Abstract
Objective
Botulinum toxin type A (Botox) injection has been used to manage pain. However, it remains to be proved whether Botox injection is effective to relieve residual limb pain (RLP) and phantom limb pain (PLP).
Design
Randomized, double-blinded pilot study.
Setting
Medical College and an outpatient clinic in Department of Physical Medicine and Rehabilitation.
Participants
Amputees (n=14) with intractable RLP and/or PLP who failed in the conventional treatments.
Interventions
Study amputees were randomized to receive 1 Botox injection versus the combination of Lidocaine and Depomedrol injection. Each patient was evaluated at baseline and every month after the injection for 6 months.
Main Outcome Measure
The changes of RLP and PLP as recorded by VAS, and the changes of the pressure pain tolerance as determined by a pressure algometer.
Results
All patients completed the protocol treatment without acute side effects, and monthly assessments of RLP, PLP, and pain tolerance after the treatment. The time trend in the outcomes was modeled as an immediate change owing to the treatment followed by a linear tread afterward. Repeated measures were incorporated using mixed effects modeling. We found that both Botox and Lidocaine/Depomedrol injections resulted in immediate improvements of RLP (Botox: P=0.002; Lidocaine/Depomedrol: P=0.06) and pain tolerance (Botox: P=0.01; Lidocaine/Depomedrol: P=0.07). The treatment effect lasted for 6 months in both groups. The patients who received Botox injection had higher starting pain than those who received Lidocaine/Depomedrol injection (P=0.07). However, there were no statistical differences in RLP and pain tolerance between these 2 groups. In addition, no improvement of PLP was observed after Botox or Lidocaine/Depomedrol injection.
Conclusions
Both Botox and Lidocaine/Depomedrol injections resulted in immediate improvement of RLP (not PLP) and pain tolerance, which lasted for 6 months in amputees who failed in conventional treatments.
Keywords: residual limb pain, phantom limb pain, amputees, botox, lidocaine, depomedrol
Phantom limb pain (PLP) and residual limb pain (RLP) are common in amputees.1 RLP could occur immediately after an amputation due to surgery, and in the late phase due to scar and neuroma formation. PLP is estimated to be between 60% and 65% with incidences especially high in individuals who experienced a traumatic loss of limb, or have a preexisting painful limb condition.2-6 PLP and RLP can be extremely debilitating. Over 50% of amputees report RLP interfering with their normal functions4 and negatively affecting their ability to wear prosthesis, delaying rehabilitation efforts, limiting participation in activities of daily living, and affecting psychosocial life.1,7
The management of RLP and PLP can be very challenging. Even with current treatment options, the success rate is low.3 Conventional pain managements for PLP and RLP include oral medications, physical modalities such as massage, biofeedback, transcutaneous electrical stimulation, and desensitization therapy, local injections, regional nerve blocks, and neuroablative procedures.1,6-9 On the basis of the focal nature of RLP, lidocaine injections are favored due to almost immediate pain relief. However, the effects are temporary. Lidocaine combined with corticosteroid had been used to try to achieve a longer effect, but its effectiveness varied from weeks to a year, and some of them have shown limited success.8,10-12
Botulinum toxin type A (Botox) (Allergan) is a type of neurotoxin that causes focal chemo-denervation.13 Botox has been shown to be effective in management of several pain conditions.14-21 Recently, several case studies22-24 reported pain relief in RLP and PLP after injection of Botox into muscular and cutaneous tissues. However, the true effectiveness of Botox injection on RLP and PLP remains to be confirmed through a double-blind randomized clinical study. In this study, using both subjective and objective measurements, we piloted a prospective doubleblinded randomized pilot study to examine the effectiveness of Botox injection, and to compare its effectiveness to Lidocaine combined with Depomedrol injection on RLP and PLP in amputees who failed in conventional treatments.
METHODS
This study was performed as a prospective, doubleblind, randomized pilot study. The study protocol was reviewed and approved by the Institutional Review Board at Medical College of Wisconsin and all investigators completed training in both human research and patient privacy. All participants were informed about the study procedure and consented to participate.
