Abstract
Purpose
Although the National Institutes of Health (NIH) has made extensive investments in educational programs related to clinical and translational science (CTS), there has been no systematic investigation of the number and characteristics of PhD programs providing training to future leaders in CTS. The authors undertook to determine the number of institutions that, having had received NIH-funded Clinical and Translational Science Awards (CTSAs), currently had or were developing PhD programs in CTS; to examine differences between programs developed before and after CTSA funding; and to provide detailed characteristics of new programs.
Method
In 2012, CTS program leaders at the 60 CTSA-funded institutions completed a cross-sectional survey focusing on four key domains related to PhD programs in CTS: program development and oversight; students; curriculum and research; and milestones.
Results
Twenty-two institutions had fully developed PhD programs in CTS, and 268 students were earning a PhD in this new field; 13 institutions were planning a PhD program. New programs were more likely to have fully developed PhD competencies and more likely to include students in medical school, students working only on their PhD, students working on a first doctoral degree, and students working in T1 translational research. They were less likely to include physicians and students working in clinical or T2 research.
Conclusions
Although CTS PhD programs have similarities, they also vary in their characteristics and management of students. This may be due to diversity in translational science itself or to the relative infancy of CTS as a discipline.
In 2005, the National Institutes of Health (NIH) called for proposals to develop clinical and translational science (CTS) centers at institutions nationwide.1 As first articulated in the NIH Roadmap,2 the rationale for funding this initiative was that, by making the boundaries between basic and clinical research more permeable, research findings could be more rapidly translated into broad improvements in public health.3 Every year, the NIH gives $500 million in Clinical and Translational Science Awards (CTSAs) to create and support these centers, representing the largest publicly funded outlay aimed at stimulating translational research.4
In 2006, the NIH awarded its first 12 CTSAs through the National Center for Research Resources. As of 2011, the NIH reached its goal of funding 60 institutions through the CTSA program, which is now under the auspices of the National Center for Advancing Translational Sciences.5 A primary mission of CTSA-funded institutions is establishing CTS as a discipline; the key metric for judging their success will be the number and quality of translational investigators they train.6 The overall initiative was based on the recognition that, to promote CTS objectives, a qualitatively different type of investigator was required, one for whom the necessary expertise could no longer be acquired on the job.1,7
To fulfill the education and training goals of this new discipline, the majority of CTSA-funded institutions have established certificate programs and master’s degree programs in CTS.8 Because a doctoral program is the most academically rigorous form of training in the core principles of CTS, the NIH encourages institutions that do not have PhD programs in CTS to develop them. It also encourages institutions that already have CTS-related PhDprograms to consolidate these programs under CTSA program oversight.1
Anecdotal evidence indicates that institutions have faced challenges to creating these programs, including financial barriers, lack of institutional support, and competition for students from similar programs. They have also faced challenges to developing and strengthening the programs, including difficulties in defining CTS and delineating it from other disciplines, lack of agreement about core competencies and related curricula, and problems in demarcating and defining the core faculty.9–11 Yet, despite the fact that some CTSAs were awarded as long as six years ago, no systematic investigation has been done of the development and characteristics of PhD programs in CTS.
We believed that the maturation of the CTSA programs made this an ideal time to address several key questions about the evolution of PhD programs in CTS. We therefore developed a cross-sectional survey to gather data from leaders of CTS programs at the 60 institutions that have received CTSAs. Our specific goals were to determine how many of these institutions currently had or were developing PhD programs in CTS; to examine potential differences between programs developed before and after the CTSA funding initiative (previously existing versus newly developed programs); and to describe in detail the characteristics of the new programs. We believed that the findings from our survey would serve as a marker of the impact of CTSA funding on the development and deployment of trained investigators in CTS.
Method
Survey development and content
Our survey development team consisted of individuals who have expertise in survey design, academic program administration, and data analysis and also have experience in developing a PhD program in CTS.
The purpose of our survey was to gather data about the CTSA-funded institutions and the characteristics of their CTS programs. The survey focused on four key domains related to PhD programs in CTS: 1) program development and oversight; 2) students; 3) curriculum and research; and 4) milestones. Each domain covered multiple topics. For example, the program development and oversight domain assessed the year that program planning began, year the first students were enrolled, the school that confers the PhD, whether there are faculty positions in the departmental home of the PhD, committee structure, financial support for students, mentoring models, and outcomes. The student domain assessed the program’s admission requirements, numbers and sociodemographic characteristics of students, expected and actual time for program completion, and student outcomes (e.g., number of program graduates and types of intended postgraduate careers). The curriculum and research domain assessed whether the program had required core courses or specialty tracks, minimum credits for graduation, whether independent research was required, and when independent research was expected to begin. The milestones domain assessed whether or not there were preliminary, comprehensive, and candidacy examinations, the format and timing of these examinations, and allowable formats for the dissertation.
