Abstract
Hypertension during preeclampsia is associated with increased maternal vascular sensitivity to angiotensin II (ANGII). This study was designed to determine mechanisms whereby agonistic autoantibodies to the ANGII type I receptor (AT1-AA) enhance blood pressure (MAP) and renal vascular sensitivity to ANGII during pregnancy. First, we examined MAP and renal artery resistance index (RARI) in response to chronic administration of ANGII or AT1-AA or AT1-AA+ANGII in pregnant rats compared to control pregnant rats. In order to examine mechanisms of heightened sensitivity in response to AT1-AA during pregnancy we examined the role of endogenous ANGII in AT1-AA infused pregnant rats, Endothelin-1 and oxidative stress in AT1-AA+ANGII treated rats. Chronic ANGII increased MAP from 95 +/−2 in NP rats to 115 +/−2 mmHg. Chronic AT1-AA increased MAP to 118+/−1 mmHg in NP rats which further increased to 123+/−2 with AT1-AA+ANGII. Increasing ANGII from (10−11-10−8) decreased Af-Art diameter 15-20% but sharply decreased Af-Art diameter 60% in AT1-AA pretreated vessels. RARI increased from 0.67 in NP rats to 0.70 with AT1-AA infusion, which was exacerbated to 0.74 in AT1-AA + ANGII infused rats. AT1-AA-induced hypertension decreased with Enalapril but was not attenuated. Both tissue ET-1 and ROS increased with AT1-AA+ANGII compared to AT1-AA alone and blockade of either of these pathways had significant effects on MAP or RARI. These data support the hypothesis that AT1-AA, via activation of ET-1 and oxidative stress and interaction with endogenous ANGII, are important mechanisms whereby MAP and renal vascular responses are enhanced during preeclampsia.
Keywords: Angiotensin II, AT1-AA, Preeclampsia
Introduction
Preeclampsia is a devastating disease, occurring in up to 8% of US pregnancies, and is a major cause of maternal and perinatal morbidity and mortality. While much research has been directed at discovering the underlying pathophysiology of this disease, we are only now beginning to understand some of the possible mechanisms involved. One widely studied theory is that placental ischemia leads to an inflammatory response and the release of multiple vasoactive mediators which lead to the pathology seen in women with preeclampsia1-3. Many hypothesize that the agonistic autoantibody which is targeted to the angiotensin II (ANGII) type 1 receptor (AT1-AA), produced by preeclamptic women, plays an important role in mediating hypertension during pregnancy 4-13. Further, we hypothesize that the AT1-AA is important in causing enhanced blood pressure sensitivity to ANGII. We have previously shown by infusing purified rat AT1-AA into pregnant rats we can mimic many of the features seen in preeclamptic women such as increased anti-angiogenic factors and activated ET-1 and oxidative stress pathways 9-14. It has been known for many years that the response to ANGII is diminished during normal pregnancy. In contrast, women who develop preeclampsia will show an increased sensitivity to ANGII 15-17. In fact, increased sensitivity to ANGII is present postpartum in women with a history of hypertensive pregnancy16. The exact mechanism behind this observation is unknown. We believe, however, that AT1-AA plays an integral role. Studies have demonstrated that previously preeclamptic women continue to produce AT1-AA up to at least 6 months postpostum17. In addition, we recently demonstrated that although the AT1-AA and ANGII stimulate endothelial cells to secrete the vasoconstrictor ET-1, exposure of endothelial cells to AT1-AA+ANGII in combination, drastically enhances cellular ET-1 secretion 10. In the same study, we demonstrated that acute infusion of ANGII into AT1-AA-induced hypertensive pregnant rats, dramatically increased blood pressure approximately 40mmHg above that of AT1-AA alone. Further, we demonstrated that AT1-AA with high dose ANGII (435 ng/kg/min) chronically increased blood pressure above that achieved with ANGII or AT1-AA alone in pregnant rats. Though we previously demonstrated a synergistic effect of AT1-AA and ANGII on the AT1 receptor to further increase blood pressure during pregnancy, mechanisms of this heightened increased were not defined. The aim of this current study was to further examine the hypothesis that chronic AT1-AA enhances renal and blood pressure sensitivity to ANGII during pregnancy via stimulation of renal vasoconstriction, ET-1 or ROS. In order to examine this hypothesis, we examined blood pressure, renal vasoconstriction, and local ET-1 message and oxidative stress in response to chronic AT1-AA+ANGII during pregnancy. An alternative approach taken to examine the synergistic effect between AT1-AA and ANGII was to measure pregnancy blood pressure and renal responses in the presence of chronic blockade of the endogenous renin-angiotensin system during chronic AT1-AA-induced hypertension.