Patient Selection
The participants were recruited from the Department of Physical Medicine and Rehabilitation at Medical College of Wisconsin, Milwaukee, WI. The enrollment period began in December, 2005 and lasted until December, 2007. Inclusion criteria were adult lower extremity amputees (18 or more years old) with clinical diagnosis of daily RLP and/or PLP greater than 5/10 on visual analog score (VAS) that were interfering with their prosthetic use, activities of daily living, and/or sleep and mood. All study amputees are single amputees who failed in conventional treatments, including oral medications, local anesthetic injections, and physical modalities such as massage, biofeedback, transcutaneous electrical stimulation, and desensitization therapy. Exclusion criteria were a previous history of Botox injections, bleeding disorder, myasthenia gravis, Eaton-Lambert syndrome, amyotrophic lateral sclerosis, allergy or sensitivity to Botox, and ischemic distal residual limb; signs and symptoms of infection at the injection site or systemic infection; and evidence of pregnancy during the study. Sixteen participants were initially screened for this study. One participant was excluded due to the previous history of Botox injection, and another one was not eligible because of history of myasthenia gravis. Fourteen participants who qualified our inclusion criteria were included in this study.
Study Procedures
All eligible patients were randomly assigned to receive either Botox injection or Lidocaine/Depomedrol injection. One physician was chosen to implement the protocol treatment for all patients after the randomization. However, patients and evaluators (who were not the treating physician) were blinded to the type of treatment that was prescribed to the patient.
Focal tender points of RLP were first identified and marked on the residual limb during the physical examination. Then photographs of the residual limb were obtained. The pain tolerance (the maximum tolerable pressure) was measured using an algometer (Wegner instruments). The pressure pain tolerance was determined by using a handheld pressure algometer (Wegner instruments) with a circular probe area of 1 cm2. When pressure pain tolerance was reached, the evaluator froze the digital display immediately upon receiving the patient’s report. Each value was determined 3 times and then averaged. Each patient received intramuscular and cutaneous/subcutaneous injection of Botox or Lidocaine/Depomedrol as detailed below.
Each patient was evaluated at 0 (immediately before injection), 1, 2, 3, 4, 5, and 6 months after the injection. Each evaluation included history and physical examination by 1 treating physician who delivered the protocol treatment to all patients, but was blinded for data collection and analysis. Others included the completion of the clinic follow-up form, measurement of algometry pain tolerance and VAS pain scale, prosthetic form, postinjection patient log, review of the patient’s pain log form, and the pain level after the injections. These were performed and collected by our evaluator, a research associate or resident physician who was previously trained, and was blind to the type of treatment this patient received.
Medications
Clostridium Botulinum type A neurotoxin (Botox) from Allergan was used for this study. Botox was reconstituted with 0.9% normal saline to a concentration of 50 units/mL. An injection dosage of 1 mL, equal to 50 units of Botox was used for each injection site, with total units ranging from 250 to 300 units. Lidocaine/Depomedrol injection: 1% lidocaine (AstraZeneca LP) and 40 mg/mL of DepoMedrol (methylprednisolone acetate Injectable Suspension, Pharmacia & Upjohn Co) were used. A total amount of 1mL mixture of 0.75mL of 1% lidocaine and 0.25mL of Depomedrol 40 mg/mL (10 mg) was injected into each painful site on the residual limb. Maximal of 6 painful sites were injected for each patient in both groups.
In our study, Botox or Lidocaine/Depomedrol was injected into both muscular and nonmuscular structures such as cutaneous/subcutaneous tissues and neuroma in addition to muscles under the electromyography guidance since all of our patients had muscle tightness with focal muscle fasciculation, focal tenderness, and/or neuroma formation. The tender spots at residual limb were identified and marked. Neither were pain medications adjusted, nor were other interventions provided during the study period.
Outcome Measurements
The primary outcome measurements included changes of intensity of RLP and PLP as recorded by VAS, and the changes of the pressure pain tolerance as determined by a pressure algometer.
