Abstract
Aim
Early prediction of (non-)response to infliximab therapy can improve therapeutic benefit by avoiding unnecessary periods of high disease activity during ineffective therapy. This prospective cohort study therefore aimed to study the predictive value of (1) disease activity alone and (2) infliximab serum trough concentrations in addition to disease activity 6 weeks after start of treatment for achieving low disease activity after 6 months.
Methods
Disease activity and infliximab serum trough concentrations were assessed in all rheumatoid arthritis (RA) patients at 2, 6 and 26 weeks after initiation of infliximab therapy. Receiver operating characteristic (ROC) curves and Youden indices were used to calculate specificity for prediction of good response after 6 months while aiming for maximum sensitivity.
Results
Fifty-seven consecutive RA patients starting with infliximab therapy were included. After 6 months, 15 (26%, 95 % CI 15, 38%) patients reached good European League against Rheumatism (EULAR) response. A disease activity score <4.2 at 6 weeks after initiation of therapy was a moderate predictor for reaching EULAR response after 6 months (sensitivity 100%, specificity 49%). Infliximab serum trough concentrations (>2.5 mg l−1) as predictor complimentary to disease activity (<4.2) slightly increased the specificity from 49% to 54% without changing the sensitivity (100%). As 39% of the patients did not fulfill at least one of these criteria at week 6, these patients could already be switched to another therapy after 6 weeks.
Conclusions
The combination of disease activity and infliximab serum trough concentrations could be a fair predictor to identify early (after 6 weeks) patients who have insufficient response after 6 months of therapy.
Keywords: infliximab trough concentrations, infliximab, prediction response, rheumatoid arthritis
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Although non-responders in rheumatoid arthritis should be identified as early as possible in order to prevent joint damage and functional decline, a good prediction model for predicting (non-)response is absent.
WHAT THIS STUDY ADDS
This study adds empirical evidence that disease activity scores in combination with infliximab serum trough concentrations at 6 weeks could to be a valuable and feasible instrument to optimize early detection of non-responders to infliximab therapy: although this result requires validation in another cohort.
Introduction
Infliximab gives rapid, sustained clinical response, retards radiographic progression and improves functional status in patients with rheumatoid arthritis (RA) [1–3]. In the two pivotal randomized controlled trials (RCTs) a major clinical response was found in, respectively, 21–46% of the patients using 3 mg kg−1 infliximab every 8 weeks [1–3]. However, these results also implicate that up to 54–79% of patients with RA do not reach the clinically relevant 50% improvement. These non-responders should be identified as early as possible, as achieving good clinical response early in the disease process is key to minimizing joint damage and functional decline which is characteristic of RA.
Although previous studies in RA identified a variety of baseline variables to identify responders to infliximab therapy (gender, smoking, disability, genetics, cytokine concentrations, particular immune cells, non-steroidal anti-inflammatory (NSAID) and methotrexate (MTX) use, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) concentrations), none of these variables was consistently related to treatment response and correlation coefficients were low [4–6]. Until now, only initial treatment response to infliximab (already after 6 weeks) seems to be a moderate predictor for achievement of low disease activity and continuation of anti-TNF treatment [7].
Measuring infliximab serum trough concentrations could have added value to identify early long term responders and non-responders. However, although several cross sectional publications suggest that assessment of infliximab serum trough concentrations may be useful for optimization of infliximab treatment [8–12], no prospective study for infliximab so far has attempted to explore the test characteristics of infliximab serum trough concentrations to predict initial response. A validated prediction model to predict response before starting infliximab therapy is therefore not available at this point.
Therefore, we set up a prospective cohort of RA-patients starting with infliximab therapy in order to study the predictive value of disease activity scores alone or combined with infliximab trough concentrations in order to predict early which patient could achieve low disease activity after 6 months.
Methods
Patients
All patients with RA, according to the American College of Rheumatology (ACR) 1987 revised criteria, starting with infliximab (3 mg kg−1) at the Sint Maartenskliniek (Nijmegen, the Netherlands) were included in this prospective study [13]. Patients were enrolled between February 2007 and May 2008. No other inclusion or exclusion criteria were used. The observation period started the day of inclusion and was censored on the 24 September 2008, or sooner when treatment was discontinued for any reason, or when the patient stopped attending.