Methods
The animals used in this study were pregnant Sprague-Dawley rats obtained from Harlan Sprague Dawley (Indianapolis, IN). The animals were housed in a temperature-controlled room (23°C) with a 12:12h light/dark cycle. All procedures performed during this study were in accordance with the National Institute of Health guidelines for use and care of animals and all protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center (Jackson, MS).
Protocol to determine effect of AT1-AA and ANGII on hypertension during pregnancy
This protocol was performed in order to determine if the combination of chronic AT1-AA on ANGII heightened blood pressure response during pregnancy compared to the effect of AT1-AA or ANGII alone. AT1-AA was infused via mini-osmotic pump as previously published 10,12-14. Mean arterial pressure MAP and Renal artery resistance index (RARI) were measured in six groups of rats: NP (n=18), AT1-AA infused (1:50, n=8), ANGII infused (50ng/kg/min, n=8), and AT1-AA+ANGII infused (1:50; n=10; 1:100; n=6; 1:200, n=6). To determine the effect of lower concentrations of AT1-AA alone on blood pressure during pregnancy, MAP was also measured in lower doses of AT1-AA infused (1:100 n=4, 1:200 n=4) pregnant rats without ANGII. On day 12 of gestation miniosmotic pumps (model 2002, Alzet Scientific Corporation, Palo Alto, CA) infusing either column purified rat AT1-AA, ANGII or the combination of AT1-AA+ ANGII were inserted intraperitoneally into NP rats. On day 18 of gestation, all rats were surgically instrumented with a carotid catheter for subsequent arterial pressure measurement. At day 19 of gestation arterial pressure was measured. Subsequently, a blood sample was collected, kidneys and placentas were harvested and litter size and pup weights were recorded while dams were under anesthesia using isoflurane (Webster, Sterling MA) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products, Madison, WI).
Protocol to determine the effect of AT1-AA and ANGII on renal sensitivity during pregnancy
This protocol examined the synergistic effect of AT1-AA+ANGII on kidneys from normal pregnant rats. The methods are the same as we previously described to isolate and perfuse the afferent arteriole (Af-Art) with intact glomerulus 21,22.
Next we measured the chronic effects of AT1-AA with ANGII on renal artery resistance index (RARI) during pregnancy. This protocol utilized multiple groups of animals: normal pregnant (NP, n=3), animals chronically exposed to AT1-AA (1:50) from day 12-19 (n=3) and animals chronically exposed to both AT1-AA (1:50) + ANGII (50ng/kg/min) (n=3), animals chronically exposed to both AT1-AA (1:100) + ANGII (50ng/kg/min) (n=3), animals chronically exposed to AT1-AA (1:200) + ANGII (50ng/kg/min) (n=3) from day 12-19 . On day 19, the resistive index of the renal arteries were determined using Power Doppler measurements taken with a Vevo 770 unit (Visual Sonics, Toronta, Ontario) with 30 Hz transducer and insonating angle <30°. The resistance index was calculated by determining both the peak systolic velocity (PSV) and enddiastolic velocity (EDV). The following formula was then used: RI = (PSV-EDV)/PSV. Measurements were taken at two levels of the renal arteries on each side of the animal with 3 measurements taken at each level for a total of 12 measurements per animal.
Determination of tissue ET-1 and ROS Levels
AT1-AA is thought to lead to hypertension and enhanced renal vascular sensitivity through multiple pathways including increased levels of ET-1 and oxidative stress. This protocol sought to determine the effects of AT1-AA and ANGII on local preproendothelin. We had previously shown that AT1-AA significantly stimulates PPET-1 mRNA in placenta, aorta and renal cortices of AT1-AA-induced hypertensive pregnant rats, therefore we examined these tissues to determine if AT1-AA further stimulated ET-1 above that of ANGII or AT1-AA alone during pregnancy. Levels of preproendothelin were determined in placentas, renal cortices and aortas as previously described12. Results were calculated using the δ/δCT method and expressed as fold increase.
We have previously shown that placental oxidative stress is significantly elevated in AT1-AA-induced hypertensive pregnant rats, therefore, this portion of the study sought to determine if AT1-AA further stimulated placental ROS above that seen with ANGII or AT1-AA alone during pregnancy. Superoxide production in the placenta was measured by using the lucigenin technique.11,14 The protein concentration was measured using a Pierce (Rockford, IL) protein assay with BSA standards. The data are expressed as RLU per min per milligram protein.