Statistical Analysis
Mixed effects modeling with patient-specific random effects for RLP and PLP, and additional within-patient location random effects for pain tolerance was used for statistical analysis. The time trend in the outcomes was modeled as an immediate change due to the treatment followed by a linear trend afterwards. The model was fitted to both groups simultaneously to obtain more precise variability estimates, however, the effect of the 2 treatments was allowed to differ. The scores of VAS and pain tolerance were averaged over the points within a person when reporting averages, but not during analysis. The analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Due to the small sample size, which limits the power, and preliminary nature of this study, we reported differences significant at a 10% level.
RESULTS
Clinical information of 14 study amputees enrolled into this study is summarized in Table 1. One patient in the Botox group dropped at 14 weeks owing to a vascular revision surgery in another limb. Another patient in the Botox group failed to follow-up in the 5-month visit after injection because of psychological exacerbation unrelated to his RLP and PLP. One patient in Lidocaine/Depomedrol did not complete the evaluation at the 6-month visit owing to noncompliance.
TABLE 1.
Clinical Data of 14 Study Amputees
| Characteristics | Botox Group (7 Patients) | Lidocaine/Depomedrol Group (7 Patients) |
|---|---|---|
| Male | 4 | 6 |
| Female | 3 | 1 |
| Average age (range) | 47 (20-75) | 50 (37-65) |
| RLP only | 3 | 3 |
| RLP and PLP | 3 | 5 |
| Transtibial amputation | 6 | 4 |
| Transfemoral amputation | 0 | 4 |
| Patients who completed 4 mo of study | 6 | 6 |
| Patients who completed 6 mo of study | 4 | 6 |
PLP indicates phantom limb pain; RLP, residual limb pain.
Table 2 shows the summary statistics of changes from baseline in the 2 groups. Data analysis showed that both Botox and Lidocaine/Depomedrol injections resulted in immediate improvements (at 1-month follow-up) of RLP (Botox: P=0.002; Lidocaine/Depomedrol: 0.06) and the pain tolerance (Botox: P=0.01; Lidocaine/Depomedrol: P=0.07). The treatment effect lasted for 6 months after the treatment in each group, respectively (Figs. 1, 2), with no evidence of a time trend (P=0.59 and P=0.96, respectively). Amputees randomized to receive Botox injection had higher starting pain (lower score in the pain tolerance) than those randomized to receive Lidocaine/Depomedrol injection (P=0.07) (Fig. 2). These analyses are summarized in Table 3. Visit-specific comparison analysis showed that the degree of RLP improvement was similar in the initial time points of visit (at 1 to 2 mo) between these 2 treatment groups, with higher effect size in the patients treated with Botox injection than Lidocaine/Depomedrol at the time points of visit (at 3 to 5 mo), and then became similar at the last time point of visit (at 6 mo) (Fig. 1). However, there was no statistical difference in pain tolerance between these 2 groups (Fig. 2). In addition, no immediate improvement of PLP was observed after Botox or Lidocaine/Depomedrol injection (Botox: P=0.49; Lidocaine/Depomedrol: P=0.42) (Fig. 3). Similarly there is no posttreatment trend effect in either group for VAS-PLP (Botox: P=0.90; Lidocaine/Depomedrol: 0.18).
TABLE 2.
Change From Baseline in Phantom Limb Pain VAS, Residual Limb Pain VAS, and Paint Tolerance by Treatment
| Month | Change in Phantom Limb Pain
|
Change in Residual Limb Pain
|
Change in Pain Tolerance
|
|||
|---|---|---|---|---|---|---|
| Botox | L/D | Botox | L/D | Botox | L/D | |
| 1 | −0.8 (0.5) | 1.3 (1.0) | −3.1 (0.6) | −1.6 (0.7) | 0.9 (0.5) | 0.4 (0.2) |
| 2 | −0.9 (0.7) | 1.5 (1.3) | −3.0 (1.3) | −1.9 (0.9) | 0.7 (0.3) | 0.4 (0.4) |
| 3 | −0.3 (0.3) | 2.0 (1.7) | −4.5 (1.3) | −1.1 (1.6) | 1.1 (0.7) | 0.6 (0.7) |
| 4 | −0.5 (0.5) | 0.6 (1.8) | −4.8 (1.2) | −1.9 (1.1) | 1.3 (0.8) | 0.2 (0.3) |
| 5 | −0.8 (0.6) | 1.2 (1.5) | −4.6 (1.6) | −1.1 (1.0) | 1.4 (1.1) | 0.1 (0.3) |
| 6 | −0.5 (0.3) | 0.9 (1.0) | −4.4 (1.5) | −2.8 (1.0) | 1.2 (1.1) | 0.5 (0.5) |
Values presented as mean (SE).
