased urate clearance associated with lower renal function. Essentially, our results indicate that the twin effects of decreasing renal function on plasma oxypurinol and urate balance each other. Dosage with diuretics also did not significantly alter the parameters in the dose–response equation. Again, the reason for the lack of effect is the common action on the renal clearance. In this case, diuretics decrease the renal clearance of both urate [34] and oxypurinol [32] without increasing the output of urate [16]. Higher plasma concentrations of urate may be produced by diuretics but, again, appear to be countered by increased plasma concentrations of oxypurinol.
For many years it has been recommended that the dosage of allopurinol should be reduced in patients with decreased renal function although several studies have shown that this commonly does not lead to adequate control of hyperuricaemia in gouty patients [9, 10]. Higher doses are often required. Our results confirm these findings and confirm that titrating dosage to target urate, irrespective of renal function, is appropriate [7, 10], although the safety of this approach is yet to be examined thoroughly. However, reduced initial dosage in renal impairment may decrease the adverse effects of allopurinol [12].
In fitting the data to both the multiple regression and dose–response equations, the intercept, UR, was greater than zero (Table 1, Supplementary Table S5). This result supports the concept of a production of urate resistant to inhibition by oxypurinol [1]. Alternatively, the apparent resistant level may be due to a maximal or saturable level of inhibition of xanthine oxidoreductase. For the purposes of predicting the optimal dose of allopurinol, however, the parameter, UR, can simply be viewed as one that improves the fit to a theoretical equation.
Limitations to our analyses include, firstly, that all variables (UP, UT, D and CLcr) were assumed to be independent. Secondly, many patients had their doses of allopurinol titrated as part of their gout treatment with the goal of achieving target plasma urate. Thus, an individual patient could be represented up to four times in regression analyses (Supplementary Table S1). Thirdly, the number of patients with renal impairment (GFR < 60 ml min−1) and patients taking diuretics was relatively small (n = 17 and 10, respectively). Further studies should include more patients with moderate to low renal function and patients also taking diuretics. Finally, the goodness-of-fit of our non-linear regressions was assessed by the value of r2 and this can be somewhat misleading. However, by reporting the OFV and conducting the F test, we demonstrated that adding a term containing CLcr to the model did not significantly improve the fit (Supplementary Table S3).
Patients with gout are commonly under treated with allopurinol. This problem was also evident in the present study where 65% achieved plasma concentrations of urate below the recommended concentration of 0.36 mmol l−1 [2] when the dose was the standard 300 mg allopurinol daily. Even fewer patients (33%) achieved a target concentration below 0.30 mmol l−1 [4].
These targets have been selected as concentrations that will lead to prevention of further attacks of gout and resolution of tophi. Unfortunately, the necessity of increased doses in achieving these concentrations has not been emphasized sufficiently. In particular, very high doses of allopurinol are likely to be required if the plasma urate before allopurinol treatment is greater than 0.6 mmol l−1. For example, to achieve a target plasma urate of 0.3 mmol l−1, equation 3 indicates that the required dose of allopurinol will be well above 600 mg daily if the pre-treatment plasma urate is above 0.6 mmol l−1 (Table 2). Similarly, with a pre-treatment plasma urate in this range, the dose of allopurinol of 400 mg should be sufficient if the target plasma urate is 0.36 mmol l−1. This may be a more realistic target and dosage of allopurinol. Clearly, the available formulation sizes (100 mg, 300 mg) and whether the tablets are scored will influence what the best approximation to the predicted dose could be. However, no matter what the estimated final dose is, allopurinol should be started at a low dose and increased gradually [3, 12].
In summary, the dose of allopurinol required to lower plasma urate to recommended target concentrations is dependent only on the baseline, pre-treatment plasma concentration of urate. Surprisingly, CLcr is not influential with respect to the final maintenance dosage. However, as recently established, CLcr should determine the starting dose of allopurinol [3, 12]. A higher baseline plasma urate will require a higher maintenance dose of allopurinol. We have provided a simple equation (equation 3) that can be used to estimate the continuing allopurinol dose likely to effectively lower the plasma urate concentrations to target.
