Abstract
Aim(s)
The current investigation aims to provide new insights into fetal exposure to tacrolimus in utero by evaluating maternal and umbilical cord blood (venous and arterial), plasma and unbound concentrations at delivery. This study also presents a case report of tacrolimus excretion via breast milk.
Methods
Maternal and umbilical cord (venous and arterial) samples were obtained at delivery from eight solid organ allograft recipients to measure tacrolimus and metabolite bound and unbound concentrations in blood and plasma. Tacrolimus pharmacokinetics in breast milk were assessed in one subject.
Results
Mean (±SD) tacrolimus concentrations at the time of delivery in umbilical cord venous blood (6.6 ± 1.8 ng ml−1) were 71 ± 18% (range 45–99%) of maternal concentrations (9.0 ± 3.4 ng ml−1). The mean umbilical cord venous plasma (0.09 ± 0.04 ng ml−1) and unbound drug concentrations (0.003 ± 0.001 ng ml−1) were approximately one fifth of the respective maternal concentrations. Arterial umbilical cord blood concentrations of tacrolimus were 100 ± 12% of umbilical venous concentrations. In addition, infant exposure to tacrolimus through the breast milk was less than 0.3% of the mother's weight-adjusted dose.
Conclusions
Differences between maternal and umbilical cord tacrolimus concentrations may be explained in part by placental P-gp function, greater red blood cell partitioning and higher haematocrit levels in venous cord blood. The neonatal drug exposure to tacrolimus via breast milk is very low and likely does not represent a health risk to the breastfeeding infant.
Keywords: breast milk, neonatal exposure, placental transfer, pregnancy, tacrolimus, unbound drug concentration
WHAT IS KNOWN ABOUT THIS SUBJECT
Utilizing a non-specific assay, tacrolimus has been reported to cross the placenta resulting in fetal exposure. Tacrolimus has also been reported to transfer into breast milk.
WHAT THIS STUDY ADDS
This is the first study to report unbound (active) tacrolimus and metabolite concentrations in the umbilical cord blood and breast milk. It provides insight into the factors influencing drug transfer in pregnancy and lactation as well as interpretation of tacrolimus concentrations in the newborn.
Introduction
Tacrolimus is an immunosuppressive agent widely used for the prevention of rejection in solid organ transplant recipients [1]. Although usually managed successfully, pregnancy following transplantation is associated with many complications [2–4]. Published data characterizing tacrolimus and metabolite transfer across the placenta, metabolism by the fetus and excretion into breast milk are limited. Utilizing a non-specific immunoassay, mean umbilical cord plasma concentrations have been reported to be 0.71 ± 0.77 ng ml−1 (49% of maternal plasma concentrations) [2]. However, clinical immunoassays are not specific for the parent compound nor are they accurate at low concentrations [5, 6]. Since tacrolimus crosses the placenta, one potential concern is the effect of tacrolimus in utero exposure on the developing fetus [4].
Tacrolimus is a substrate for P-glycoprotein (P-gp), one of several drug transporters expressed and active on placental syncytiotrophoblasts [7]. Some of these transporters, such as P-gp, minimize fetal exposure by effluxing drug back into the maternal circulation [8]. Umbilical cord blood is part of the fetal circulation. Umbilical cord venous blood brings oxygen and nutrients from the placenta to the fetal inferior vena cava and the umbilical arteries return fetal blood from the internal iliac arteries to the placenta. We utilized venous umbilical cord : maternal blood, plasma and unbound tacrolimus and metabolite concentration ratios at the time of delivery to characterize placental transfer in vivo [9]. In plasma, tacrolimus has been shown to bind to α1-acid glycoprotein and albumin [10, 11]. There is a low percentage of unbound tacrolimus in plasma (5.4 ± 0.7% during mid and late pregnancy and 2.8 ± 0.4% in maternal plasma post-partum) [12]. Furthermore, tacrolimus concentrates in erythrocytes, with a blood : plasma ratio ranging from 4 to 42, resulting in an even lower fraction unbound in whole blood [13, 14]. It is unbound drug that is available to bind receptors and cross membranes, including those involved in drug transfer across the placenta and into the breast milk [15]. To our knowledge, only one study has evaluated in vivo placental transfer of tacrolimus utilizing a highly sensitive and specific assay, although metabolite concentrations were not reported [16].
Maternal CYP3A4/5 metabolic activities in the liver and small intestine are the major determinants of steady-state circulating maternal drug concentrations following oral drug administration [17–20]. In adult healthy volunteers, tacrolimus clearance is reported to be 0.040 ± 0.009 l h–1 kg−1 [21]. Extensive pre-systemic metabolism and P-gp efflux limits the oral bioavailability of tacrolimus in non-pregnant women and men to approximately 14 ± 6% [22, 23]. Paediatric patients (0.7–13.2 years of age) have been reported to have a faster clearance 0.138 ± 0.071 l h–1 kg−1 and require a higher weight adjusted dosage despite their higher absolute bioavailability (31 ± 24%) than adults [21]. Recently, the pharmacokinetics of tacrolimus in infants less than 30 days of age were published [16]. Placental transfer resulted in neonatal concentrations similar to maternal concentrations. Tacrolimus concentrations in the infants declined by ∼15% per day [16]. This finding likely reflects initial metabolism by CYP3A7, which is then gradually replaced by CYP3A4 metabolism after birth [24]. In the fetus, CYP3A7 is the major cytochrome P450 enzyme expressed in the liver, accounting for ∼50% of the total cytochrome P450 content [20]. CYP3A7 is less efficient at metabolizing tacrolimus than either CYP3A4 or CYP3A5 (relative catalytic efficiencies of 29% and 18%, respectively) [18]. Measurement of tacrolimus and metabolite concentration gradients between the umbilical vein and artery can provide information on fetal metabolism of tacrolimus and its impact on fetal exposure [9].