L/D indicates Lidocaine/Depomedrol injection; VAS, visual analog score.
FIGURE 1.
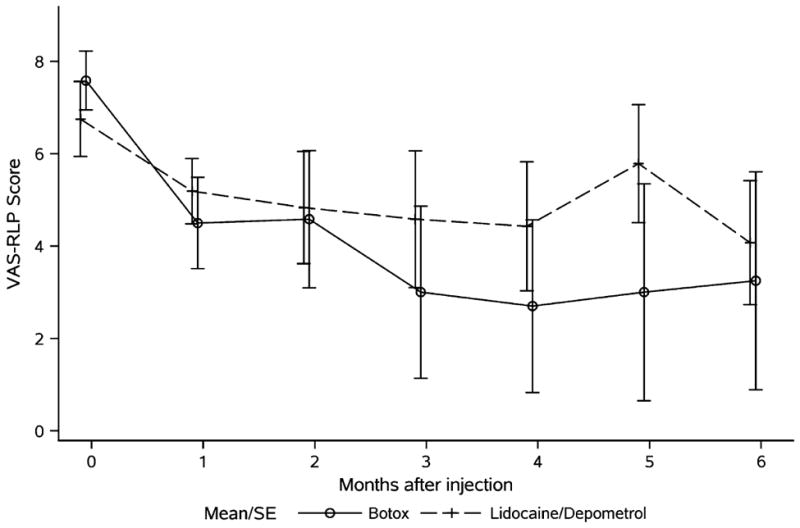
Average VAS-RLP (VAS-R) in amputees treated with Botox injection (solid line) and Lidocaine/Depomedrol injection (dotted line). RLP indicates residual limb pain; VAS, visual analog score.
FIGURE 2.
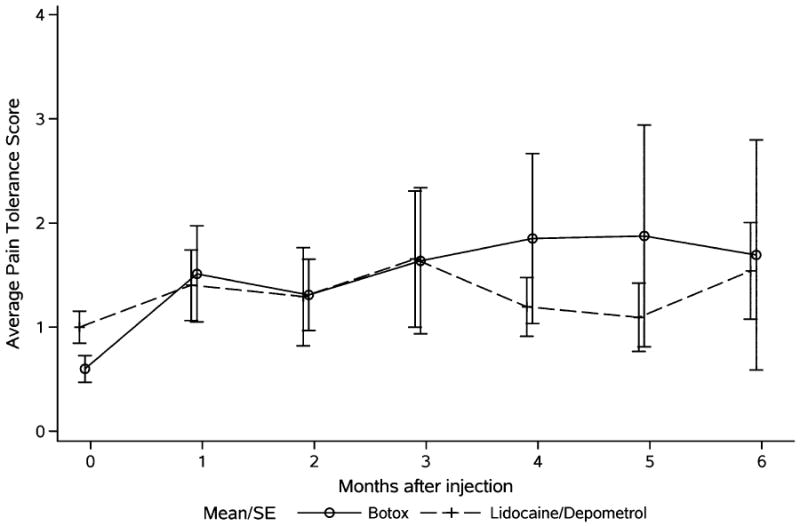
Average pain tolerance scores in amputees treated with Botox injection (solid line) and Lidocaine/Depomedrol injection (dotted line).
TABLE 3.