Protocol to determine the effect of blocking endogenous ANGII on hypertension and renal vascular sensitivity in response to AT1-AA
This protocol sought to determine the effects of blocking endogenous ANGII in animals exposed to AT1-AA. In this protocol an angiotensin converting enzyme inhibitor, enalapril, was administered via the drinking water of pregnant rats. MAP and RARI were measured in four groups of pregnant rats: normal pregnant (NP) (N=21), NP+AT1-AA (N=9), NP+Enalapril (N=7) and NP+AT1-AA+Enalapril (N=8). Beginning day 12, AT1-AA (1:50) was infused via miniosmotic pumps and Enalapril was administered via drinking water to NP rats (Enalapril, 250 mg/L adlibitum in drinking water). RARI was determined and carotid catheters inserted on day 18. MAP and was measured on day 19 as described above.
Protocol to determine the effect of blocking ETA on hypertension and RARI in rats exposed to AT1-AA+ANGII
AT1-AA causes blood pressure increases during pregnancy via ET-1 and activation of ETA receptor. This protocol was designed to evaluate the effect that blocking ETA would have on the changes that are seen in pregnant rats exposed to AT1-AA+ANGII. NP (n=20) animals were infused with AT1-AA (1:50) and ANGII (50ng/kg/min) via miniosmotic pumps which were placed on day 12. Also beginning on day 12, ETA (5mg/kg/day) was added to the animal’s drinking water. The RARI were then determined and carotid artery catheters inserted on day 18 and MAP was determined on day 19.
Protocol to determine the effect of administration of a superoxide dismutase mimetic on hypertension and RARI in animals exposed to AT1-AA+ANGII
Administration of the SOD mimetic Tempol blocked AT1-AA-induced hypertension in a previous study performed by our laboratory. This protocol sought to determine the effects of blunting the ROS by administering Tempol (superoxide dismutase mimetic) on AT1-AA+ANGII infused rats. NP animals (n=8) were infused with AT1-AA (1:50) and AngII (50ng/kg/min) via miniosmotic pumps placed on day 12 as previously described and Tempol (30mg/kg/day) was added to their drinking water. RARI were determined and carotid catheters were placed on day 18. The MAP was measured on day 19.
Statistics
The mean for each series was determined. A commercial software package (STATA, Statacorp) was then utilized to determine whether there was a significant difference between the groups by performing an ANOVA. Statistical significance was considered a P<0.05. Statistical analysis was performed with STATA (RI) and ANOVA (MAP, PPET-1 and ROS).
Results
The effect of AT1-AA on ANGII-induced renal sensitivity and hypertension during pregnancy
We have consistently shown that chronic infusion of rat AT1-AA (1:50) significantly increases blood pressure in normal pregnant rats 9-13. In addition, we demonstrate chronic infusion of ANGII leads to hypertension in normal pregnant animals. ANGII increased MAP 20mmHg over baseline, while AT1-AA (1:50) increased blood pressure 23 mmHg above NP rats. Importantly, this hypertensive response was mildly enhanced when AT1-AA (1:50)+ANGII were infused simultaneously reaching almost 10mmHg above that of ANGII control rats (Figure 1). This response is less than that previously shown with 435ng/kg/min ANGII infusions+AT1-Ab (generated antibody mimicking the actions of AT1-AA). This response was closer to 20mmHg greater than the ANGII dose used in that study, which could be attributed to the higher ANGII and longer time frame of the study. Never the less the increase was 8 mmHg above that with ANGII alone and although higher than either AT1-AA or ANGII alone, was not significantly greater when compared statistically. (Figure 1).
Figure 1. AT1-AA interacts with ANGII to increase blood pressure and enhance renal vasoconstriction during pregnancy.
Chronic infusion of ANGII or AT1-AA alone increases blood pressure significantly above that of NP rats. Combination of AT1-AA+ANGII chronic infusion causes an even greater blood pressure response in pregnant rats. Chronic infusion of AT1-AA increases renal artery resistive index significantly above that of NP rats. Combination of AT1-AA+ANGII chronic infusion causes an even greater resistive index than AT1-AA or ANGII alone. Significance equal P<0.05, compared to NP.
The mean RARI for normal pregnant animals was 0.671+/−0.012 and for those treated with ANGII, for animals chronically exposed to AT1-AA was 0.703+/− 0.012 and for animals which received both AT1-AA and ANGII, RARI was 0.740+/− 0.013. ANOVA was performed to determine any difference among these means. This revealed that there was a statistically significant difference between the RARI in NP animals compared to animals exposed to both AT1-AA+ANGII (P=0.002). The difference between animals exposed to only AT1-AA and those exposed to both AT1-AA +ANGII was not significantly higher (P=0.33), but does suggest that the two synergistically enhance renal resistance. As with blood pressure, RARI with lower AT1-AA doses (1:100 and 1:200) combined with ANGII was not different than RARI with ANGII infusion (Figure 1).