Tacrolimus crosses into breast milk [4, 5] and some women have been advised against breastfeeding during therapy, despite apparently low infant exposure [2, 25]. In the current study, we characterized both the tacrolimus milk : blood and milk : plasma area under the concentration–time curve ratios. In addition, we determined unbound drug concentration in milk and plasma to gain an understanding of the mechanism by which tacrolimus is transferred into human milk across the mammary epithelium [26].
Methods
Subjects of the study
The study was approved by the Institutional Review Boards at the University of Washington, Georgetown University and the University of Texas Medical Branch at Galveston and was conducted in accordance with their guidelines. All subjects gave written informed consent. Eight pregnant subjects participated in the tacrolimus placental transfer study. One additional subject participated in the breast milk sample collection for measurement of tacrolimus concentrations.
Sample collection
A total of eight pregnant subjects participated in the placental transfer study. Maternal and umbilical cord (arterial and venous) blood samples were collected immediately after delivery in seven cases. The mean time interval between dosing and sample collection was 4.9 h (range 1.7 to 10.3 h) for maternal blood and 4.5 h (range 1.6 to 10.1 h) for umbilical cord blood. For one other subject, whose delivery occurred 4.5 h after tacrolimus dosing, maternal blood was collected 30 min before delivery while venous cord blood was collected 30 min after delivery.
For one additional subject who did not participate in collection of labour and delivery samples, a breast milk pharmacokinetic study was completed 45 weeks and 1 day after delivery. Sequential blood samples (5 ml) were collected over one dosing interval (just before (0 h) and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12 h after a regular oral dose), processed and stored as previously described [12]. Breast milk collections were performed using the Medela Classic double electric breast pump, into glass bottles every 3 h, over one dosing interval. Both breasts were completely emptied of milk during each collection. The subject did not breastfeed her infant during the study day. Glass pipettes were used for all transfers to avoid adsorptive loss of tacrolimus.
Tacrolimus and metabolite analysis
Tacrolimus and metabolite concentrations in blood, plasma and milk samples were quantified utilizing a previously reported LC-MS/MS assay [12, 27]. Calibration curves for tacrolimus and its metabolites were generated by plotting the peak area ratios of tacrolimus or metabolite to internal standard against known standard tacrolimus or metabolite concentrations. Standard curve concentrations for tacrolimus ranged from 0.005 ng ml−1 to 40 ng ml−1. The limit of quantitation was 0.005 ng ml−1 for tacrolimus, and in the range of 0.003–0.04 ng ml−1 for 13-DMT, 15-DMT, 31-DMT and 12-HT in blood, urine and plasma samples. Intra-day and inter-day coefficients of variation for the assays were all less than 10%.
Unbound fractions in plasma (fup) and blood (fub) were determined as previously reported [12]. An ultracentrifugation procedure adapted from Nakai et al. [28] was used to determine unbound fraction of tacrolimus in milk (fum).
Pharmacokinetic analysis
Non-compartmental pharmacokinetic analysis was performed using WinNonlin software version 5.2 (Pharsight, Mountain View, CA). Measured milk concentrations for each 3 h interval were used to determine the amount excreted (milk concentration × milk volume). The estimated amount excreted daily was the sum of the 3 h intervals over the 12 h sampling period multiplied by 2. The tacrolimus dose ingested by a typical, exclusively breastfed 3-month-old infant was also estimated, by assuming a breast milk ingestion of 150 ml kg–1 day−1. The tacrolimus dose ingested by the actual infant was also calculated based on the measured concentrations and excreted milk volume.
To elucidate the difference in whole blood : plasma (Kb : p) concentration ratios between maternal and cord blood, the partitioning of tacrolimus between blood cells and plasma (Kbc/p) was estimated by Kbc/p = (Kb/p/Hct) − (1/Hct) + 1 [29]. Kb : p is the blood : plasma concentration ratio and Hct represents the haematocrit. For maternal blood, Hct was not available for samples at term. Instead, pre-term haematocrit values (28.7 ± 3.8%, range 25%–35%) for the same individual (56 ± 53 days before delivery) were used for six subjects. For one other subject whose haematocrit was not available, the mean value (28.7) was used. For venous umbilical cord blood, an average reported haematocrit value of 51% was used for all calculations [30].
Statistical analysis
Descriptive statistics are presented as mean ± SD, unless otherwise indicated. Statistical comparisons were conducted using a paired two-sided Student's t-test (GraphPad Prism 5, La Jolla, CA, USA). A P value less than 0.05 was considered significant.
Results
Patient demographics
The women participating in this study are a subset of women previously reported describing the pharmacokinetics of tacrolimus during pregnancy [12]. A total of eight pregnant subjects (three non-Hispanic White, three Hispanic, one non-Hispanic Black, one Asian), age 25.4 ± 6.2 years and height 158.4 ± 8.8 cm, participated in the placental transfer study. All subjects were solid organ transplant recipients (four kidney, one kidney/pancreas, one kidney/heart, two liver) and were receiving tacrolimus orally for immunosuppression. The dosing intervals were 8 h (n = 4), 12 h (n = 3) or 24 h (n = 1). The mean gestational age at delivery was 36.8 ± 2.9 weeks.
The liver transplant recipient who participated in the breast milk sample collection weighed 55.1 kg on her study day. The infant weight was not measured.
Placental transfer of tacrolimus
Maternal (n = 8) and umbilical cord blood (n = 7 for venous and n = 5 for arterial) were collected at the time of delivery. The average tacrolimus dosage was 7.5 ± 2.6 mg day−1 (range 4–12 mg day−1). For the one patient who gave birth to twins, two individual umbilical cord venous blood samples were collected, analyzed separately, then averaged, as their tacrolimus concentrations differed by only 4.9%. Tacrolimus was assayed in plasma from maternal (n = 7) and umbilical venous cord (n = 6) samples while plasma samples from cord arterial blood were haemolyzed and therefore not included in analysis. Unbound tacrolimus concentrations were determined in maternal (n = 7) and umbilical cord venous (n = 5) plasma samples. Total and unbound tacrolimus concentrations are presented in Table 1.