Model-based Estimates of Immediate Change (at 1-Month Follow-up) and Longer-Term Trends (Monthly Follow-up up to 6 Months) in VAS-RLP (VAS-R) (Figure 1), Pain Tolerance Scores (Figure 2), and VAS-PLP (VAS-P) (Figure 3) in Single Amputees Treated With Botox Injections and Lidocaine/Depomedrol Injection (L/D)
| VAS of Phantom Limb
|
VAS of Residual Limb
|
Pain Tolerance
|
||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| Baseline | ||||||
| Botox vs. L/D | −0.8 (−3.8, 2.1) | 0.58 | 0.8 (−1.7, 3.4) | 0.53 | −0.4 (−0.8, 0.0) | 0.07 |
| Immediate effect | ||||||
| Botox | −0.8 (−2.9, 1.4) | 0.49 | −3.0 (−4.9, −1.2) | 0.002 | 0.9 (0.2, 1.5) | 0.01 |
| L/D | 0.8 (−1.1, 2.7) | 0.42 | −1.6 (−3.1, 0.0) | 0.06 | 0.5 (0.0, 1.1) | 0.07 |
| Post-treatment trend | ||||||
| Botox | 0.0 (−0.5, 0.5) | 0.90 | −0.2 (−0.7, 0.4) | 0.59 | 0.0 (−0.2, 0.2) | 0.96 |
| L/D | 0.3 (−0.1, 0.7) | 0.18 | 0.0 (−0.5, 0.4) | 0.96 | −0.1 (−0.2, 0.1) | 0.51 |
PLP indicates phantom limb pain; RLP, residual limb pain; VAS, visual analog score.
FIGURE 3.
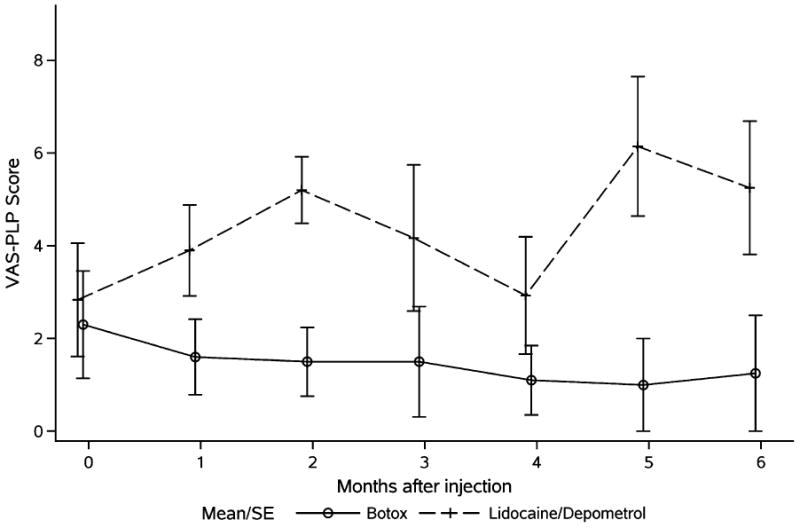
Average VAS-PLP (VAS-P) in amputees treated with Botox injection (solid line) and Lidocaine/Depomedrol injection (dotted line). PLP indicates phantom limb pain; VAS, visual analog score.
DISCUSSION
RLP and PLP are common painful conditions in amputees. The management of these types of pain is often difficult and challenging. Current treatments have failed to provide effective pain relief owing to either unresponsiveness or short lasting effect of pain reduction.2,5-8 Lidocaine is a well-known anesthetic used for all kinds of local injections. It is effective but short-lived. Steroid injection has been routinely used for treatment of Morton neuroma, 12 and was recently reported for the treatment of a painful stump neuroma.11 Corticosteroid, including Depomedrol combined with the local anesthetic agent such as lidocaine may extend the effect of pain relief through additional anti-inflammatory mechanisms in many painful conditions.25-27 This is because Depomedrol as an antiinflammatory agent might inhibit the inflammatory process by limiting the capillary dilatation and permeability of vascular structures present in the mechanism of RLP.25-27 The length of the treatment effect has been reported to vary from weeks to months.10,11 However, the benefits of corticosteroid injection in the management of RLP and PLP remain to be defined. On the basis of the above suggested mechanism and potential benefits of adding steroid, we have used the combination of Lidocaine and Depomedrol as a control arm to be compared with Botox injection in this study. The significant improvements of RLP and pain tolerance were observed in amputees treated with Lidocaine/Depomedrol injection, suggesting this combination regimen is a treatment option in amputees with RLP who failed in conservative (noninterventional) treatments. The improvement of RLP and pain tolerance lasted for approximately 6 months. This is clearly longer than Lidocaine injection alone as the pharmacologic effect of lidocaine usually last for no more than 1 hour. One possible explanation, although many exist, is that inflammatory or neuro-inflammatory mechanism may be involved in the development of RLP.