In drafting the survey, we had input from several members of the Education Key Function Committee of the CTSA Consortium. This CTSA education committee supports the advancement of integrated and interdisciplinary education and training in CTS by facilitating the dissemination of new teaching ideas and curricula and educational resource sharing (www.ctsacentral.org/committee/education-and-career-development). We elicited feedback about the survey’s overall content and wording of specific items and worked in an iterative fashion to incorporate changes. We pilot-tested the survey with members of this committee in September 2011 and made additional refinements based on their comments.
The final survey consisted of 49 items to be administered online. An item near the beginning of the survey asked respondents whether their institution had or was planning a PhD program in CTS. This item was designed to serve as a screening item. If respondents answered no, they were thanked for their participation and were not asked to complete the majority of the remaining items. If respondents answered yes, they were asked whether their program was fully developed or still in the planning stages. If it was fully developed, they were asked, “Was the program developed as a result of CTSA funding or did it already exist?” The response to this item allowed us to divide the respondents into our two study groups: 1) institutions with previously existing PhD programs in CTS and 2) institutions with newly developed ones.
Survey administration and data analysis
We sent an e-mail describing the study to all 60 directors of CTSA research education, training, and career development cores. Contact information for directors was obtained through a combination of searches of each program’s website and personal telephone contact with each program. In this November 2011 e-mail, we asked each director to have the institutional representative who was most knowledgeable about existing or planned PhD programs in CTS to complete the survey at a secure online site within three weeks. Most surveys were completed within this time frame. Using follow-up e-mails and telephone calls, we achieved a 100% response rate. In the few cases in which we needed clarification of survey responses, we obtained the information through personal communication with program administrators. To complete our study, we also needed information about didactic coursework that formed the basis of the PhD programs. In January 2012, we retrieved this information from program course listings on each institution’s website and through telephone calls with program leaders.
Raw questionnaire data were aggregated into an Excel file for analysis. Analyses were primarily descriptive. Frequencies and percentages were calculated for all program and student characteristics.
Results
CTSA-funded institutions
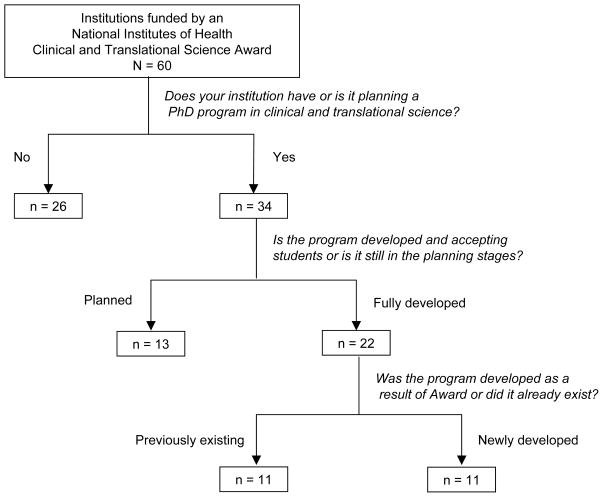
Of the 60 CTSA-funded institutions (Figure 1), 26 did not have and were not planning to have a PhD program in CTS. The most common reasons for this pertained to lack of demand and lack of funding: general lack of demand (5 programs or 19%), demand for the program was filled by other PhD programs in the same geographic area (3 or 12%), demand for the program was filled by similar PhD programs at the same institution (8 or 31%), insufficient funding for students (7 or 27%), and insufficient funding for the program itself (8 or 31%). An additional reason cited was difficulty in defining CTS as a field (42%). In an open-ended follow-up question asking about additional reasons for not initiating a PhD program, some institutions indicated that there was no need for a CTS doctoral degree, that master’s level training was sufficient, or that PhD programs should be discipline-based and that training in CTS did not warrant a stand-alone degree.
Figure 1.