In additional efforts to examine the role of AT1-AA to enhance renal ANGII sensitivity during pregnancy, we measured vasoconstriction from isolated Af-Art from normal pregnant rats in dose response to ANGII alone and in combination with purified rat AT1-AA. We first established a dose response of increased constriction of the Af-Art with increasing ANGII alone (10−11-10−8) or increasing AT1-AA alone (1:200, 1:150, and 1:100). We chose nonconstrictor doses of ANGII and AT1-AA to determine direct changes in afferent arterioles that would impact renal sensitivity in the presence of ANGII. The diameter of the Af-Art was reduced to 8.7 ±0.8 μm with AT1-AA+ANGII vs. 12.1 ±0.4 μm with Ang II alone (p <0.01) vs 13.7±1.2 μm with AT1-AA alone Figure 2. This equated to a 56% decrease in Af-Art diameter over baseline. In the presence of 7-aa peptide, an AT1-AA specific inhibitor, the constriction of the arteriole induced by ANGII and AT1-AA was blunted; the diameter of the Af-Art was 10.7 ±0.8 μm (p <0.05 vs Ang II plus AT1-AA; n = 8). Although lower doses of AT1-AA were not sufficient to increase blood pressure, data from these direct studies indicate the importance of low concentrations of AT1-AA to heighten renal vascular sensitivity at the level of the arterioles to ANGII during pregnancy.
Figure 2. AT1-AA blocking peptide blunts AT1-AA-enhanced ANGII- induced afferent arteriolar constriction.
Administration of non constrictor doses of ANGII or AT1-AA has no effect on aft art isolated from pregnant rats. However combination of AT1-AA+ANGII has profound constrictor effects that are significantly blunted by an AT1-AA seven amino acid blocking peptide. Signiciance equals P<0.05 compared to baseline.
Effect of AT1-AA and ANGII on levels of preproendothelin and ROS
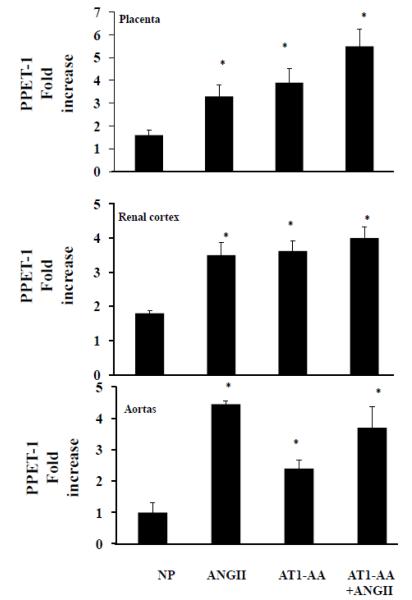
Using real-time PCR, we measured local preproendothelin (PPET-1). Levels change significantly in renal, placental and aorta tissues between NP when compared to ANGII, and both AT1-AA treated groups (P<0.05) (Figure 3). We note significant increases of preproendothelin in the renal cortices and placenta of AT1-AA+ANGII groups compared to ANGII alone or NP groups. Interestingly in the PPET-1 aorta of ANGII-infused rats remained unchanged compared to aorta of rats exposed to AT1-AA+ANGII, however, these levels were significantly greater than PPET-1 in the aorta of NP rats.
Figure 3. Tissue levels of PPET-1 are increased with AT1-AA+ANGII.
AT1-AA+ANGII increased ET-1 transcript in the renal cortices and aorta of pregnant rats Significance equal P<0.05, compared to NP.
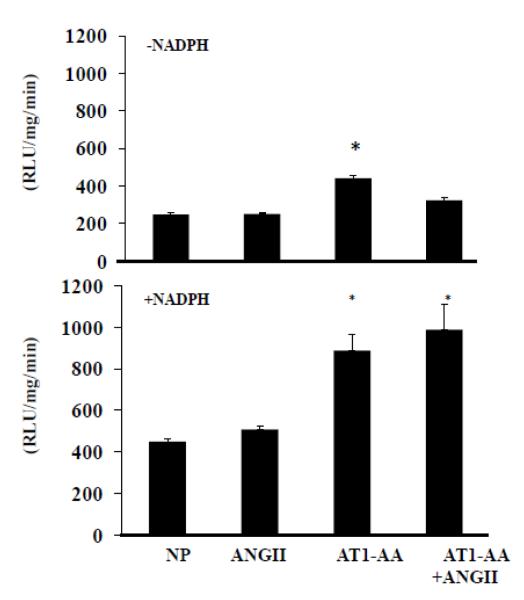
AT1-AA alone significantly stimulates basal placental oxidative stress to a much greater degree than ANGII alone or when compared to NP rats. Importantly, NADPH stimulated placental ROS was further enhanced from AT1-AA+ ANGII-infused rats compared to AT1-AA or ANGII alone (Figure 4). Collectively, these data indicate that there is increased ET-1 and oxidative stress seen in the tissues of animals exposed to both AT1-AA and ANGII.
