Abstract
Similar to other bacteria, Brucella strains require several biologically essential metals for their survival in vitro and in vivo. Acquiring sufficient levels of some of these metals, particularly iron, manganese and zinc, is especially challenging in the mammalian host, where sequestration of these micronutrients is a well-documented component of both the innate and acquired immune responses. This review describes the Brucella metal transporters that have been shown to play critical roles in the virulence of these bacteria in experimental and natural hosts.
Keywords: Brucella, iron, manganese, zinc, magnesium, nickel, bacterial iron acquisition
Introduction
The Brucella spp. are Gram-negative bacteria that cause disease in a variety of mammalian hosts (Roop et al., 2009). Although these bacterial strains are presently divided into 10 ‘nomenspecies’ for diagnostic and epidemiological reasons, comparative genomic studies indicate that they are highly related at the genetic level (O'Callaghan and Whatmore, 2011). The brucellae are members of the α-proteobacteria (Moreno et al., 1990), a phylogenetic group of bacteria that includes plant symbionts (Sinorhizobium, Rhizobium, and Bradyrhizobium spp.), plant pathogens (Agrobacterium spp.), and mammalian pathogens (Brucella and Bartonella spp.). It has become readily apparent that there are remarkable parallels between the interactions of these bacteria and their eukaryotic hosts (Batut et al., 2004), and studies of their comparative biology have made significant contributions to our understanding of the pathogenesis of Brucella infections (Sola-Landa et al., 1998; O'Callaghan et al.,1999; LeVier et al., 2000; Sieira et al., 2000).
Brucella melitensis, Brucella suis, and Brucella abortus strains cause abortion and infertility in goats, sheep, swine, and cattle, respectively, and are a great concern to the agricultural communities in areas of the world where these infections are not controlled by surveillance and eradication programs (Corbel, 1997). As they are easily transmitted to humans through direct contact with infected animals or their products, these strains also represent a serious public health threat in regions where they remain endemic in food animals. In fact, brucellosis is considered to be the world's leading zoonotic disease (Pappas et al., 2006). B. melitensis, B. suis, and B. abortus strains also possess biological characteristics that have historically made them attractive as agents of biological warfare (Franz et al., 1997), and currently make them a potential threat for use in bioterrorism (Valderas and Roop, 2006). Specifically, they are easy to aerosolize, they have a very low infectious dose, and the disease they produce is difficult to treat in humans (Ariza et al., 2007) and impractical to treat in animals (Nicoletti et al., 1989).
Brucella ovis and Brucella canis strains are also important veterinary pathogens. B. ovis causes epididymitis and infertility in rams and occasionally abortion in ewes (Blasco, 2003), and B. canis produces abortion and infertility in dogs (Wanke, 2004). B. canis infections associated with contact with infected dogs have been reported in humans (Lucero et al., 2010), although these infections occur much less frequently and appear to be less severe than those caused by B. melitensis, B. suis, or B. abortus. Human disease caused by B. ovis, on the other hand, has not been documented.
Brucella pinnipedialis and Brucella ceti strains are naturally found in marine mammals (Dawson et al., 2008). Reproductive tract pathology has been associated with B. ceti infections in cetaceans (e.g. dolphins and porpoises), but whether or not B. pinnipedialis causes disease in pinnipeds (e.g. seals and sea lions) is presently unknown (Nymo et al., 2011). Human infections with B. ceti strains have been reported (Sohn et al., 2003), but the source of these infections is unclear. Other Brucella strains have been isolated from wild rodents [e.g. Brucella neotomae (Stoenner and Lackman, 1957) and Brucella microti (Scholz et al., 2008)], and human clinical specimens [Brucella inopinata (Scholz et al., 2010)], but neither the capacity of the B. neotomae or B. microti strains to produce human disease, nor the natural host for B. inopinata strains, is known.
The mammalian host as a metal-restricted environment
With a few notable exceptions, all living things require magnesium, manganese, iron, copper, zinc, cobalt, and nickel as micronutrients to support their cellular metabolism and physiology (Summers, 2009; Waldron and Robinson, 2009). These metals play important structural roles in proteins and other cellular components. Owing to their redox activity at physiological pH, iron and copper serve critical functions in proteins that are components of electron transport chains or other proteins that undergo oxidation–reduction reactions. Unfortunately, iron and copper also have the capacity to react with the reactive oxygen species H2O2 and O2– in a series of reactions known as Fenton chemistry. These reactions produce the highly toxic OH• radical, which can cause extensive damage to cellular proteins, nucleic acids and lipids (Summers, 2009). Improper incorporation of metals in proteins can also lead to their inactivation (Waldron and Robinson, 2009). To avoid these latter two problems, organisms possess homeostasis systems that ensure that they only accumulate the levels of metals they need to meet their physiological requirements. These homeostasis systems consist of efflux systems; chaperones, transfer and storage proteins that hold these metals in unreactive or non-toxic forms; and transcriptional and translational regulators that tightly regulate expression of the genes encoding these metal import, export and storage systems.