Flowchart of survey responses. In 2011–12, all 60 institutions that had received a National Institutes of Health Clinical and Translational Science (CTS) Award responded to a survey. The survey posed cascading questions meant to sort out those institutions that had PhD programs in CTS, and to determine if each program had been created before receiving the CTS Award (11 programs) or after (11 programs). For a list of the 22 CTS PhD programs, see the footnote that accompanies this article.
Of the remaining 34 CTSA-funded institutions, 13 were in various stages of planning a PhD program in CTS, whereas 21 already had a developed PhD program in this field. One institution had two programs so Figure 1 shows 22 fully developed programs. Of the 22 PhD programs in CTS, 11 had been created before CTSA funding was received (previously existing programs), and 11 had been created after funding was received (newly developed programs).*
Key differences between previously existing and newly developed PhD programs
Although the 11 previously existing and 11 newly developed PhD programs in CTS had many similarities, they also had some key differences (Table 1). For example, newly developed programs were more likely to include students in medical school, students working only on their PhD, students working on a first doctoral degree, and students working in T1 translational research (research that uses laboratory understanding of disease mechanisms to develop new methods for diagnosis, therapy, and prevention and to first test them in humans). They were less likely to include physicians, to include students working in clinical or T2 translational research (using results from clinical studies to inform everyday clinical practice and health decision making), and to expect doctoral students to begin their research in the first year of the program. Although newly developed programs were more likely to have lab rotations as a required part of PhD training and were also more likely to have fully developed PhD competencies, they tended to assign a smaller percentage of the total required PhD credits to didactic coursework (34% of total credits for newer programs versus 52% for previously existing programs).
Table 1.
Key Differences Between Previously Existing and Newly Developed PhD Programs in Clinical and Translational Science That Have Received a National Institutes of Health Clinical and Translational Science Award, 2012
| Variable | Program characteristics | Student characteristics | ||
|---|---|---|---|---|
|
| ||||
| 11 previously existing programs: No. (%) | 11 newly developed programs: No. (%) | 187 students in previously existing programs: No. (%) * | 81 students in newly developed programs: No. (%) | |
| Student type | ||||
| Students who have an MD | 8 (73) | 6 (55) | 148 (79) | 13 (16) |
| Students in medical school | 2 (18) | 8 (73) | 4 (2) | 21 (26) |
| Students working only on their PhD | 6 (55) | 10 (91) | 35 (19) | 47 (58) |
| Degree type | ||||
|
|
||||
| First doctoral degree† | 7 (64) | 11 (100) | 39 (21) | 68 (84) |
|
|
||||
| Second doctoral degree | 8 (73) | 6 (55) | 148 (79) | 13 (16) |
|
|
||||
| Research type‡ | ||||
|
|
||||
| Basic research | — | — | 34 (20) | 4 (6) |
| T1 translational research | 5 (45) | 9 (82) | 17 (10) | 31 (46) |
| Clinical research | 5 (45) | 4 (36) | 59 (35) | 15 (22) |
| T2 translational research | 7 (64) | 3 (27) | 43 (26) | 16 (24) |
| Clinical practice | — | — | 15 (9) | 2 (3) |
|
|
||||
| Independent research is pursued from the beginning of the program | 3 (27) | 0 (0) | — | — |
|
|
||||
| Program requires lab rotations | 6 (55) | 9 (82) | — | — |
|
|
||||
| Program has fully developed PhD competencies | 3 (27) | 6 (55) | — | — |
Students in the program at Rockefeller University were excluded from this analysis because the vast majority of them were focused on non–translational basic science research.
Medical students also earning PhDs were included in this category.
Data on student research type were not reported for 19 students (10%) in previously existing programs and 13 students (16%) in newly developed programs and are therefore not included in the table.
Consistent with these results, the analysis of the characteristics of students in the PhD programs showed that students in the newer programs were more likely to be working solely on their PhD degree (i.e., were not physicians or medical students), to be working on their first doctoral degree, and to be engaged in T1 research.
Characteristics of the newly developed PhD programs
Program development and oversight
Of the eleven institutions with newly developed PhD programs in CTS, seven began planning their programs in 2004–2006, but most did not enroll students until after this period (Table 2). At the time of the survey, the majority of institutions planned to confer PhD degrees through their graduate school but did not have faculty positions available in the departmental home in which degree candidates would work. Most of the programs had admissions and curriculum committees but not steering or disciplinary committees; most supported their students through a combination of departmental funds, federal funds awarded to mentors, training grants, career development grants, and employee education benefits; and most used a team mentoring model. Most were not tracking any outcomes for program graduates, but this may change in the future because most had not yet graduated any PhD students. Of the programs that were tracking outcomes, the majority were following career progress.