Table 1.
Tacrolimus concentrations (ng ml−1) and concentration ratios in maternal, umbilical cord blood and plasma samples. Results reported as mean ± SD (range)
| Concentration | Maternal venous | Umbilical cord venous | Umbilical cord arterial |
|---|---|---|---|
| Blood | 9.0 ± 3.4 | 6.6 ± 1.8 | 5.7 ± 1.0 |
| (4.1, 13.7) | (4.9, 10.1) | (4.6, 7.25) | |
| (n = 8) | (n = 7) | (n = 5) | |
| Plasma | 0.40 ± 0.20 | 0.09 ± 0.04 | |
| (0.23, 0.80) | (0.05, 0.16) | ||
| (n = 7) | (n = 6) | ||
| Unbound drug | 0.017 ± 0.010 | 0.003 ± 0.001 | |
| (0.006, 0.030) | (0.002, 0.006) | ||
| (n = 7) | (n = 5) | ||
| Ratio | Venous umbilical cord : maternal | Umbilical cord arterial : venous | |
| Blood | 0.71 ± 0.18 | 1.00 ± 0.12 | |
| (0.44, 0.99) | (0.81, 1.14) | ||
| (n = 7) | (n = 5) | ||
| Plasma | 0.23 ± 0.11 | ||
| (0.13, 0.44) | |||
| (n = 6) | |||
| Unbound drug | 0.19 ± 0.10 | ||
| (0.09, 0.36) | |||
| (n = 5) |
The mean tacrolimus concentrations in maternal blood and venous umbilical cord blood were 9.0 ± 3.4 ng ml−1 and 6.6 ± 1.8 ng ml−1, respectively; i.e. venous umbilical cord blood tacrolimus was 71 ± 18% (range 45–99%) of maternal blood concentrations (Figure 1). In comparison, venous cord plasma tacrolimus was only 23 ± 11% (range 12–44%) of maternal plasma concentrations. The percent unbound of tacrolimus in maternal plasma (4.4 ± 2.8%) was comparable with that in umbilical cord venous plasma (3.6 ± 0.8%). Accordingly, the percent of unbound tacrolimus in venous cord to maternal plasma was 19 ± 10% (range 9–36%), similar to the concentration ratio calculated based on total plasma concentrations (23 ± 11%).
Figure 1.
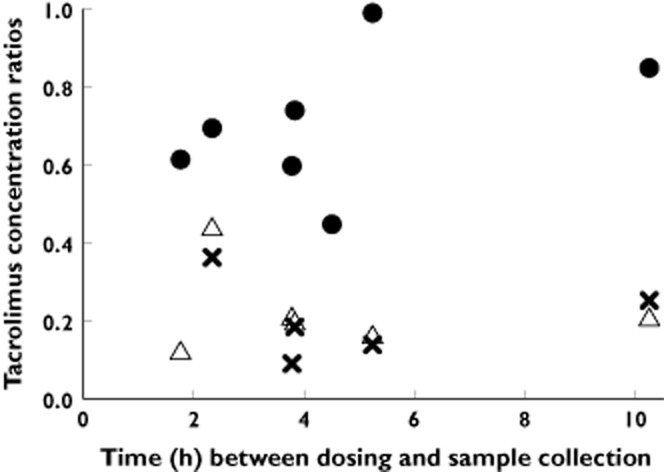
Tacrolimus venous umbilical cord blood : maternal blood, venous umbilical cord plasma : maternal plasma and venous umbilical cord unbound : maternal unbound concentration ratios in eight patients, shown by the time after last dose of tacrolimus to blood sampling. For each ratio, the different data points represent an observation in a different subject. •, venous cord blood : maternal blood (n = 7); ▵, venous cord plasma : maternal plasma (n = 6);  , venous cord unbound : maternal unbound (n = 5)
, venous cord unbound : maternal unbound (n = 5)
The tacrolimus venous umbilical cord blood : plasma concentration ratios (Kb : p) (83.0 ± 24.2, range 46.9–107.5) were, on average, 274% higher than maternal ratios (22.2 ± 5.7, range 16.0–29.8, P < 0.003, Figure 2A). Tacrolimus partition ratio between blood cells and plasma was calculated to be 75.4 ± 19.0 (range 49.3–97.1) in maternal blood and 161.9 ± 47.5 (range 91.0–209.7) in venous umbilical cord blood (P < 0.02, Figure 2B).
Figure 2.