Treatment protocol
All patients who started infliximab fulfilled the Dutch criteria for reimbursement of anti-tumour necrosis factor alpha (anti-TNFα) therapy: 1) moderate or high disease activity (disease activity score (DAS)28 > 3.2) and 2) having failed at least two disease modifying anti-rheumatic drugs (DMARDs) including MTX in an optimal dose up to 25 mg per week with folic acid supplementation. Patients started with exactly 3 mg kg−1 infliximab at weeks 0, 2 and 6 and subsequently every 8 weeks thereafter.
Outcomes
Primary outcome measure was fulfilment of good EULAR response criteria, 6 months after infliximab start, which requires both a DAS28 score ≤3.2 and a decrease in DAS28 > 1.2 compared with baseline [14].
Serum trough concentrations were collected 1 h prior each infusion for the assessment of serum infliximab and anti-infliximab antibodies. Infliximab antibody levels in serum were determined by an enzyme-linked immunosorbent assay [9]. This assay had an adequate test performance (within day precision 6.9% to 13.8%; accuracy (RE) −8.4 to 4.0%) with a lower limit of detection of 0.037 mg l−1 and limits of quantification between 0.5 mg l−1 and 50 mg l−1 [15]. These assays are inexpensive (€39) and readily accessible (Sanquin Research, Amsterdam, the Netherlands).
Serum anti-infliximab antibody levels were determined by a previously described radioimmunoassay [16]. The cut-off level for a positive signal was set at 12 AU ml−1 (mean + 3 SD of blank serum values). The laboratory staff was blinded for patient characteristics.
The following baseline data were recorded: demographic variables, year of disease onset, previous and concomitant DMARD treatment and systemic corticosteroid and MTX dosage. At inclusion and at each follow-up visit the dose of administered infliximab, adverse effects and co-medication were registered. Trained and calibrated research nurses assessed the DAS28 of the patients before each infliximab infusion (measurement error 0.35 after calibration).
Ethical considerations
Approval from the Medical Research Ethics Committee (MREC) was sought for. The committee decided that this approval was not necessary because DAS28 guided treatment of infliximab was performed as usual care for all patients meeting the requirements of the Dutch legislation and no extra venous puncture was necessary.
All patients were informed in writing and consented.
Statistical analysis
Descriptive statistics were provided using mean (± SD) or median (p25–p75) values depending on the (non-) parametric distribution of measured variables. We used Mantel-Haenszel χ2-tests to evaluate differences in proportions and Student's t-tests to evaluate differences in means. Baseline characteristics associated with EULAR response after 6 months (P < 0.1) were included in the prediction model.
The 95% confidence interval (95% CI) for the area under the receiver operating characteristic (ROC) curves were used to test whether DAS28 scores and infliximab serum trough concentrations were discriminating between EULAR-responders and non-responders. If the CI did not include the 0.5 value, the predictor was considered to have an ability to distinguish between responders and non-responders.
In order to calculate cut-off values with optimal sensitivity and specificity for the DAS28 scores and infliximab serum trough concentrations, Youden indices (J = sensitivity + specificity – 1) were calculated for every measurement as a possible cut-off point. Subsequently, Youden indices were calculated for all possible combinations of DAS28 scores and infliximab serum trough concentrations with maximal Youden indices. The combination with the highest Youden index is the most discriminative combination of a DAS28 score and infliximab serum trough concentration in order to predict EULAR response [17].
Results
Fifty-seven consecutive RA patients starting infliximab therapy were included. Baseline demographic and clinical data are summarized in Table 1. Three patients discontinued treatment after the second infusion due to lack of efficacy. After 6 months, 15 (26.3%, 95% CI 14.9, 37.7%) patients reached good EULAR response. Initial DAS28 scores (responders 4.9 (± 0.2) vs. non-responders 5.1 (± 0.2)), age (responders 58.8 (± 0.3) vs. 56.3 (± 0.2) years) and disease duration (responders 10.1 (± 2.5) vs. 8.1 (± 1.2) years) did not significantly differ between responders and non-responders. None of the other baseline variables (gender, anti-CCP status, rheumatoid factor status, DMARD use and MTX use) was significantly associated with good EULAR response.
Table 1.