Botox has been used to treat the spasticity and other hypertonic muscular diseases for years by selectively preventing the release of acetylcholine at the nerve-muscle junction. Recently, Botox has been explored as a tool to alleviate pain, including RLP and PLP.21,23 Several studies22-24 reported that intramuscular and/or cutaneous injections of Botox produced significant relief in RLP and PLP. Our data are congruent with these studies and showed a significant reduction in RLP in the study amputees. The effect of RLP relief was significant (>50%) and seemed to be more effective in the first 3 months after Botox injection, which was still effective up to 6-month time point of visit. It is well known that the antispasticity effect of Botox usually lasts for about 3 months via inhibition on the synaptic transmission of acetylcholine at the motor end-plate13,28,29 before the compensatory nerve sprouting and regeneration process completes. But the effect of pain relief may last longer than 3 months as observed in our study and others.21 The underlying mechanism of Botox in promoting pain relief remains a subject of investigation. More than 3-month lasting effect observed by us and others21 may suggest mechanisms other than the cholinergic participated in the relief of RLP. Botox may affect both cholinergic fibers and noncholinergic nociceptive sensory fibers. Pain relief after Botox injection might be due to reduced myofascial pain related to muscle spasm/tightness evidenced by focal muscle fasciculation as Botox injected directly into the muscular structures under electromyography guidance. It may also minimize ectopic discharges from neuroma, and decreased release of nociceptors in peripheral pain pathways15,30-33 when Botox was administered at the neuroma site or near the nociceptive structures either intramuscularly or cutaneously and subcutaneously. Animal models suggest that Botox reduces the release of substance P, glutamate, c-Fos gene expression,30 and calcitonin generelated peptide peripherally,15,34 and at the dorsal root ganglion.15,30 We speculate that both anticholinergic effect and direct inhibition of sensory neurons may explain the effect of pain relief in our amputees.
The comparison analysis showed that the degree of RLP improvement was more in favor of Botox injection in the management of RLP than Lidocaine/Depomedrol injection; however, this could be related to small sample size. Clearly additional effort is needed to recruit more patients to confirm our observation. Results from this study suggest that Botox injection should be considered at least as an alternative option for amputees with RLP who failed in conventional treatments.
Of note is that we did not observe significant improvement in PLP (VAS-P) in either group treated with Botox or Lidocaine/Depomedrol) injection (Fig. 3), and, in fact, PLP appeared to increase in the Lidocaine/Depomedrol group, though not statistically significantly. This observation is in contrast to the previous case reports22-24 that the Botox is effective in PLP and other case report27 that Lidocaine/Depomedrol is effective in PLP. However, given the nature of this prospective double-blinded randomized evaluation in 14 amputees, we feel that the Botox or Lidocaine/Depomedrol might not be the preferred choice in the management of PLP, although low baseline PLP score, a small sample size, and variant of amputee patients can be blamed for this observation.
CONCLUSIONS
Both Botox and Lidocaine/Depomedrol injections resulted in immediate improvement of RLP (not PLP) and pain tolerance, which is stable for at least 6 months, in amputees who previously failed in the conventional treatment. Botox injection seems to improve RLP more than Lidocaine/Depomedrol injection in 3 to 6 months after the treatment. Neither Botox injection nor Lidocaine/Depomedrol is considered to be the preferred choice for PLP. Certainly a larger study is required to confirm our observation in future.