Acknowledgments
The authors thank Drs Mona Manghani, Darren Roberts and Ms Michelle Hennessey for their assistance in recruiting patients for the present study. The work was supported by an Arthritis Australia National Research Grant (Ray and Pam Robinson Award for Rheumatology, 2008), the National Health and Medical Research Council Program Grant 568612 and the Lexy Davies Bequest.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1 Plasma concentrations of urate pre-treatment (UP), during treatment (UT) with various doses (D) of allopurinol and creatinine clearances (CLcr)
Table S2 Final parameter estimates and median estimates from non-parametric bootstrap (n = 1000 replicates) analysis of optimum model for equation 1
Table S3 The effects of creatinine clearance (CLcr) determined by the Cockcroft & Gault (with LBW) and MDRD equations to the best fit parameters to equation 1*
Table S4 Literature data of plasma concentrations of urate pre-treatment (UP), and during treatment (UT) with various doses (D) of allopurinol and creatinine clearances (CLcr)
Table S5 Multiple linear regressions of UT vs. D, UP, CLcr
References
- 1.Day RO, Graham GG, Hicks M, McLachlan AJ, Stocker SL, Williams KM. Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. Clin Pharmacokinet. 2007;46:623–644. doi: 10.2165/00003088-200746080-00001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, Gerster J, Jacobs J, Leeb B, Liote F, McCarthy G, Netter P, Nuki G, Perez-Ruiz F, Pignone A, Pimentao J, Punzi L, Roddy E, Uhlig T, Zimmermann-Gorska I. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1312–1324. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M, Gogia M, Perez-Ruiz F, Taylor W, Liote F, Choi H, Singh JA, Dalbeth N, Kaplan S, Niyyar V, Jones D, Yarows SA, Roessler B, Kerr G, King C, Levy G, Furst DE, Edwards NL, Mandell B, Schumacher HR, Robbins M, Wenger N, Terkeltaub R. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, Hingorani A, Jaques R, Nuki G. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46:1372–1374. doi: 10.1093/rheumatology/kem056a. [DOI] [PubMed] [Google Scholar]
- 5.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 6.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 7.Vazquez-Mellado J, Morales EM, Pacheco-Tena C, Burgos-Vargas R. Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis. 2001;60:981–983. doi: 10.1136/ard.60.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panomvana D, Sripradit S, Angthararak S. Higher therapeutic plasma oxypurinol concentrations might be required for gouty patients with chronic kidney disease. J Clin Rheumatol. 2008;14:6–11. doi: 10.1097/RHU.0b013e318164dceb. [DOI] [PubMed] [Google Scholar]
- 9.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006;33:1646–1650. [PubMed] [Google Scholar]
- 10.Stamp LK, O'Donnell JL, Zhang M, James J, Frampton C, Barclay ML, Chapman PT. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63:412–421. doi: 10.1002/art.30119. [DOI] [PubMed] [Google Scholar]
- 11.Arellano F, Sacristan JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337–343. doi: 10.1177/106002809302700317. [DOI] [PubMed] [Google Scholar]
- 12.Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, Dalbeth N. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529–2536. doi: 10.1002/art.34488. [DOI] [PubMed] [Google Scholar]