Table 2.
Program Development and Oversight Characteristics of 11 PhD Programs in Clinical and Translational Science Developed after Receiving a National Institutes of Health Clinical and Translational Science Award, 2012
| Characteristic | No. (%) |
|---|---|
| Year PhD planning began | |
| 2004–2006 | 7 (64) |
| 2007–2009 | 4 (36) |
| Year first students enrolled | |
| 2004–2006 | 1 (9) |
| 2007–2009 | 5 (45) |
| 2010–2011 | 5 (45) |
| School conferring PhD | |
| Graduate school | 7 (64) |
| Medical and graduate schools, jointly | 2 (18) |
| Medical school | 1 (9) |
| Other | 1 (9) |
| Faculty positions in departmental home of degree | |
| Yes | 4 (36) |
| Committees | |
| Admissions | 10 (91) |
| Curriculum | 8 (73) |
| Advisory | 5 (45) |
| Executive | 5 (45) |
| Examination | 4 (36) |
| Steering | 2 (18) |
| Disciplinary | 1 (9) |
| Governing | 1 (9) |
| Sources of financial support for students | |
| Departmental funds | 10 (91) |
| R01 funds awarded to mentors | 10 (91) |
| Other federal funds awarded to mentors | 7 (64) |
| Other nonfederal funds awarded to mentors | 4 (36) |
| Training grants | 9 (82) |
| Employee education benefits | 7 (64) |
| Career development grants | 7 (64) |
| Internal fellowships | 6 (55) |
| External fellowships | 3 (27) |
| Mentoring model | |
| Team | 7 (64) |
| Dual | 4 (36) |
| Single | 2 (18) |
| Other | 2 (18) |
| Outcomes tracked for program graduates | |
| Career progress | 4 (36) |
| Publications | 2 (18) |
| Grants | 2 (18) |
| Career fields | 2 (18) |
Students
Even though nine of the eleven programs required standardized tests for admission of students (typically GREs or MCATs), only five required prior research experience (Table 3). While all programs allowed entering students to transfer some credits, the mean number allowed was 16.9 credits. Of the programs, ten included students who were working only on their PhD, eight included students in medical school, and six included physicians at various levels of training. Most programs did not require students to pay their own tuition. Although most of them expected students to graduate in about five years, four programs reported having had students leave before completion and four had graduated students from the program with an average time to graduation of 4.4 years (52.8 months). The majority expected their graduates to enter academic research or academic clinical positions following PhD program completion.
Table 3.
Student-Related Characteristics of 11 PhD Programs in Clinical and Translational Science Developed after Receiving a National Institutes of Health Clinical and Translational Science Award, 2012
| Characteristic | No. (%) |
|---|---|
| Admission requirements | |
| Standardized tests | 9 (82) |
| Prior research experience | 5 (45) |
| Maximum number of transferable credits* | |
| 0–12 | 4 (36) |
| 13–24 | 4 (36) |
| 25–36 | 3 (27) |
| Part-time students allowed | |
| Yes | 5 (45) |
| Student type | |
| Students who have an MD | 6 (55) |
| Faculty members | 3 (27) |
| Medical residents | 0 (0) |
| Medical fellows | 3 (27) |
| Students in medical school | 8 (73) |
| Students working only on their PhD | 10 (91) |
| Percentage of students paying their own tuition | |
| 0 | 7 (64) |
| 1–10 | 0 (0) |
| 11–20 | 2 (18) |
| 20–30 | 2 (18) |
| Expected years for program completion | |
| 3 | 1 (9) |
| 4 | 1 (9) |
| 5 | 7 (64) |
| 6 | 1 (9) |
| Any students left before program completion | |
| Yes | 4 (36) |
| Any students graduated from program† | |
| Yes | 4 (36) |
| Intended postgraduate career | |
| Academic, mainly research | 8 (73) |
| Academic, mainly clinical | 6 (55) |
| Academic, mainly teaching | 3 (27) |
| Industrial, research | 3 (27) |
| Federal, research | 2 (18) |
| Consultancy | 1 (9) |
| Federal, non-research | 0 (0) |
| Private practice, clinical | 0 (0) |
| Other | 1 (9) |
The mean number of transferable credits was 16.9.