Baseline characteristics of patients
| Cohort n = 57 | |
|---|---|
| Age (years), mean (± SD) | 57 (12) |
| Women (n, %) | 36 (63) |
| Co-morbidity (n, %) | 24 (42) |
| Median disease duration (years, p25–p75) | 6.1 (2.1–16) |
| Onset RA (months) mean (± SD) | 50 ± 14 |
| Rheumatoid factor positive (n, %) | 44 (79) |
| Anti-CCP positive (n, %) | 36 (63) |
| DAS28 at baseline, mean (± SD) | 5.0 (1.0) |
| 28 SJC at baseline, median (p25–p75) | 7 (3–9) |
| 28 TJC at baseline, median (p25–p75) | 5 (1–10) |
| ESR (mm h−1) at baseline, median (p25–p75) | 32 (16–54) |
| Patient global assessment at baseline (mm), mean (± SD) | 47 (24) |
| Previous DMARDs, median (p25–p75) | 3 (2-3) |
| Previously treated with another biological (n, %) | 7 (13) |
| Concurrent corticosteroids (n, %) | 16 (28) |
| Corticosteroid dosage (mg), median (p25–p75) | 10 (6.3–10) |
| DMARD at baseline (n, %) | 39 (71) |
| Methotrexate (n, %) | 32 (59) |
| Dose (mg week−1), median (p25–p75) | 15 (12–25) |
| Concurrent non-MTX, (n, %) | 7 (13) |
| Receiving >1 current DMARD, (n, %) | 0 (0) |
Predictive value of disease activity scores at 2 and 6 weeks
After 2 weeks, DAS28-scores were already significantly lower in patients with good EULAR response after 6 months (DAS282weeks 3.5 (± 0.8)) compared with patients without good EULAR response (DAS282weeks 4.3 (± 1.1); P = 0.01). This difference increased after 6 weeks, with a DAS28 score of EULAR responders of 2.9 (± 0.9) compared with DAS28-scores of 4.1 (± 1.0) in patients without good EULAR response (P < 0.01). The decrease in disease activity between baseline and 2 and 6 weeks was not significantly associated with EULAR response at 6 months.
Figure 1 shows the ROC curve for good EULAR response after 6 months in relation to the DAS28 score at 2 and 6 weeks. After 2 weeks, a DAS28 score of 5.0 achieved a sensitivity of 100% and a specificity 31% (Youden index 0.31), implicating that none of the 12 patients with DAS282weeks > 5.0 obtained a good EULAR response whereas 17 (39%) of the 44 patients with DAS282weeks < 5.0 had a good EULAR response at 6 months. After 6 weeks, the ROC curve indicated that none of the 19 (0%) patients with DAS286weeks ≥ 4.2 obtained a good EULAR response whereas 20 (51.3%) of the 39 patients with DAS286weeks < 4.2 had no good EULAR response after 6 months. Table 2 shows the 2 × 2 table for these test results (sensitivity 100%, specificity 49%, positive prediction value (PPV) 43%, negative prediction value (NPV) 100%, Youden index 0.49).
Figure 1.
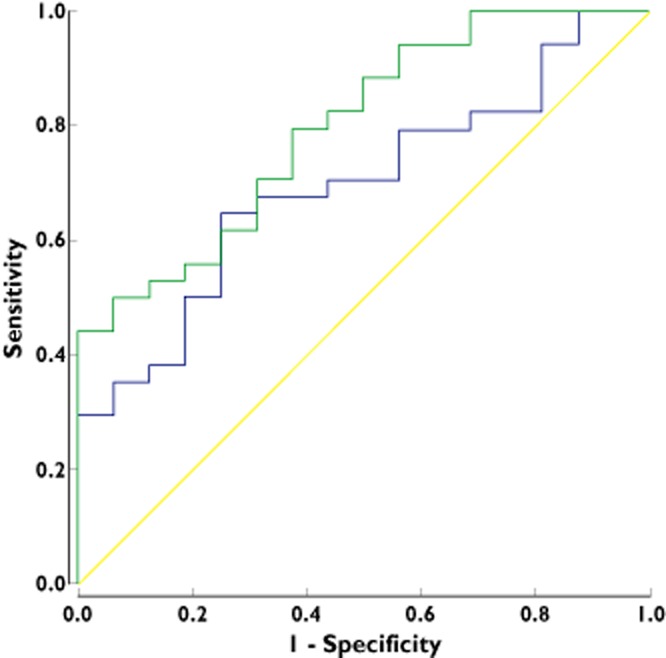
ROC curve for the EULAR good response after 6 months vs. the DAS28 score at 2 weeks (AUC: 0.70: 95% CI: 0.55, 0.84) and after 6 weeks (AUC: 0.80: 95% BI: 0.67, 0.92).  , DA28 at 2 weeks;
, DA28 at 2 weeks;  , DA28 at 6 weeks;
, DA28 at 6 weeks;  , Reference line
, Reference line
Table 2.