- 13.Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, Lan JL, Yang LC, Hong HS, Chen MJ, Lai PC, Wu MS, Chu CY, Wang KH, Chen CH, Fann CS, Wu JY, Chen YT. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MH, Stocker SL, Anderson J, Phillips EJ, Nolan D, Williams KM, Graham GG, Sullivan JR, Day RO. Initiating allopurinol therapy: do we need to know the patient's human leucocyte antigen status? Intern Med J. 2012;42:411–416. doi: 10.1111/j.1445-5994.2011.02567.x. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Ruiz F, Calabozo M, Fernandez-Lopez MJ, Herrero-Beites A, Ruiz-Lucea E, Garcia-Erauskin G, Duruelo J, Alonso-Ruiz A. Treatment of chronic gout in patients with renal function impairment: an open, randomized, actively controlled study. J Clin Rheumatol. 1999;5:49–55. doi: 10.1097/00124743-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Stamp LK, Barclay ML, O'Donnell JL, Zhang M, Drake J, Frampton C, Chapman PT. Furosemide increases plasma oxypurinol without lowering serum urate – a complex drug interaction: implications for clinical practice. Rheumatology. 2012;51:1670–1676. doi: 10.1093/rheumatology/kes091. [DOI] [PubMed] [Google Scholar]
- 17.Stocker SL, Graham GG, McLachlan AJ, Williams KM, Day RO. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in patients with gout. J Rheumatol. 2011;38:904–910. doi: 10.3899/jrheum.101160. [DOI] [PubMed] [Google Scholar]
- 18.Yue TF, Gutman AB. Effect of allopurinol (4-hydroxypyrazolo-(3,4-D)pyrimidine) on serum and urinary uric acid in primary and secondary gout. Am J Med. 1964;37:885–898. doi: 10.1016/0002-9343(64)90131-7. [DOI] [PubMed] [Google Scholar]
- 19.Rundles RW, Metz EN, Silberman HR. Allopurinol in the treatment of gout. Ann Intern Med. 1966;64:229–258. doi: 10.7326/0003-4819-64-2-229. [DOI] [PubMed] [Google Scholar]
- 20.Scott JT, Hall AP, Grahame R. Allopurinol in treatment of gout. Br Med J. 1966;2:321–327. doi: 10.1136/bmj.2.5509.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elion GB, Yu TF, Gutman AB, Hitchings GH. Renal clearance of oxipurinol, the chief metabolite of allopurinol. Am J Med. 1968;45:69–77. doi: 10.1016/0002-9343(68)90008-9. [DOI] [PubMed] [Google Scholar]
- 22.Rodnan GP, Robin JA, Tolchin SF, Elion GB. Allopurinol and gouty hyperuricemia. Efficacy of a single daily dose. JAMA. 1975;231:1143–1147. [PubMed] [Google Scholar]
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing [Computer Program] Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 27.de Klerk E, van der Heijde D, Landewe R, van der Tempel H, Urquhart J, van der Linden S. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol. 2003;30:44–54. [PubMed] [Google Scholar]
- 28.Sarawate CA, Brewer KK, Yang W, Patel PA, Schumacher HR, Saag KG, Bakst AW. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81:925–934. doi: 10.4065/81.7.925. [DOI] [PubMed] [Google Scholar]
- 29.Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 2008;67:609–613. doi: 10.1136/ard.2007.076182. [DOI] [PubMed] [Google Scholar]
- 30.Harrold LR, Andrade SE, Briesacher BA, Raebel MA, Fouayzi H, Yood RA, Ockene IS. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11:R46. doi: 10.1186/ar2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedel AA, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol. 2004;31:1575–1581. [PubMed] [Google Scholar]
- 32.Stocker SL, McLachlan AJ, Savic RM, Kirkpatrick CM, Graham GG, Williams KM, Day RO. The pharmacokinetics of oxypurinol in people with gout. Br J Clin Pharmacol. 2012;74:477–489. doi: 10.1111/j.1365-2125.2012.04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garyfallos A, Magoula I, Tsapas G. Evaluation of the renal mechanisms for urate homeostasis in uremic patients by the probenecid and pyrazinamide test. Nephron. 1987;46:273–280. doi: 10.1159/000184368. [DOI] [PubMed] [Google Scholar]
- 34.Reese OG, Jr, Steele TH. Renal transport of urate during diuretic-induced hypouricemia. Am J Med. 1976;60:973–979. doi: 10.1016/0002-9343(76)90569-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