The mean actual time to graduation was 4.4 years (52.8 months), with a range of 37–60 months.
Curriculum and research
All eleven programs had a set of required core courses, but the majority did not have specialty fields or tracks within their PhD program (Table 4). The number of credits required for graduation ranged from 30 to 150, with most programs requiring fewer than 90 credits. The mean number of credits was 71, with an average of 29 credits (average 34%) assigned to didactic coursework. Of the programs, nine required lab rotations and six had fully developed PhD competencies (Table 1). All programs required independently conducted research, but the majority had no set time point for the beginning of independent research.
Table 4.
Curriculum and Research Characteristics and Milestones of 11 PhD Programs in Clinical and Translational Science Developed after Receiving a National Institutes of Health Clinical and Translational Science Award, 2012
| Characteristic or Milestone | No. (%) |
|---|---|
| Curriculum and Research Characteristics | |
| Required core courses (n = 11) | |
| Yes | 11 (100) |
| Required courses (n = 10) | |
| Career development | 9 (90) |
| Research methods | 9 (90) |
| Analysis | 8 (80) |
| Clinical and translational science | 7 (70) |
| Regulatory issues | 7 (70) |
| Basic science | 3 (30) |
| Communication | 2 (20) |
| Specialty fields or tracks (n = 11) | |
| Yes | 2 (18) |
| Minimum credits for graduation (n = 11)* | |
| 30–59 | 3 (27) |
| 60–89 | 4 (36) |
| 90–119 | 1 (9) |
| 120–150 | 1 (9) |
| Require independent research separate from mentor (n = 11) | |
| Yes | 10 (91) |
| Onset of independent research (n = 11) | |
| From the beginning of the program | 0 (0) |
| After conducting formal, supervised research | 1 (9) |
| After comprehensive exams | 3 (27) |
| No set point | 6 (55) |
| Milestones | |
| Preliminary exam (n = 11) | |
| Yes | 5 (45) |
| Format of preliminary exam (n = 5) | |
| Oral review | 1 (20) |
| Written examination | 1 (20) |
| Oral examination | 1 (20) |
| Grant application | 1 (20) |
| Other | 1 (20) |
| Timing of preliminary exam (n = 5) | |
| After completion of core curriculum | 2 (40) |
| After a certain number of credits | 1 (20) |
| After 1 year in the program | 1 (20) |
| Other time point | 1 (20) |
| Comprehensive exam (n = 11) | |
| Yes | 6 (55) |
| Allowable format for comprehensive exam (n = 6) | |
| Oral examination | 4 (67) |
| Grant application | 3 (50) |
| Written examination | 2 (33) |
| Literature review | 2 (33) |
| Written study design | 2 (33) |
| Oral review | 1 (16) |
| Other | 2 (33) |
| Candidacy exam (n = 11) | |
| Yes | 10 (91) |
| Allowable format for candidacy exam (n = 10) | |
| Oral examination | 8 (80) |
| Grant application | 5 (50) |
| Oral review | 3 (30) |
| Written examination | 3 (30) |
| Literature review | 1 (10) |
| Written study design | 1 (10) |
| Other | 2 (20) |
| Allowable dissertation format (n = 11) | |
| Traditinal full-length dissertation | 10 (91) |
| Compilation of manuscripts | 6 (55) |
The mean number of credits was 71.
Milestones
Of the eleven programs, five had a preliminary exam, six had a comprehensive exam, and ten had a candidacy exam. The formats for these varied widely (Table 4). For example, for the comprehensive exam, the most common formats were an oral examination and a written grant application. While ten programs allowed a traditional full-length dissertation, six allowed a compilation of manuscripts (average of 2.8 manuscripts) as an alternative to a full-length dissertation.
Discussion
Our study represents the first systematic investigation of the characteristics of PhD programs in CTS and of the impact of CTSA funding on the development of these programs. Our survey of the 60 CTSA-funded institutions suggests that the NIH investment to establish CTS programs nationwide has led to considerable activity. In 2011, when the survey was completed, 21 recipients of CTSAs had fully developed PhD programs in CTS (11 created before and 11 created after CTSA funding; one institution had two programs), 13 other institutions were planning PhD programs, and 268 students were earning a PhD in this new field. The three key barriers to establishing programs were lack of demand, lack of financial resources, and difficulty defining the scope and content of a PhD program in CTS. The first and second of these barriers may lead an institution to view the mobilization of resources required to create a PhD program as either unnecessary or impractical, and the third may reflect the relatively recent emergence of CTS as a field of study.