2 × 2 table for prediction of EULAR response after 6 months vs. DAS28 score at 6 weeks (left) and vs. DAS28 score and/or infliximab serum trough concentrations at 6 weeks (right)
| DAS28 response (after 6 months) | DAS28 response (after 6 months) | ||||
|---|---|---|---|---|---|
| Good | Non/moderate | Good | Non/moderate | ||
| DAS28 < 4.2 | 15 (28%)a | 20 (37%)b | DAS28 < 4.2 and infliximab trough concentration >2.5 mg l−1 | 15 (28%)a | 18 (33%)b |
| DAS28 ≥ 4.2 | 0 (0%)c | 19 (35%)d | DAS28 ≥ 4.2 and/or infliximab trough concentration ≤2.5 mg l−1 | 0 (0%)c | 21 (39%)d |
| Sensitivity = a/(a + c) | 100% | Sensitivity | 100% | ||
| Specificity = d/(b + d) | 49% | Specificity | 54% | ||
| PPV (positive predictive value) = a/(a + b) | 43% | PPV | 45% | ||
| NPV (negative predictive value) = d/(c + d) | 100% | NPV | 100% | ||
Predictive value of infliximab serum trough concentrations
Figure 2 depicts the infliximab serum trough concentrations in EULAR responders and non-responders after 2, 6 and 14 weeks. Patients with a good EULAR response after 6 months tended to have higher infliximab serum trough concentrations (2 weeks 23.4 mg l−1 (± 20.8), 6 weeks 12.3 mg l−1 (± 6.1)) compared with non- or partially-responding patients (2 weeks 16.0 mg l−1 (± 10.4), 6 weeks: 9.0 mg l−1 (± 6.8)).
Figure 2.
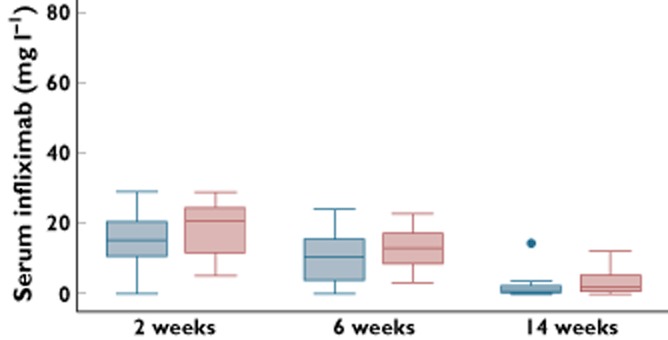
Infliximab serum trough concentrations in EULAR responders and non-responders after 2, 6 and 14 weeks.  , EULAR non-responders;
, EULAR non-responders;  , EULAR-responders
, EULAR-responders
After 6 weeks, all patients with infliximab serum trough concentrations <2.5 mg l−1 (n = 9) did not attain infliximab response, while 15 of 46 patients with infliximab serum trough concentrations >2.5 mg ml−1 reached good EULAR response (ROC area 0.67 (95% CI 0.52, 0.82), sensitivity 100%, specificity 23%, PPV 35%, NPV 100%, Youden index: 0.23). The most optimal cut off score was however an infliximab serum trough concentration of 11 mg l−1 (sensitivity: 73%, specificity: 64%, PPV: 42%, NPV: 83%: Youden index 0.37). Of note, these were serum trough concentrations 4 weeks after the last infusion, and these are not directly comparable with regular 8 week interval trough concentrations.
After 14 weeks, infliximab serum trough concentrations tended to be higher in the group of EULAR responders compared with the non-responders (median (interquartile range) 0.9 (0.05–2.6) vs. 2.0 (0.7–5.4) mg l−1, P = 0.06).