Acknowledgments
The statistical analysis of this study is supported by CTSA grant 1UL1RR031973 (part of the NIH/NCRR). Allergan Inc provided an unrestricted educational grant to the Department of Physical Medicine and Rehabilitation at the Medical College of Wisconsin. Allergan Inc did not impose any impediment, directly or indirectly, on the publication of the study results.
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated.
The authors declare no conflict of interest.
References
- 1.Sherman RA. Phantom Pain. New York: Plenum Press; 1997. [Google Scholar]
- 2.Nikolajsen L, Jensen TS. Phantom limb pain. Br J Anesth. 2001;87:107–116. doi: 10.1093/bja/87.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Flor H, Birbaumer N, Sherman R. Phantom limb pain. Pain: Clinical Update. International Association for the Study of Pain. 2000;VIII(3):1–4. [Google Scholar]
- 4.Rhamachandran VS, Hirstein W. The perception of phantom limbs. The D.O. Hebb Lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 5.Katz J. Prevention of phantom limb pain by regional anesthesia. Lancet. 1997;349:519–520. doi: 10.1016/s0140-6736(97)80081-5. [DOI] [PubMed] [Google Scholar]
- 6.Sala C, Andreose JS, Fumagalli G, et al. Calcitonin gene-related peptides: possible role in formation and maintenance of neuromuscular junctions. J Neurosci. 1995;15:520–528. doi: 10.1523/JNEUROSCI.15-01-00520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman RA. The use of electromyographic and temperature biofeedback for treatment of cramping and burning phantom limb pain. Applications in Chronic Pain, Physical Medicine and Rehabilitation 1997 [Google Scholar]
- 8.Nicholson B. Evaluation and treatment of central pain syndromes. Neurology. 2004;62(suppl 2):S30–S36. doi: 10.1212/wnl.62.5_suppl_2.s30. [DOI] [PubMed] [Google Scholar]
- 9.West M, Wu H. Pulsed radiofrequency ablation for the management of residual limb pain and phantom limb pain. Pain Practice. 2010 doi: 10.1111/j.1533-2500.2009.00353.x. online early view, since March. [DOI] [PubMed] [Google Scholar]
- 10.Shankar H. Ultrasound demonstration of vascularity changes with changes in pin perception in a stump neuroma. Clin J Pain. 2009;25:253–255. doi: 10.1097/AJP.0b013e31818b2b12. [DOI] [PubMed] [Google Scholar]
- 11.Ernberg LA, Adler RS, Lane J. Ultrasound in the detection and treatment of a painful stump neuroma. Skeletal Radiol. 2003;32:306–309. doi: 10.1007/s00256-002-0606-9. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen MR, Kitaoka HB, Patzer GL. Nonoperative treatment of plantar interdigital neuroma with a single corticosteroid injection. Clin Orthop. 1996;326:188–193. doi: 10.1097/00003086-199605000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Lew MF. Review of the FDA- Approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clin J Pain. 2002;18:S142–S146. doi: 10.1097/00002508-200211001-00005. [DOI] [PubMed] [Google Scholar]
- 14.Smith HS, Audette J, Royal MA. Botulinum toxin in pain management of soft tissue syndromes. Clin J Pain. 2002;18:S147–S154. doi: 10.1097/00002508-200211001-00006. [DOI] [PubMed] [Google Scholar]
- 15.Cui M, Khanijou S, Rubino J, et al. Subcutaneous administration of botulinum toxin A reduces formalin- induced pain. Pain. 2004;107:125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Juan FJ. Use of botulinum toxin A for musculoskeletal pain in patients with whiplash associated disorders. BMC Musculoskelet Disord. 2004;5:5. doi: 10.1186/1471-2474-5-5. doi:10.1186:1471-2474-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic J. Treatment of cervical dystonia with botulinum toxin. Movement Disord. 2002;19:S109–S115. doi: 10.1002/mds.20024. [DOI] [PubMed] [Google Scholar]