Our survey revealed several key differences between programs developed before and after the CTSA initiative. One key difference was that the newly developed programs included more students in medical school, students working only on their PhD, and students working on a first doctoral degree. They included fewer physicians. The fact that they were serving a different and potentially less mature student population may explain why the newer programs were less likely to begin independent research at the onset of the program. A second key difference was that the newer programs were more likely to focus on T1 research, whereas the older programs tended to focus on clinical and T2 research. This difference may be a function of the fact that the older programs attracted more students with MD degrees, a group that also may have been more likely to focus on clinical investigation rather than the translation of basic science findings into public health improvements. And a third key difference was that the newer programs were more likely to have established competencies. This may be a reflection of the fact that during their development, these new programs were directly confronted with the challenge of defining and categorizing the content of a PhD program in CTS.
Our most important aim was to describe the characteristics of those PhD programs developed as a direct result of CTSA funding, rather than those adapted from existing programs. Because both the framework and the content of the eleven newly developed programs were fully formulated with CTS goals in mind, they most clearly represent what national leaders in CTS believe should comprise CTS training. Although the eleven newer programs had many similarities, they varied widely not only with regard to the characteristics of their students but also with regard to the management of the students and programs. For example, the survey showed that programs varied in terms of how they funded their students, how many credits they allowed their students to transfer from other institutions, whether they required their students to take preliminary and comprehensive exams, what types of careers they expected their graduates to enter, and how intensely they tracked or planned to track the outcomes of their programs. In addition, although the survey found clear agreement that a set of core courses and independent research should be required, it found less consensus about the content of required courses or the time point at which independent research should begin. Most programs, for example, required research methods and career development courses, but incrementally fewer required analytic, clinical and translational science, regulatory, basic science, and communication courses.
Unlike PhD programs in more well-established areas, such as immunology, biochemistry, economics, and psychology, the PhD programs in CTS vary greatly in student characteristics, core course content, and program milestones. On the one hand, this diversity may reflect the nature of CTS, which encompasses a broad range of research types and activities, and program-to-program variation will continue even as the programs mature. On the other hand, the variation may be attributable to the relative infancy of CTS as a discipline or to the fact that most programs are housed in medical schools without a long history of or clear template for establishing and maintaining non–basic-science PhD programs. If this is the case, the maturation of the discipline may lead to the convergence of the characteristics and requirements of the CTS programs.
A key strength of our study is that we based our findings on a 100% response rate from CTSA-funded institutions throughout the United States. One limitation is that our data do not allow us to fully explain whether differences between existing and newly developed programs are a result of differences in the programs’ goals, their entrance requirements, or the type of students they ultimately attract. A second limitation is the lack of information on PhD program graduates, particularly for the newly developed programs, which still have only a few graduates. This presents an opportunity for future research and program comparison.
Conclusions
While PhD programs in CTS have numerous similarities, they also have interesting variations that are related to the characteristics and management of students and may be due to diversity in translational science itself or to the relative infancy of CTS as a discipline.
Although we believe that our results offer insight into the development and characteristics of PhD programs in CTS, we recognize that a full understanding of the evolution and final configuration of currently functioning and planned PhD programs in this field will require additional investigations.
Acknowledgments
For their help in developing the survey used in this study, we are grateful to the following members of the Education Key Function Committee of the Clinical and Translational Science Awards Consortium: Karl E. Anderson, MD (University of Texas, Galveston); Melissa D. Begg, ScD (Columbia University, New York); Michael F. Fleming, MD, MPH (Northwestern University, Chicago); Thomas Hulsey, ScD (Medical University of South Carolina, Charleston); Michael Lichtenstein, MD, MSc (University of Texas Health Science Center at San Antonio); and Ellie Schoenbaum, MD (Albert Einstein College of Medicine, Bronx).
Funding/Support: The project described was supported by the National Institutes of Health (NIH) through grant numbers UL1 RR024153 and UL1TR000005.