Anti-infliximab antibodies were formed in three (after 6 weeks) and nine patients (14 weeks). None of the patients with anti-infliximab antibodies after 6 weeks reached EULAR response after 26 weeks, whereas only one patient with anti-infliximab antibodies after 14 weeks reached EULAR response after 6 months.
Additional predictive value of combining infliximab serum trough concentrations and disease activity scores
Figure 3 displays the two graph ROC curves visualizing the relationship between DAS28 scores (A) and infliximab serum trough concentrations (B) on the one hand and sensitivity, specificity and Youden's index for predicting EULAR response after 6 months on the other hand. After calculating Youden indices for every individual potential cut off point, four DAS28 scores maximized Youden indices (3.2, 3.4, 3.6 and 4.2), whereas six infliximab serum trough concentrations maximized Youden indices (2.5, 3.3, 5.2, 6.7, 8.5 and 11.0). The 24 (4 × 6) combinations of these predictors were subsequently tested as possible cut-off levels. Sensitivity, specificity and Youden indices of these possible cut off values are depicted in Table 3.
Figure 3.
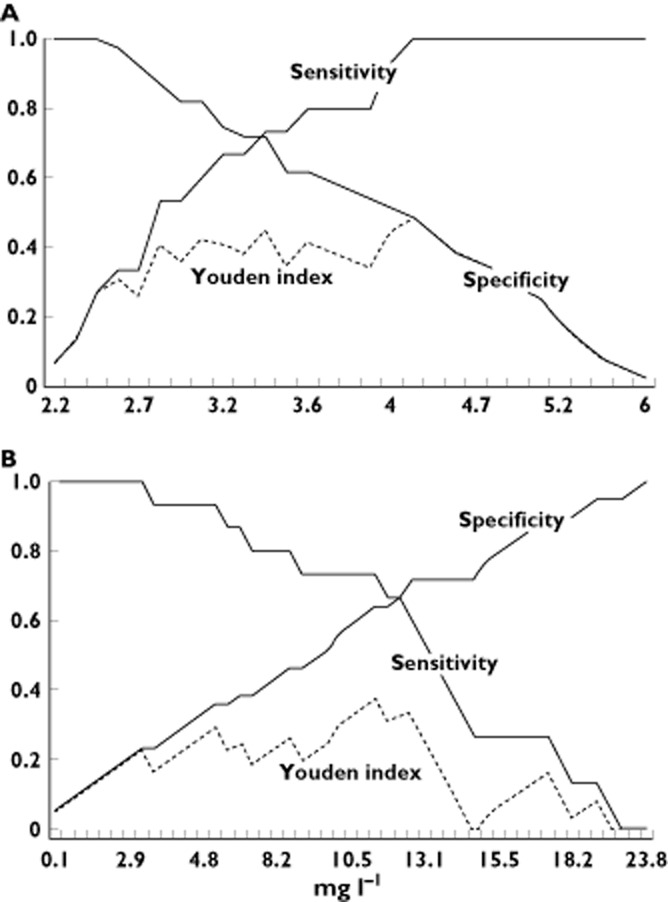
Two-graph ROC visualizing the relationship between DAS28 scores (A, x-axis) and infliximab serum trough concentrations (B, x-axis) and sensitivity, specificity and Youden index for predicting EULAR response after 6 months
Table 3.