- 18.Lang A. Botulinum toxin type A therapy in chronic pain disorders. Arch Phys Med Rehabil. 2003;84(suppl 1):S69–S73. doi: 10.1053/apmr.2003.50121. [DOI] [PubMed] [Google Scholar]
- 19.Fishman L, Anderson C, Rosner B. Botox and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabilit. 2002;81:936–942. doi: 10.1097/00002060-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Porta M. Botulinum toxin type A injections for myofascial pain syndrome and tension type headache. Eur J Neurol. 1999;6:S103–S109. [Google Scholar]
- 21.Ney JP, Difazio M, Sichani A, et al. Treatment of chronic low back pain with successive injections of Botulinum Toxin A over 6 months: a prospective trial of 60 patients. Clin J Pan. 2006;22:363–369. doi: 10.1097/01.ajp.0000174267.06993.3f. [DOI] [PubMed] [Google Scholar]
- 22.Kern U, Martin C, Scheicher S, et al. Effects of botulinum toxin type B on Stump Pain and Involuntary Movements of the Stump. Am J Phys Med Rehabilit. 2004;83:396–399. doi: 10.1097/01.phm.0000124444.32257.04. [DOI] [PubMed] [Google Scholar]
- 23.Kern U, Martin C, Scheicher S, et al. Botulinum toxin type A influences stump pain after limb amputations. J Pain Sympt Manage. 2003;26:1069–1070. doi: 10.1016/j.jpainsymman.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Jin LJ, Kollewe K, Krampfl K, et al. Treatment of phantom limb pain with botulinum toxin type A. Pain Med. 2009;10:300–303. doi: 10.1111/j.1526-4637.2008.00554.x. [DOI] [PubMed] [Google Scholar]
- 25.Bogduk N. A narrative review of intra-articular corticosteroid injections for low back pain. Pain Med. 2005;6:287–296. doi: 10.1111/j.1526-4637.2005.00048.x. [DOI] [PubMed] [Google Scholar]
- 26.Valat JP, Rozenberg S. Local corticosteroid injections for low back pain and sciatica. Joint Bone Spine. 2008;75:403–407. doi: 10.1016/j.jbspin.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Bloomquist T. Amputation and phantom limb pain: A pain-prevention model. AANA. 2001;69:211–217. [PubMed] [Google Scholar]
- 28.Oliver D. Synaptic transmission: Inhibition of neurotransmitter release by botulinum toxins. Headache. 2003;43:S16–S24. doi: 10.1046/j.1526-4610.43.7s.4.x. [DOI] [PubMed] [Google Scholar]
- 29.Brin M, Aioki KR. Botulinum toxin type A: Pharmacology. In: Mayer NH, Simpson DM, editors. Spasticity: Etiology, Evaluation, Management and the Role of Botulinum Toxin. New York: WE MOVE; 2002. pp. 110–124. [Google Scholar]
- 30.Aoki RK. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43:S9–S15. doi: 10.1046/j.1526-4610.43.7s.3.x. [DOI] [PubMed] [Google Scholar]
- 31.Welsh MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to clostridium botulinum neurotoxins. Toxicology. 2000;38:245–258. doi: 10.1016/s0041-0101(99)00153-1. [DOI] [PubMed] [Google Scholar]
- 32.Blersch W, Schulte-Mattler W, Przywara S, et al. Botulinum toxin A and the cutaneous nociception in humans: a prospective, double-blind, placebo-controlled, randomized study. J Neurol Sci. 2002;205:59–63. doi: 10.1016/s0022-510x(02)00313-1. [DOI] [PubMed] [Google Scholar]
- 33.Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin J Pain. 2001;17:2–10. doi: 10.1097/00002508-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Enneking FK, Scarborough MT, Radison EA. Local aesthetic infusion through nerve sheath catheters for analgesia following upper extremity amputation. Reg Anesth. 1997;22:351–356. doi: 10.1016/s1098-7339(97)80011-9. [DOI] [PubMed] [Google Scholar]