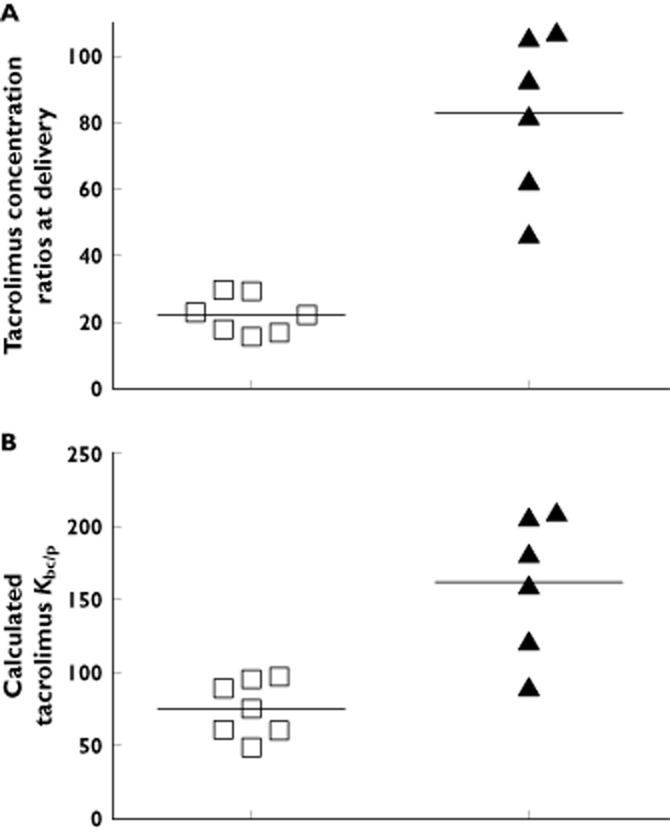
Tacrolimus maternal blood : plasma (n = 7), venous umbilical cord blood : plasma (n = 6) concentration ratios in patients, taking tacrolimus at the time of delivery (A) and the calculated Kbc/p, the tacrolimus partitioning ratio between blood cells and plasma, in maternal blood (n = 7) and venous umbilical cord blood (n = 6) (B). The horizontal lines represent the mean ratios. A) □, maternal blood : maternal plasma; ▴, venous cord blood : venous cord plasma; B) □, maternal blood Kbc/p; ▴, Venous Cord Blood Kbc/p
The umbilical cord venous : maternal (Figure 1) or arterial : venous drug concentration ratios did not vary as a function of time between dosing and sample collection (n = 5 for observations for which both arterial and venous umbilical concentrations were available). Arterial umbilical cord blood concentrations of tacrolimus were 100 ± 12% of venous concentrations (range 81–113%).
Of the four primary metabolites of tacrolimus, the pharmacologically inactive metabolites 13-O-desmethyl tacrolimus (13-DMT) and 15-O-desmethyl tacrolimus (15-DMT) could be measured in all umbilical cord samples, while the active metabolite 31-O-desmethyl tacrolimus (31-DMT) was quantifiable in four out of eight venous cord samples (including two individual umbilical cord venous blood samples from one patient who gave birth to twins) at very low concentrations (0.02 ± 0.01 ng ml−1) (see supplementary Table S1 for metabolite concentrations). The umbilical cord venous : maternal concentration ratios of 31-DMT, 13-DMT and 15-DMT in blood were 0.42 ± 0.23, 0.67 ± 0.14 and 1.05 ± 0.43, respectively. Similar to the parent drug, venous umbilical cord plasma concentrations of 13-DMT and 15-DMT were only 13 ± 8% and 9 ± 7% of maternal plasma concentrations, respectively. The umbilical cord arterial : venous blood ratios of 13-DMT and 15-DMT were both 1.13 ± 0.10.
Excretion of tacrolimus into milk
The amount of tacrolimus excreted in breast milk over a 12 h steady-state dosing interval was determined in one patient, treated with 1.5 mg of oral tacrolimus twice daily. Tacrolimus concentration in breast milk peaked later than blood or plasma (6 h, 1 h and 1 h respectively). Peak and trough concentrations in milk were 1.11 and 0.78 ng ml−1, respectively (Figure 3A). The average concentration of tacrolimus in milk was 0.93 ng ml−1, comparable with previously reported values [5, 25]. The milk : blood and milk : plasma AUC ratios in this subject were 0.13 and 2.89, respectively. Tacrolimus percent unbound in milk and plasma were 3.7 ± 0.6% (n = 3) and 2.7 ± 0.4% (n = 3), respectively. The calculated unbound tacrolimus milk : plasma AUC ratio was 3.96 (Figure 3B).
Figure 3.
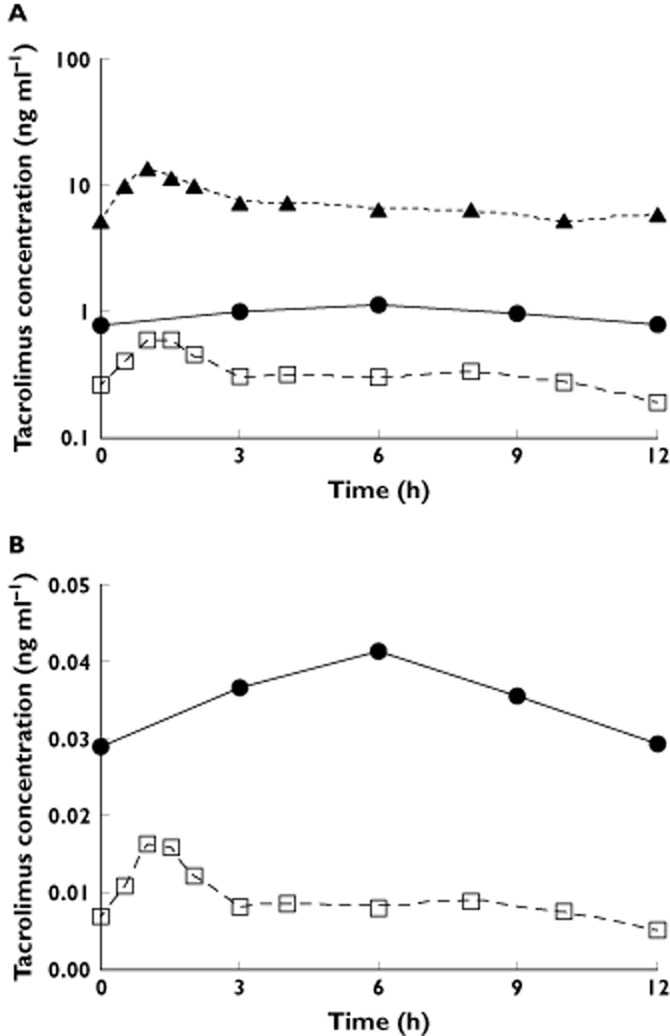
Tacrolimus concentration (logarithmic scale) in maternal blood, plasma and breast milk (A) and steady-state unbound tacrolimus concentrations in maternal plasma and breast milk (B) of one subject over a single dosing interval. The subject was treated with 1.5 mg of tacrolimus twice daily for immunosuppression. A)  , milk;
, milk;  , plasma;
, plasma;  , blood; B)
, blood; B)  , milk unbound drug;
, milk unbound drug;  , plasma unbound drug
, plasma unbound drug
Daily tacrolimus excretion into milk was estimated to be 32.0 ng, corresponding to 0.059% of the mother's weight-adjusted dose (54.4 μg kg–1 day−1). The tacrolimus exposure via breast milk for an average 3-month-old exclusively breast-fed infant was estimated to be 0.14 μg kg–1 day−1, i.e. 0.3% of the mother's weight-adjusted dose. The mean concentration of 13-DMT in milk was 0.03 ng ml−1 (trough to peak concentration ranged from 0.01 to 0.04 ng ml−1), much lower than that of parent drug. The other metabolites were all under their limits of quantitation in breast milk.