Sensitivity, specificity and Youden indices of all possible combinations of DAS28 scores and infliximab serum trough concentrations with maximal Youden indices
| DAS28 score | Infliximab serum trough concentration (mg l−1) | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|
| 3.2 | 2.5 | 0.67 | 0.74 | 0.41 |
| 3.2 | 3.3 | 0.64 | 0.74 | 0.34 |
| 3.2 | 5.2 | 0.53 | 0,77 | 0.30 |
| 3.2 | 6.7 | 0.47 | 0.79 | 0.26 |
| 3.2 | 8.5 | 0.40 | 0.82 | 0.22 |
| 3.2 | 11.0 | 0.33 | 0.87 | 0.21 |
| 3.4 | 2.5 | 0.73 | 0.74 | 0.48 |
| 3.4 | 3.3 | 0.67 | 0.74 | 0.41 |
| 3.4 | 5.2 | 0.60 | 0.77 | 0.37 |
| 3.4 | 6.7 | 0.53 | 0.79 | 0.33 |
| 3.4 | 8.5 | 0.47 | 0.82 | 0.29 |
| 3.4 | 11.0 | 0.40 | 0.87 | 0.27 |
| 3.6 | 2.5 | 0.80 | 0.67 | 0.47 |
| 3.6 | 3.3 | 0.73 | 0.67 | 0.40 |
| 3.6 | 5.2 | 0.67 | 0.72 | 0.38 |
| 3.6 | 6.7 | 0.60 | 0.74 | 0.34 |
| 3.6 | 8.5 | 0.53 | 0.79 | 0.33 |
| 3.6 | 11.0 | 0.47 | 0.82 | 0.29 |
| 4.2 | 2.5 | 1.00 | 0.54 | 0.54 |
| 4.2 | 3.3 | 0.93 | 0.51 | 0.45 |
| 4.2 | 5.2 | 0.87 | 0.59 | 0.46 |
| 4.2 | 6.7 | 0.80 | 0.62 | 0.42 |
| 4.2 | 8.5 | 0.73 | 0.67 | 0.40 |
| 4.2 | 11.0 | 0.67 | 0.74 | 0.41 |
As indicated in Table 3, the Youden index was maximized with DAS scores after 6 weeks ≥4.2 and/or infliximab serum trough concentrations ≤2.5 mg l−1 (sensitivity 100%, specificit: 54%, PP: 45%, NP:100%, Youden index 0.54). Consequently, none of the 21 of 54 (39%) patients with either DAS286weeks ≥ 4.2 and/or infliximab serum trough concentrations ≤2.5 mg l−1 reached EULAR response after 6 months.
Discussion
Early prediction of response to infliximab therapy can improve therapeutic benefit by avoiding unnecessary periods of high disease activity, costs and side effects. The results of this study could help to identify early responders as we found that the combination of either a DAS28 score of ≥4.2 and/or infliximab serum trough concentrations ≤2.5 mg l−1 6 weeks after initiation of therapy was a fair predictor (sensitivity 100%, NPV 100%) for not achieving low disease activity, with also acceptable specificity (54%). As 39% of the patients starting infliximab fulfilled this criterion at week 6 (54% of the non-responders), these patients could potentially be switched to another therapy after 6 weeks. This implicates that infliximab serum trough concentrations (measured just before the third infusion at 6 weeks) should be measured in 27 patients to find one extra non-responding patients (number needed to measure = 27). Early identification of non-responding patients enables the patient to get alternative effective treatment and increases the cost effectiveness of the treatment by shortening the period of moderate/high disease activity despite costly medication.
Increasing the dose in these non-responding patients is less sensible, at least in RA. Increasing the dose has a much lower chance of response in rheumatic diseases [18, 19] and is associated with more adverse events and costs [20] than switching to another biological, and is therefore not cost effective.
In order to improve the specificity of this prediction model, we considered inclusion of other baseline variables that have been reported to be associated, at least to some extent, with response. However, as none of the baseline variables (like gender, disability, NSAID and MTX use, genetics, particular immune cells, RF and anti-CCP concentrations) were related to low disease activity after 6 months, baseline variables were unable to increase the specificity of this prediction model. Other candidate predictors that could be considered include genetic predictors, especially polymorphism in the TNF (receptor) region. Thus far, however, none of these markers has unequivocally been shown to be associated with response to anti-TNF [21]. Furthermore, the use of these genetic markers is at this moment not feasible in clinical practice, because they are not widely accessible and because assessment is too time consuming and costly.
Although this prediction model suggests that DAS28 scores combined with infliximab serum trough concentrations could predict early half of the non-responding patients, several requirements are to be met before this prediction model could be successfully used in clinical practice. Firstly, it should be noted that the proposed prediction model was developed in a relatively small population and should be validated in another cohort of patients with RA treated with infliximab, as it is to be expected that the sensitivity and specificity will be somewhat lower after replication in another cohort. The next step should address feasibility. This seems adequate, as the DAS28 is a widely adopted measure for disease activity that can be measured instantaneously. The measurement of infliximab serum trough concentrations is regularly available in the Netherlands, is not very expensive and takes 2 weeks.
In conclusion, disease activity scores in combination with infliximab serum trough concentrations at 6 weeks seem to be a valuable and feasible instrument to optimize early detection of non-responders to infliximab therapy although this result requires validation in another cohort.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
The authors would like to thank the rheumatologists and specialist nurses of the Department of Rheumatology of Sint Maartenskliniek Nijmegen for their support.