Figure 4. Placental oxidative stress is further exacerbated with AT1-AA+ANGII.
AT1-AA stimulated placental oxidative stress much greater than ANGII compared to normal pregnant rats. This response was even greater in rats treated with both AT1-AA+ANGII Significance equal P<0.05, compared to NP.
Effect of blocking ETA receptors on hypertension and RARI in rats chronically treated with AT1-AA and ANGII
The hypertensive response with chronic AT1-AA+ANGII was significantly blunted ETA receptor blockade , MAP was 105mmHg (p<0.001) (Figure 5). This was still greater than that of NP rats, indicating that endothelin is not the sole mediator of increased blood pressure in response to chronic AT1-AA+ANGII during pregnancy. Furthermore, AT1-AA+ANGII pregnant rats showed a drastically elevated RARI of 0.739 which was unchanged with ETA receptor blockade (0.753). This was not significantly different from rats exposed to the combination of AT1-AA and ANGII who were not treated with ETA (p>0.05) (Figure 5). These data support the pressure data with ETA blockade, both of which indicate the complex response of pregnancy to elevated AT1-AA with ANGII that may occur with ET-1 activation but not dependent upon Endothelin.
Figure 5. ET-1 and ROS play an important to enhance renal and blood pressure sensitivity to ANGII during pregnancy.
MAP blunted in the presence of ETA. (*) indicates significant differnce compared to AT1-AA+ANGII (p<0.05). Surprisingly, the RARI was unchanged in animals exposed to both AngII and AT1-AA when given ETA in their drinking water. This did not reach statistical significance.The addition of Tempol to the drinking water of rats exposed to AngII and AT1-AA was noted to blunt the hypertesive response though this difference did not reach statistical significance. Tempol did however, normalize the RARI seen with AT1-AA+ANGII. (*) indicates significant difference compared to AT1-AA+ANGII (p<0.05).
The effect of administration of a superoxide dismutase mimetic on hypertension and RARI in animals exposed to AT1-AA+ANGII
When pregnant rats were treated with AT1-AA+ANGII+Tempol, hypertension was only slightly decreased (118 mmHg) compared to AT1-AA+ANGII (123mmHg) which was not statistically significant (p>0.05) (Figure 5). AT1-AA+ANGII pregnant rats showed a drastically elevated RARI of 0.739 which was normalized with Tempol, RARI was 0.649 (p<0.001) (Figure 5). These data support the hypothesis that oxidative stress is an important player in renal sensitivity and hypertension during pregnancy when exposed to chronic AT1-AA+ANGII.
A role for endogenous ANGII in AT1-AA-induced hypertension
Although AT1-AA increases blood pressure, this next protocol was undertaken to examine if endogenous ANGII interacts with the AT1-AA to elevate blood pressure and renal vascular sensitivity during pregnancy. Infusion of AT1-AA increased blood pressure in NP rats from 95 to 118 mmHg. Administration of Enalapril lowered MAP in NP rats to 91mmHg. AT1-AA-induced blood pressure increased to 100 mmHg when Enalapril was administered, which was significantly greater than that of NP rats treated with Enalapril, thus indicating that the AT1-AA increases blood pressure independently of ANGII. Figure 1 demonstrates that AT1-AA works synergistically with ANGII to significantly increase renal sensitivity during pregnancy above that seen with AT1-AA or ANGII alone. Futhermore, Figure 1 demonstrates that together AT1-AA + ANGII mildly increase blood pressure above that with ANGII or AT1-AA alone. Although AT1-AA increased the RARI in NP rats (Figure 1), administration of Enalapril normalized RARI (0.67+/−.02) to AT1-AA during pregnancy. Although these data indicate an important role for the endogenous renin angiotensin system to indeed play a role in blood pressure and renal sensitivity when AT1-AAs are elevated in pregnant rats, it also supports the hypothesis that AT1-AA can cause PE like symptoms during pregnancy independent of ANGII.