Discussion
Fetal exposure to tacrolimus has been evaluated in a limited number of studies [2, 16]. The current investigation characterizes whole blood, plasma and unbound tacrolimus concentrations in maternal and umbilical cord blood samples at delivery, providing new insights into fetal tacrolimus exposure. This study also presents an additional report of an infant being breast fed by a mother receiving tacrolimus-based immunosuppressive therapy.
Many factors are known to influence drug transfer across the placenta, including placental barrier permeability in relation to the physicochemical properties of the drug, facilitated or active drug transport, and drug biotransformation [9]. Tacrolimus is lipophilic with a molecular weight of 804 Da. Although its molecular weight exceeds the relative cut off (500 Da) for easy diffusion across the placenta, some degree of passive diffusion is expected [31]. For fetal development, the distribution, function and toxicity of tacrolimus in different organs, such as the interruption of T cell development at critical phases within the fetal thymus, potential functional changes in the fetal brain, renal insufficiency and neonatal hyperkalaemia need to be considered [2, 32]. Although prematurity occurred in 53% of 170 infants born to kidney transplant recipients taking tacrolimus, and 46% had low birth weight [33], the incidence of major malformations in the National Transplantation Pregnancy Registry was not much higher than that in the general population [32]. Jain et al. concluded that neonatal complications were minor and congenital anomalies were rare, the latter supported by the absence of congenital anomalies in a report from British Columbia [34, 35]. Instead, the greatest concerns for the infants exposed to tacrolimus in utero appear to stem from maternal complications such as hypertension, pre-eclampsia and premature rupture of membranes which might be related in part to placental or maternal endothelial effects and changes in blood flow ultimately requiring preterm delivery. Any evaluation of the contribution of tacrolimus to maternal complications in pregnancy is confounded by the other maternal conditions.
Tacrolimus is a P-gp substrate [7] and thus subject to the actions of this placental transporter, which limits fetal exposure to xenobiotics in the maternal circulation by active efflux at the microvillous, maternal-facing syncytiotrophoblast plasma membrane [36]. Indeed, the observed mean unbound drug concentration ratio between umbilical cord venous blood and maternal blood was 0.19 ± 0.10, suggesting efficient tacrolimus efflux. The placental transfer of ciclosporin, another icalcineurin inhibitor and P-gp substrate [7], has also been studied. The umbilical cord : maternal blood ciclosporin concentration ratio at delivery has been reported to be 0.2 at 6 h after the last dose [37], 0.56 at 8 and 10 h after the last dose [38], and approximately 1 at 20 h after the last dose [39]. Although an unbound concentration ratio was not reported, these data for ciclosporin, like ours for tacrolimus, are consistent with fetal-to-maternal P-gp mediated drug efflux. Because the placenta is at its thinnest and the expression of P-gp is substantially decreased towards term, fetal tacrolimus exposure at the time of our study may be maximal compared with exposure at earlier gestational ages [9, 40].
The differences observed in the venous umbilical cord : maternal concentration ratio based upon blood (0.71 ± 0.18), plasma (0.23 ± 0.11) and unbound drug (0.19 ± 0.10) measurements may be explained by greater partitioning of tacrolimus into cord blood cells and a higher haematocrit in cord blood, compared with the maternal blood. Kbc/p expresses a drug's binding to constituents in RBCs and other blood cell types vs. binding to plasma proteins (viz. albumin and α1-acid glycoprotein) [29]. Previous studies have shown that RBC uptake accounts for >80% of the cellular fraction of tacrolimus in blood [41]. Hence, the two-fold higher Kbc/p value in venous umbilical cord blood compared with that in maternal blood most likely reflects a greater uptake of tacrolimus into fetal RBCs, compared with maternal RBCs. The mechanism of this difference in RBC : plasma partitioning is unclear. The absolute lymphocyte count is on average 2.7 times greater in human umbilical cord blood than in adult peripheral blood [42]. However, because tacrolimus associated with the lymphocyte fraction is very low (0.61%) compared with the erythrocyte fraction (83.2%) [41], it cannot account for the two-fold higher Kbc/p. One limitation for the current analysis is that haematocrit was not available for samples at term, but values for the same subject 56 ± 53 days before delivery were used by assuming that haematocrit is relatively stable during the third trimester. This assumption is based on average haematocrit values that were reported to increase by only ∼2 percentage points over the third trimester of pregnancy [43].
The concentration of tacrolimus in whole blood does not necessarily reflect its concentration at the intracellular site of action for calcineurin inhibition within lymphocytes [44–46]. In utero immunosuppression from maternal tacrolimus administration may correlate better with the unbound tacrolimus concentration. In this and other studies, tacrolimus concentration in plasma has been shown to reflect unbound concentration better than the concentration in whole blood [47]. It has been shown that the dose of tacrolimus required for a 50% inhibition of IL-2 release by T cells was 10-fold higher in cultures with RBCs than without [48], suggesting that fetal immunosuppression may be limited by greater drug RBC partitioning and a higher haematocrit in cord blood.