Authors' contributions
Authors' contributions: BvdB, AdB, GW, AM, YH, PR, BB and FH conceived and designed the study. All authors were involved in carrying out the study and interpreting the results. BvdB performed the statistical analysis and drafted the manuscript. All authors read, commented on and approved the final manuscript.
References
- 1.St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, Keystone E, Schiff M, Kalden JR, Wang B, Dewoody K, Weiss R, Baker D Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset Study Group. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 2.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen LE, Kapetanovic MC, Gülfe A, Söderlin M, Saxne T, Geborek P. Predictors of response to anti-TNF therapy according to ACR and EULAR criteria in patients with established RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology. 2008;47:495–499. doi: 10.1093/rheumatology/ken002. [DOI] [PubMed] [Google Scholar]
- 5.Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res Ther. 2011;13:R126. doi: 10.1186/ar3431. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery P, Dörner T. Optimising treatment in rheumatoid arthritis: a review of potential biological markers of response. Ann Rheum Dis. 2011;70:2063–2070. doi: 10.1136/ard.2010.148015. [DOI] [PubMed] [Google Scholar]
- 7.Gülfe A, Kristensen LE, Geborek P. Six and 12 weeks treatment response predicts continuation of tumor necrosis factor blockade in rheumatoid arthritis: an observational cohort study from southern Sweden. J Rheumatol. 2009;36:517–521. doi: 10.3899/jrheum.080509. [DOI] [PubMed] [Google Scholar]
- 8.Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006;54:3782–3789. doi: 10.1002/art.22214. [DOI] [PubMed] [Google Scholar]
- 9.Wolbink GJ, Vis M, Lems W, Voskuyl AE, de Groot E, Nurmohamed MT, Stapel S, Tak PP, Aarden L, Dijkmans B. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:711–715. doi: 10.1002/art.21671. [DOI] [PubMed] [Google Scholar]
- 10.Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, Bendtzen K. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739–1745. doi: 10.1136/ard.2008.092833. [DOI] [PubMed] [Google Scholar]
- 11.Svenson M, Geborek P, Saxne T, Bendtzen K. Monitoring patients treated with anti-TNF-alpha biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology. 2007;46:1828–1834. doi: 10.1093/rheumatology/kem261. [DOI] [PubMed] [Google Scholar]
- 12.Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165–178. doi: 10.1136/annrheumdis-2012-202545. [DOI] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–1850. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Van den Bemt BJF, Van Eerd JE, Den Broeder AA, Hekster YA, Van Riel PLCM, Stapel SO, Wolbink GJ. Validation of a precise and accurate enzyme-linked immunosorbent assay for the bioanalysis of infliximab in human serum. 2009. In: Optimizing Pharmacotherapy in Patients with Rheumatoid Arthritis (Thesis). Nijmegen: 62–71.
- 16.Wolbink GJ, Vis M, Lems W, Voskuyl AE, de Groot E, Nurmohamed MT, Stapel S, Tak PP, Aarden L, Dijkmans B. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:711–715. doi: 10.1002/art.21671. [DOI] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Pavelka K, Jarosová K, Suchý D, Senolt L, Chroust K, Dusek L, Vencovský J. Increasing the infliximab dose in rheumatoid arthritis patients: a randomised, double blind study failed to confirm its efficacy. Ann Rheum Dis. 2009;68:1285–1289. doi: 10.1136/ard.2008.090860. [DOI] [PubMed] [Google Scholar]
- 19.van den Bemt BJ, den Broeder AA, Snijders GF, Hekster YA, van Riel PL, Benraad B, Wolbink GJ, van den Hoogen FH. Sustained effect after lowering high dose infliximab in patients with rheumatoid arthritis: a prospective dose titration study. Ann Rheum Dis. 2008;67:1697–1701. doi: 10.1136/ard.2007.083683. [DOI] [PubMed] [Google Scholar]
- 20.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs JD, Ferraccioli G. The need for personalised medicine for rheumatoid arthritis. Ann Rheum Dis. 2011;70:4–7. doi: 10.1136/ard.2010.135376. [DOI] [PubMed] [Google Scholar]