Discussion
Although ANGII levels are normal or even below normal in preeclampsia there is a marked increase in the vascular sensitivity to ANGII, however, the mechanisms underlying these observations remain unclear10, 14-20. Previous studies showed marked ANGII sensitivity among preeclamptic women compared to normal pregnant women 15. In a subsequent study this enhanced sensitivity was present 6 months to 18 months postpartum in previously preeclamptic women17. Our laboratory believes the AT1-AA to play a causal role to enhance vascular sensitivity to ANGII in these women. We have previously shown that infusion of purified rat AT1-AA into normal pregnant rats increased blood pressure via activation of ET-1 and placental oxidative stress pathways 12,14. Furthermore, we have shown that hypertension in response to chronic infusion of AT1-AA was attenuated with either the SOD mimetic, Tempol, or the ETA receptor blocker. In this study we present exciting data that chronic AT1-AA enhances ANGII-induced renal vasoconstriction. In addition, although not reaching statistical significant, AT1-AA mildly increased blood pressure response to ANGII in pregnant rats. Figure 1 support this hypothesis and provides important evidence that ANGII has a more profound renal response when administered simultaneously with AT1-AA. In addition, Figure 1 shows that together AT1-AA+ANGII increase blood pressure greater however not statistically significant than with ANGII alone. Although either AT1-AA or ANGII increase blood pressure during pregnancy, the exacerbation of renal vascular response to AT1-AA+ANGII indicate an important interaction of the molecules with the AT1-receptor to heighten vasoconstriction in the kidney that could have long lasting effects and play a role in the development of cardiovascular disease and hypertension among previously preeclamptic women. Importantly, when we infused lower, noncontrictor (1:100, 1:200) doses of AT1-AA with ANGII, increases in blood pressure were not profound and change in the RARI was not different. These data highlight the importance of levels of AT1-AA necessary to achieve the blood pressure response during preeclampsia. Infusion of the 1:50 dose has been consistent with achieving circulating levels of AT1-AA similar to that seen in preeclamptic (RUPP) rat models and to that seen in preeclamptic women. In addition, this dose has consistently increased blood pressure and other circulating factors known to play a role in the pathophysiology of preeclampsia thereby demonstrating the importance of the levels of AT1-AA, independent of ANGII, to cause much of the pathophysiology in preeclampsia 12-14.
Doppler sonography was used to measure the resistance index of the renal artery (RARI). Again, although infusion of the AT1-AA alone acts on the kidney to increase the RARI, this response was exacerbated in pregnant rats chronically exposed to AT1-AA +ANGII. We further examined the effects of the AT1-AA to enhance renal vasoconstriction by examining the synergistic effects of the AT1-AA on ANGII-induced afferent arteriolar constriction. We demonstrate that even at low concentrations, levels that do not increase blood pressure during pregnancy, the AT1-AA strongly enhances ANGII-induced afferent arteriolar vasoconstriction in isolated arterioles from normal pregnant rats. Notice in Figure 2, under control conditions, increasing concentrations of ANGII from 10−11 to 10−9 decreased arteriole diameter by 15-20 %. In sharp contrast, arterioles that were pretreated with a non-constrictor dose of the AT1-AA decreased diameter by approximately 60% with increasing concentrations of ANGII from 10−11 to 10-9 . These effects were significantly blunted by the seven amino acid AT1-AA blocking peptide, thus indicating the importance of the AT1-AA to heighten renal vasoconstriction to ANGII during pregnancy.