Differential protein binding between the fetal and maternal circulations can be a critical determinant of placental drug transfer since it is the unbound drug that equilibrates across the placenta [9, 49]. Concentrations of both albumin and α1-acid glycoprotein, the plasma proteins that bind tacrolimus [10, 11], increase gradually throughout gestation in fetal plasma and vary across trimester in the maternal circulation [50]. However, we observed no significant difference in the unbound fraction of tacrolimus between maternal and umbilical cord plasma. Similarly, Hutson et al. suggested that protein binding may not be a significant factor in establishing the umbilical cord : maternal concentration ratio at steady-state when efflux transporters limit drug transfer [9].
Arterial and venous umbilical cord blood concentrations for tacrolimus were comparable, suggesting that fetal liver CYP3A7 does not seem to play a quantitatively important role in fetal tacrolimus disposition. Furthermore, we did not observe a difference in the primary metabolite concentration (13-DMT and 15-DMT) in the umbilical cord arterial and venous blood (supplementary Table S1). Studies with greater sample size are needed to examine further whether significant metabolism of tacrolimus, and local metabolite formation, occurs in the fetus.
The safety of tacrolimus for the nursing infant warrants special consideration [32]. The amount of tacrolimus excreted in breast milk in our case report was only 0.059% of the maternal weight-adjusted dose. For an exclusively breast-fed infant at 3 months of age, the estimated tacrolimus dose ingested via breast milk was estimated be to 0.3% of the maternal weight-adjusted dose. This is consistent with a previous case report of a breast-fed infant receiving approximately 0.5% of the maternal weight-adjusted dose [25]. Additionally, French et al. [5] described a 2.5-month-old infant exposed to tacrolimus through breast milk who was developing well physically and neurologically, Armenti et al. [51] reported five nursing infant cases with no reports of problems in the children and Jain et al. [2] reported colostrum concentrations comparable with our results and satisfactory post-natal growth and development. Recently, Bramham et al. [16] reported that breast-fed infants did not have higher tacrolimus concentrations compared with bottle-fed infants and concluded that ingestion of tacrolimus by infants via breast milk is negligible. Our data indicate that the infant exposure to tacrolimus via breast milk is extremely low and should not lead to adverse effects in the nursing infant, although long term follow up studies in these children require further investigation. In the first neonatal week, clinicians can expect to see some residual tacrolimus in newborn blood samples carrying over from in utero exposure, which is much higher than exposure via breast milk.
The observation that the total milk : plasma and unbound milk : plasma AUC ratios were 2.89 and 3.96, is consistent with a previously reported calculated milk : plasma ratio of 2.2 [26]. These ratios suggest active transport of tacrolimus in the mammary gland and/or movement into milk through fat from the mammary gland. Both P-glycoprotein and breast cancer resistance protein (BCRP) are located in the apical membrane of mammary epithelial cells, where they can actively extrude a variety of compounds [52]. Although the multidrug transporter BCRP is strongly induced in the mammary gland of humans during lactation and is responsible for the active secretion of substrates into milk [53], tacrolimus was not shown to be its substrate [54]. For P-gp, its mRNA [55] and protein expression are detected in human mammary gland epithelial cells, but the protein expression levels are thought to be too low to support quantifiable drug transporting activity [56]. Edwards et al. [57] demonstrated protein expression of P-gp in lactating rat mammary tissue, but concluded that it does not have a significant role in the secretion of nelfinavir into rat milk. Nonetheless, the unbound milk : plasma concentration ratio data are most consistent with some form of mammary efflux activity, mediated by P-gp or other unidentified transporters.
In conclusion, fetal tacrolimus exposure, expressed as the umbilical cord venous blood : maternal blood concentration ratio, averaged 71% of maternal exposure at term, while the mean umbilical cord venous plasma and unbound drug concentrations were approximately one fifth of the respective maternal concentrations. These observations may be explained in part by placental P-gp mediated efflux, by greater red blood cell partitioning and higher haematocrit levels in the fetus. There was no clear evidence of significant fetal tacrolimus metabolism. In addition, the neonatal drug exposure to tacrolimus in breast milk was very low, suggesting no health risk to the breastfeeding infant.