Footnotes
The 11 institutions that had PhD programs in CTS before receiving a CTSA are Johns Hopkins University, Mount Sinai School of Medicine, Rockefeller University, Tufts University, University of California at Los Angeles, University of California at San Francisco, University of Iowa, University of Massachusetts Medical School, University of Texas Health Science Center at Houston, University of Texas Medical Branch (Clinical Science), and Yale University. The 11 institutions that developed PhD programs in CTS after receiving a CTSA are Albert Einstein College of Medicine and Dentistry, Mayo Clinic College of Medicine, Medical College of Wisconsin, The Ohio State University, University of Arkansas for Medical Sciences, University of Kentucky Research Foundation, University of Pittsburgh, University of Rochester School of Medicine, University of Texas Medical Branch (Human Pathophysiology and Translational Medicine), University of Wisconsin at Madison, and Virginia Commonwealth University.
Other disclosures: None.
Ethical approval: Not applicable.
Disclaimer: The NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Previous presentations: Dr. Switzer presented data from this study at the annual, jointly sponsored ACRT-SCTS meeting on April 19, 2012 in Washington, D.C.
Contributor Information
Dr. Galen E. Switzer, Professor of medicine, psychiatry, and clinical and translational science, and director, PhD Program in Clinical and Translational Science, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania. He is also associate director, Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System, Pittsburgh, Pennsylvania.
Dr. Georgeanna F.W.B. Robinson, Assistant director, Institute for Clinical Research Education, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Dr. Doris M. Rubio, Professor of medicine, biostatistics, nursing, and clinical and translational science, and director, Data Center, Center for Research on Health Care; co-director, Institute for Clinical Research Education; director, Office for Evaluation, and director, Office of Lifelong Learning, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
Dr. Nicole R. Fowler, Assistant professor of medicine and assistant director, PhD Program in Clinical and Translational Science, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
Dr. Wishwa N. Kapoor, A Falk Professor of Medicine, professor of health policy and management, and chief, Division of General Internal Medicine; vice chair, Department of Medicine; director, Institute for Clinical Research Education; director, Center for Research on Health Care; co-director, RAND-University of Pittsburgh Health Institute; and co-director, Clinical and Translational Science Institute, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.Zerhouni EA. Translational and clinical science: Time for a new vision. N Engl J Med. 2005;353(15):1621–1623. doi: 10.1056/NEJMsb053723. [DOI] [PubMed] [Google Scholar]
- 2.Zerhouni EA. The NIH roadmap. Science. 2003;302(5642):63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 3.Collins FS. Reengineering translational science: The time is right. Sci Transl Med. 2011;3(90):90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selker HP. Beyond translational research from T1 to T4: beyond “separate but equal” to integration (Ti) Clin Transl Sci. 2010;3(6):270–271. doi: 10.1111/j.1752-8062.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Center for Advancing Translational Sciences. [Accessed May 15, 2013.]; http://www.ncats.nih.gov/about/org/organization.html.
- 6.Reis SE, Berglund L, Bernard GR, Califf RM, Fitzgerald GA, Johnson PC National Clinical and Translational Science Awards Consortium. Reengineering the national clinical and translational research enterprise: the strategic plan of the National Clinical and Translational Science Awards Consortium. Acad Med. 2010;85(3):463–469. doi: 10.1097/ACM.0b013e3181ccc877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers FJ, Begg MD, Fleming M, Merchant C. Strengthening the career development of clinical translational scientist trainees: a consensus statement of the Clinical and Translational Science Award (CTSA) Research Education and Career Development Committees. Clin Transl Sci. 2012;5(2):132–137. doi: 10.1111/j.1752-8062.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huskins WC, Weavers KM, Gorden JF, Gabriel SE. Creating the future: training the next generation of translational science teams. Clin Transl Sci. 2008;1(2):94–95. 98. doi: 10.1111/j.1752-8062.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore DW, Dillmore TC, Robinson GFWB. Advancing knowledge and research: developing a doctoral program in clinical and translational science. Clin Transl Sci. 2011;4(5):359–362. doi: 10.1111/j.1752-8062.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Califf RM, Berglund L Principal Investigators of National Institutes of Health Clinical and Translational Science Awards. Linking scientific discovery and better health for the nation: the first three years of the NIH’s clinical and translational science awards. Acad Med. 2010;85(3):457–462. doi: 10.1097/ACM.0b013e3181ccb74d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pienta KJ, Spork AL, Scheske J. The Clinical and Translational Science Awards (CTSAs) are transforming the way academic medical institutions approach translational research: the University of Michigan experience. Clin Transl Sci. 2011;4(4):233–235. doi: 10.1111/j.1752-8062.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]