Although we show the AT1-AA exacerbates the renal response when ANGII is present, we know that ANGII is not elevated in preeclamptic women. Therefore, we wanted to determine the effect of AT1-AA when the endogenous renin angiotensin system is inhibited. We found that administration of Enalapril decreased the blood pressure response in NP rats and dampened the AT1-AA-induced hypertension during pregnancy (Figure 6). Although Enalapril decreased blood pressure in NP controls and lowered AT1-AA-induced hypertension, there was still a significant increase in hypertension in response to AT1-AA infusion compared to NP in the presence of Enalapril. Importantly, these data indicate the increase in blood pressure in response to elevated AT1-AA during pregnancy is independent of the endogenous renin angiotensin system and not contingent upon elevations in ANGII. However, administration of enalapril normalized the increase in renal artery resistive index in AT1-AA-infused pregnant rats suggesting that its renal effects could be within the smaller vessels and not necessarily in the renal artery. Never the less, these data, in concert with the above mentioned seven amino acid AT1-AA blockade of the ANGII-induced constriction of the Aft-Art, indicate the important interaction between the AT1-AA and ANGII with the AT1 receptor to illicit a response commonly seen during preeclampsia. Most importantly, these data support our hypothesis that the heightened ANGII vascular response observed in preeclamptic women is dependent upon AT1-AA. Although these data indicate the importance of this interaction, the mechanisms underlying this abnormality remain undefined. Our previously published data indicate an enhanced endothelial response occurs as a result of AT1-AA-enhanced ANGII sensitivity. Additionally, we have also shown a significant increase in placental oxidative stress associated with AT1-AA-induced hypertension. Previous studies from ours and other laboratories demonstrate that AT1-AA behaves like ANGII activating the AT1 receptor leading to downstream signaling mechanisms. In this study we demonstrate that with both AT1-AA +ANGII, renal and pressor mechanisms are potentiated during pregnancy, thus, the area of mechanistic investigation became ET-1 and oxidative stress as avenues of exacerbated renal and arterial responses to AT1-AA+ANGII during pregnancy. In Figures 3 and 4 we demonstrate that both local ET-1 production and placental oxidative stress are exacerbated in the AT1-AA+ANGII treated group compared to AT1-AA or ANGII alone. Blockade of ETA receptors blunted blood pressure but had no effect on RARI in AT1-AA+ANGII group (Figure 5). Administration of Tempol had more profound effects to attenuate RARI in the AT1-AA+ANGII group than Tempol had on the blood pressure response in this group and more profound than ETA blockade had on RARI in the AT1-AA+ANGII group (Figure 5). Interestingly, combined with the Enalapril data, these studies collectively indicate that there is no single mechanism or pathway stimulated by AT1-AA found to be responsible for the enhanced vascular sensitivity and renal responses during pregnancy. These studies, much like the clinical scenario of preeclampsia, highlight the complexity of this disease and multifaceted pathology elicited by the AT1-AA. Importantly, the endogenous ANGII system, activation of ET-1 and ROS all play an important role in renal vasoconstriction and hypertension when AT1-AA are present during pregnancy. Inhibition of the renal response by Enalapril highlights the importance of ANGII in this interaction. Likewise, blockade of the afferent arteriolar constriction by the AT1-AA blocking 7-amino acid indicates the importance of the AT1-AA to mediate vasoconstrictor response in the afferent arteriole as well. Although both components play an important role in the renal response it will be important to determine the relevance of downstream ANGII signaling, such as calcium influx or receptor internalization, in the presence of the AT1-AA. Furthermore, studies designed to determine the stoichiometry or association of these components will be important to ascertain how these molecules interact to cause these profound effects during pregnancy. However, until we can discover ways to inhibit the actions of the AT1-AA without interfering with normal function of the endogenous RAS in mother and fetus, we will not be able to slow the progression of this disease or improve pregnancy outcomes of women and children stricken with preeclampsia. This study emphasizes the importance of future investigation concerning AT1-AA inhibition or attenuation of its interaction with the AT1 receptor in drug discovery developing future therapeutics for preeclamptic women.
Figure 6. AT1-AA increased blood pressure independent of endogenous ANGII.
Enalapril decreased blood pressure in NP rats. In addtion Enalapril blunted the rise in blood pressure normally seen in response to chronic AT1-AA infusion. However, the blood pressure response to AT1-AA in the presence of Enalapril was still significantly greater than that of NP treated with Enalapril. (* denotes P< 0.05).
Perspectives
In conclusion, AT1-AA produced during pregnancy causes chronic increases in blood pressure and renal artery resistance. These increases are independent of ANGII, however, in the presence of ANGII, AT1-AA mildly increased blood pressure and significantly heightened renal response in the pregnant rat. This data supports our hypothesis that the AT1-AA plays a causal role in the increased vascular sensitivity of preeclamptic women to ANGII, demonstrated in the early studies performed by Norman Gant. AT1-AA and ANGII independently cause increased ET-1 activation and oxidative stress. Interestingly, in combination AT1-AA and ANGII heighten renal and placental ET-1 and placental oxidative stress. Blockade of ETA receptors or administration of an SOD mimetic either reduces blood pressure or RARI indicating that when the AT1-AA is produced during preeclampsia a multitude of pathways are activated and play a role in causing the hypertension during the pathogenesis of this disease. Neither ANGII receptor blockers, ETA receptor blockers nor SOD mimetics are used in the treatment of hypertension during pregnancy. Therefore, drug discovery targeting the blunting of the effects of the AT1-AA on the AT1 R in the presence or absence of ANGII will be essential to designing innovative treatment strategies to improve prognosis of women and children affected by this devastating disease.