Funding Source
The project described was supported by grants U10HD047892, U10HD047891, and U10HD047890 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institute of Health/National Center for Research Resources grants M01RR023942, M01RR00037, UL1RR025014, and RR023256 and NIGMS grant GM68871. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Acknowledgments
The authors thank Claudine Hernandez, Susan McKay and Kristin Puhl from the University of Washington Department of Pharmacy for coordinating the studies. The authors also thank Dr. David K. Blough from the University of Washington Department of Pharmacy for the statistical assistance.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare support from National Institute of Child Health (NIH) for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1 Tacrolimus primary metabolite concentrations (ng ml−1) in maternal and umbilical cord blood in patients taking tacrolimus at the time of delivery
References
- 1.Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63:1247–1297. doi: 10.2165/00003495-200363120-00006. [DOI] [PubMed] [Google Scholar]
- 2.Jain A, Venkataramanan R, Fung JJ, Gartner JC, Lever J, Balan V, Warty V, Starzl TE. Pregnancy after liver transplantation under tacrolimus. Transplantation. 1997;64:559–565. doi: 10.1097/00007890-199708270-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kainz A, Harabacz I, Cowlrick IS, Gadgil S, Hagiwara D. Analysis of 100 pregnancy outcomes in women treated systemically with tacrolimus. Transpl Int. 2000;13(Suppl. 1):S299–300. doi: 10.1007/s001470050347. [DOI] [PubMed] [Google Scholar]
- 4.McKay DB, Josephson MA. Pregnancy in recipients of solid organs - effects on mother and child. N Engl J Med. 2006;354:1281–1293. doi: 10.1056/NEJMra050431. [DOI] [PubMed] [Google Scholar]
- 5.French AE, Soldin SJ, Soldin OP, Koren G. Milk transfer and neonatal safety of tacrolimus. Ann Pharmacother. 2003;37:815–818. doi: 10.1345/aph.1C312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrows R, Chusney G, Loucaidou M, James A, Goel S, Borrows S, Van Tromp J, Cairns T, Griffith M, Hakim N, McLean A, Palmer A, Papalois V, Taube D. Analysis of factors influencing tacrolimus levels and immunoassay bias in renal transplantation. J Clin Pharmacol. 2007;47:1035–1042. doi: 10.1177/0091270007303765. [DOI] [PubMed] [Google Scholar]
- 7.Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268:6077–6080. [PubMed] [Google Scholar]
- 8.Wang JS, Newport DJ, Stowe ZN, Donovan JL, Pennell PB, DeVane CL. The emerging importance of transporter proteins in the psychopharmacological treatment of the pregnant patient. Drug Metab Rev. 2007;39:723–746. doi: 10.1080/03602530701690390. [DOI] [PubMed] [Google Scholar]
- 9.Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90:67–76. doi: 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- 10.Piekoszewski W, Jusko WJ. Plasma protein binding of tacrolimus in humans. J Pharm Sci. 1993;82:340–341. doi: 10.1002/jps.2600820325. [DOI] [PubMed] [Google Scholar]
- 11.Weiss HM, Fresneau M, Moenius T, Stuetz A, Billich A. Binding of pimecrolimus and tacrolimus to skin and plasma proteins: implications for systemic exposure after topical application. Drug Metab Dispos. 2008;36:1812–1818. doi: 10.1124/dmd.108.021915. [DOI] [PubMed] [Google Scholar]
- 12.Zheng S, Easterling TR, Umans JG, Miodovnik M, Calamia JC, Thummel KE, Shen DD, Davis CL, Hebert MF. Pharmacokinetics of tacrolimus during pregnancy. Ther Drug Monit. 2012;34:660–670. doi: 10.1097/FTD.0b013e3182708edf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sam WJ, Tham LS, Holmes MJ, Aw M, Quak SH, Lee KH, Lim SG, Prabhakaran K, Chan SY, Ho PC. Population pharmacokinetics of tacrolimus in whole blood and plasma in Asian liver transplant patients. Clin Pharmacokinet. 2006;45:59–75. doi: 10.2165/00003088-200645010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Zheng S, Tasnif Y, Hebert MF, Davis CL, Shitara Y, Calamia JC, Lin YS, Shen DD, Thummel KE. Measurement and compartmental modeling of the effect of CYP3A5 gene variation on systemic and intrarenal tacrolimus disposition. Clin Pharmacol Ther. 2012;92:737–745. doi: 10.1038/clpt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebert MF, Zheng S, Hays K, Shen DD, Davis CL, Umans JG, Miodovnik M, Thummel KE, Easterling TR. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2012;95:908–915. doi: 10.1097/TP.0b013e318278d367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol. 2013;8:563–567. doi: 10.2215/CJN.06400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, Malaise J, Lison D, Squifflet JP, Wallemacq P. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–154. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kamdem LK, Streit F, Zanger UM, Brockmoller J, Oellerich M, Armstrong VW, Wojnowski L. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51:1374–1381. doi: 10.1373/clinchem.2005.050047. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, Thummel KE. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34:836–847. doi: 10.1124/dmd.105.008680. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Antona C, Jande M, Rane A, Ingelman-Sundberg M. Identification and phenotype characterization of two CYP3A haplotypes causing different enzymatic capacity in fetal livers. Clin Pharmacol Ther. 2005;77:259–270. doi: 10.1016/j.clpt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.PROGRAF® product labeling. Astellas Pharma US, Inc. 2009. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050708s027,050709s021lbl.pdf (last accessed 12 April 2013)
- 22.Floren LC, Bekersky I, Benet LZ, Mekki Q, Dressler D, Lee JW, Roberts JP, Hebert MF. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin Pharmacol Ther. 1997;62:41–49. doi: 10.1016/S0009-9236(97)90150-8. [DOI] [PubMed] [Google Scholar]
- 23.Hebert MF, Fisher RM, Marsh CL, Dressler D, Bekersky I. Effects of rifampin on tacrolimus pharmacokinetics in healthy volunteers. J Clin Pharmacol. 1999;39:91–96. doi: 10.1177/00912709922007499. [DOI] [PubMed] [Google Scholar]
- 24.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37:485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 25.Gardiner SJ, Begg EJ. Breastfeeding during tacrolimus therapy. Obstet Gynecol. 2006;107:453–455. doi: 10.1097/01.AOG.0000164052.66219.c7. [DOI] [PubMed] [Google Scholar]
- 26.Koshimichi H, Ito K, Hisaka A, Honma M, Suzuki H. Analysis and prediction of drug transfer into human milk taking into consideration secretion and reuptake clearances across the mammary epithelia. Drug Metab Dispos. 2011;39:2370–2380. doi: 10.1124/dmd.111.040972. [DOI] [PubMed] [Google Scholar]
- 27.Chen YL, Hirabayashi H, Akhtar S, Pelzer M, Kobayashi M. Simultaneous determination of three isomeric metabolites of tacrolimus (FK506) in human whole blood and plasma using high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:330–341. doi: 10.1016/j.jchromb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Nakai D, Kumamoto K, Sakikawa C, Kosaka T, Tokui T. Evaluation of the protein binding ratio of drugs by a micro-scale ultracentrifugation method. J Pharm Sci. 2004;93:847–854. doi: 10.1002/jps.20012. [DOI] [PubMed] [Google Scholar]
- 29.Hinderling PH. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49:279–295. [PubMed] [Google Scholar]
- 30.Jahazi A, Kordi M, Mirbehbahani NB, Mazloom SR. The effect of early and late umbilical cord clamping on neonatal hematocrit. J Perinatol. 2008;28:523–525. doi: 10.1038/jp.2008.55. [DOI] [PubMed] [Google Scholar]
- 31.Danesi R, Del Tacca M. Teratogenesis and immunosuppressive treatment. Transplant Proc. 2004;36:705–707. doi: 10.1016/j.transproceed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 32.McKay DB, Josephson MA. Pregnancy after kidney transplantation. Clin J Am Soc Nephrol. 2008;3:S117–125. doi: 10.2215/CJN.02980707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coscia LA, Constantinescu S, Moritz MJ, Frank AM, Ramirez CB, Maley WR, Doria C, McGrory CH, Armenti VT. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2010:65–85. [PubMed] [Google Scholar]
- 34.Humphreys RA, Wong HH, Milner R, Matsuda-Abedini M. Pregnancy outcomes among solid organ transplant recipients in British Columbia. J Obstet Gynaecol Can. 2012;34:416–424. doi: 10.1016/S1701-2163(16)35237-9. [DOI] [PubMed] [Google Scholar]
- 35.Jain AB, Reyes J, Marcos A, Mazariegos G, Eghtesad B, Fontes PA, Cacciarelli TV, Marsh JW, de Vera ME, Rafail A, Starzl TE, Fung JJ. Pregnancy after liver transplantation with tacrolimus immunosuppression: a single center's experience update at 13 years. Transplantation. 2003;76:827–832. doi: 10.1097/01.TP.0000084823.89528.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Audus KL. Controlling drug delivery across the placenta. Eur J Pharm Sci. 1999;8:161–165. doi: 10.1016/s0928-0987(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 37.Bourget P, Fernandez H, Bismuth H, Papiernik E. Transplacental passage of cyclosporine after liver transplantation. Transplantation. 1990;49:663. [PubMed] [Google Scholar]
- 38.Venkataramanan R, Koneru B, Wang CC, Burckart GJ, Caritis SN, Starzl TE. Cyclosporine and its metabolites in mother and baby. Transplantation. 1988;46:468–469. doi: 10.1097/00007890-198809000-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flechner SM, Katz AR, Rogers AJ, Van Buren C, Kahan BD. The presence of cyclosporine in body tissues and fluids during pregnancy. Am J Kidney Dis. 1985;5:60–63. doi: 10.1016/s0272-6386(85)80138-4. [DOI] [PubMed] [Google Scholar]
- 40.Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27:602–609. doi: 10.1016/j.placenta.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004;57:298–309. doi: 10.1046/j.1365-2125.2003.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83:197–203. [PubMed] [Google Scholar]
- 43.Milman N, Bergholt T, Byg KE, Eriksen L, Graudal N. Iron status and iron balance during pregnancy. A critical reappraisal of iron supplementation. Acta Obstet Gynecol Scand. 1999;78:749–757. [PubMed] [Google Scholar]
- 44.Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41:813–851. doi: 10.2165/00003088-200241110-00003. [DOI] [PubMed] [Google Scholar]
- 45.Kelly PA, Burckart GJ, Venkataramanan R. Tacrolimus: a new immunosuppressive agent. Am J Health Syst Pharm. 1995;52:1521–1535. doi: 10.1093/ajhp/52.14.1521. [DOI] [PubMed] [Google Scholar]
- 46.Ichimaru N, Takahara S, Kokado Y, Wang JD, Hatori M, Kameoka H, Inoue T, Okuyama A. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis. 2001;158:417–423. doi: 10.1016/s0021-9150(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 47.Minematsu T, Sugiyama E, Kusama M, Hori S, Yamada Y, Ohtani H, Sawada Y, Sato H, Takayama T, Sugawara Y, Makuuchi M, Iga T. Effect of hematocrit on pharmacokinetics of tacrolimus in adult living donor liver transplant recipients. Transplant Proc. 2004;36:1506–1511. doi: 10.1016/j.transproceed.2004.04.097. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed M, Venkataraman R, Logar AJ, Rao AS, Bartley GP, Robert K, Dodson FS, Shapiro R, Fung JJ, Zeevi A. Quantitation of immunosuppression by tacrolimus using flow cytometric analysis of interleukin-2 and interferon-gamma inhibition in CD8(-) and CD8(+) peripheral blood T cells. Ther Drug Monit. 2001;23:354–362. doi: 10.1097/00007691-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Hill MD, Abramson FP. The significance of plasma-protein binding on the fetal maternal distribution of drugs at steady-state. Clin Pharmacokinet. 1988;14:156–170. doi: 10.2165/00003088-198814030-00004. [DOI] [PubMed] [Google Scholar]
- 50.Krauer B, Dayer P, Anner R. Changes in serum albumin and alpha 1-acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs. Br J Obstet Gynaecol. 1984;91:875–881. doi: 10.1111/j.1471-0528.1984.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 51.Armenti VT, Radomski JS, Moritz MJ, Gaughan WJ, McGrory CH, Coscia LA. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2003:131–141. [PubMed] [Google Scholar]
- 52.Breedveld P, Beijnen JH, Schellens JHM. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 54.Gupta A, Dai Y, Vethanayagam RR, Hebert MF, Thummel KE, Unadkat JD, Ross DD, Mao Q. Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother Pharmacol. 2006;58:374–383. doi: 10.1007/s00280-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 55.Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303:487–496. doi: 10.1124/jpet.102.038315. [DOI] [PubMed] [Google Scholar]
- 56.Ito S, Alcorn J. Xenobiotic transporter expression and function in the human mammary gland. Adv Drug Deliv Rev. 2003;55:653–665. doi: 10.1016/s0169-409x(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 57.Edwards JE, Alcorn J, Savolainen J, Anderson BD, McNamara PJ. Role of P-glycoprotein in distribution of nelfinavir across the blood-mammary tissue barrier and blood-brain barrier. Antimicrob Agents Chemother. 2005;49:1626–1628. doi: 10.1128/AAC.49.4.1626-1628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