Supplementary Material
Novelty and Significance.
What is new?
Administration of AT1-AA+ANGII simultaneously increased blood pressure and significantly enhanced renal vasoconstriction during pregnancy, via activation of ET-1 and oxidative stress pathways.
What is relevant?
Preeclamptic women display enhanced ANGII sensitivity during pregnancy and following delivery
Summary
This study indicates one important mechanism of AT1-AA is to heighten renal vascular sensitivity to ANGII. This increase in blood pressure in the presence of AT1-AA is multifaceted and involves ET-1 and ROS and endogenous ANGII. Designing peptides to inhibit interaction of the AT1-AA with AT1R could be an important avenue to improve pregnancy outcomes in preclamptic women.
Acknowledgments
Sources of Funding This work was supported by NIH grants HD067541 and HL51971. RD is supported by the German Research Foundation (DFG 631/7-1).
Footnotes
Disclosures None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Caritis S, Hauth J. What we have learned about preeclampsia. Semin Perinatol. 2003;27:239–246. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.LaMarca B, Gilbert J, Granger JP. Recent Progress Toward the Understanding of the Pathophysiology of Hypertension During Pregnancy. Hypertension. 2008;51:982–988. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert J, Ryan MJ, LaMarca B, SeDeek M, Murphy SR, Granger JP. Pathophysiology of Hypertension During Preeclampsia: Linking Placental Ischemia with Endothelial Dysfunction. American Journal of Physiology: Heart and Circulatory Physiology. 2007;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Ramin SM, Kellems RE. Potential Roles of Angiotensin Receptor-Activating Autoantibody in the Pathophysiology of Preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dechend R, Muller DN, Wallukat G, Homuth V, Krause M, Dudenhausen J, Luft FC. Activating Auto-Antibodies Against the AT1 Receptor in Preeclampsia. Autoimmunity Reviews. 2004;4:61–65. doi: 10.1016/j.autrev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Bräsen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Müller DN. Agonistic Autoantibodies to the AT1 Receptor in a Transgenic Rat Model of Preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 9.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzel K, Rajakumar A, Haase H, et al. Angiotensin II Type 1 Receptor Antibodies and Increased Angiotensin II Sensitivity in Pregnant Rats. Hypertension. 2011;58:77–84. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol. 2007;34:938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 12.LaMarca BBD, Parrish M, Ray L, Murphy SR, Roberts L, Wallukat Glover P, Wenzel G, CockrellK K, Martin JN, Jr., Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: Role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrish M, Murphy SR, Rutland S, Wallace K, Wenzel K2, Wallukat G2, Keiser S, Ray LF, Dechend R2, Martin JN, Granger JP, LaMarca B. The effect of immune factors, Tumor necrosis factor alpha (TNF-α) and agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA), on Soluble fms-like tyrosine-1 (sFlt-1) and soluble Endoglin (sEng) production in response to hypertension during pregnancy. Am J Hypertens. 2010;23:911–916. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrish Marc R., Wallace Kedra, Dechend Ralf, Weimer Abram, Wenzel Katrin, Wallukat Gerd, Ray Lillian F., Arany Marrietta, Tam Kiran Tam, Cockrell Kathy, Martin James N., Jr., LaMarca BB. Hypertension in Response to AT1-AA: Role of Reactive Oxygen Species in Pregnancy-Induced. American Journal of Hypertension. 2011;24:835–840. doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 15.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Hypertension. 2010;55:1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49:612–7. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 18.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. J Physiol Renal Physiol. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 19.Whalley PJ, Everett RB, Gant NF, Cox K, MacDonald PC. Pressor responsiveness to angiotensin II in hospitalized primigravid women with pregnancy-induced hypertension. AmJ Obstet Gynecol. 1983;145:481–483. doi: 10.1016/0002-9378(83)90321-6. [DOI] [PubMed] [Google Scholar]
- 20.Hladunewich MA, Kingdom J, Odutayo A, Burns K, Lai V, O’Brien T, Gandhi S, Zimpelmann J, Kiss A, Miller J, Cherney D. Postpartum assessment of the RAS in women with previous server, early-onset preeclampsia. J Clin Endocrinol Metab. 2011;96:3517–3524. doi: 10.1210/jc.2011-1125. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int. 2004;66:268–274. doi: 10.1111/j.1523-1755.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 22.Fu Y, Hall JE, Lu D, Lin L, Manning RD, Jr., Cheng L, Gomez-Sanchez C, Juncos LA, Liu R. Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors. Hypertension. 2012;59:599–606. doi: 10.1161/HYPERTENSIONAHA.111.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.