In mammals, metal homeostasis systems not only protect the host from metal toxicity, but they also deprive invading microbes of the metals they need to establish a productive infection. Sequestration of iron, for instance, is a well-documented strategy employed by mammals to limit the replication of microbial pathogens (Nairz et al., 2010). Iron that is not incorporated into host proteins is bound tightly by iron binding proteins such as transferrin and lactoferrin in the extracellular environment (Griffiths, 1999). This is predominantly an oxidizing environment, and, the vast majority of this iron is present as Fe3+ at physiological pH, and it has been estimated that the amount of ‘free’ Fe3+ in the blood and tissue fluids is <10–18 M. During infection, the protein hepcidin also inhibits the ability of the iron exporter ferroportin to release iron obtained from nutritional sources and recycled from senescent or damaged erythrocytes from the spleen, liver and intestine into the bloodstream, which further restricts the availability of iron in the extracellular environment in the host. This so-called ‘hypoferremic response’ is considered to be an important component of innate immunity (Weinberg, 1995; Nemeth et al., 2004; Weiss, 2005).
Brucella strains are primarily intracellular pathogens in their mammalian hosts. Multiple independent studies by numerous research groups have clearly shown that the capacity of these strains to survive and replicate efficiently in host macrophages is critical to their ability to produce chronic infections in a variety of natural and experimental hosts (reviewed in Roop et al., 2009). In pregnant animals, extensive intracellular replication of the brucellae within placental trophoblasts is associated with abortion and reproductive tract pathology (Enright, 1990). Within the intracellular environment in the host, iron is present as a dynamic equilibrium between Fe2+ and Fe3+, and the ratio of these two types of iron present within an intracellular compartment is dictated by the redox status and pH of that intracellular compartment as well as the activity of cellular ferric reductases and ferroxidases (Anderson and Vulpe, 2009). Three mechanisms have been identified by which mammals can deprive microbial pathogens such as the brucellae that live within phagosomal compartments in host macrophages of iron. All three of these strategies are considered to be important components of the host immune response to infection. The first involves the natural resistance associated macrophage protein (Nramp1) (Cellier et al., 2007). This protein is incorporated into the phagosomal membranes of macrophages and pumps divalent cations such as Fe2+ and Mn2+ out of the phagosomal compartment. Macrophages activated by interferon γ (IFNγ) also have reduced levels of transferrin receptors on their surface, which reduces the overall flux of iron through these host cells (Byrd and Horwitz, 1989). Finally, although there is a generalized inhibition of iron release via ferroportin from host cells during the hypoferremic response, the ferroportin activity of infected macrophages actually increases, which results in an active efflux of iron from these cells (Nairz et al., 2007).
Recent studies indicate that mammals also actively deprive invading microbes of zinc and manganese as a defense mechanism (Kehl-Fie and Skaar, 2010). The identities of the proteins responsible for the sequestration of zinc in host tissues is unclear, but calreticulin, a protein produced by neutrophils, has been shown to be important for depriving Staphylococcus aureus of manganese during the formation of abscesses in a mouse model (Corbin et al., 2008). In addition, as mentioned above, it is well documented that the capacity of Nramp1 to remove Mn2+ from the phagosomal compartment plays an important role in the capacity of macrophages to limit intracellular replication by microbial pathogens (Zaharik et al., 2004; Cellier et al., 2007).
Brucella strains require iron, manganese, zinc, and magnesium transporters for wild-type virulence in natural and experimental hosts
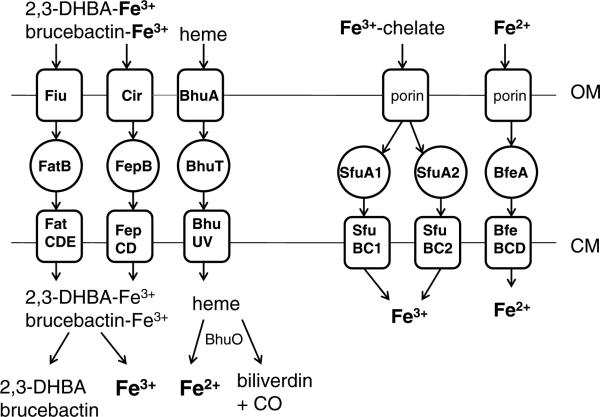
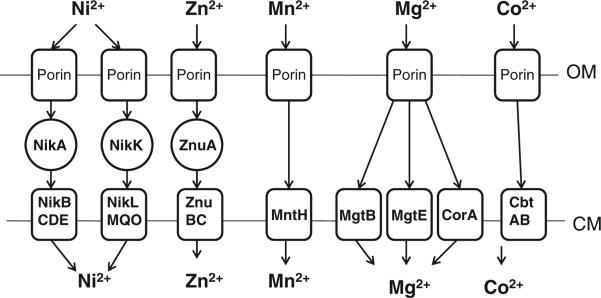
Figures 1 and 2 show the iron, manganese, zinc, nickel, cobalt, and magnesium transport systems predicted to be present in Brucella strains based on surveys of the publicly available genome sequences, and Table 1 lists the genes in the B. abortus 2308 genome sequence that encode the individual components of these systems. For a more general and comprehensive review of the genes involved in metal acquisition and homeostasis in Brucella strains, the reader is directed to a recently published book chapter (Roop et al., 2011).
Fig. 1.
Iron transporters in Brucella. Abbreviations: OM, outer membrane; CM, cytoplasmic membrane.
Fig. 2.
Nickel, zinc, manganese, magnesium and cobalt transporters in Brucella. Abbreviations: OM, outer membrane; CM, cytoplasmic membrane. MgtC is not shown in this figure because its precise role in Mg2+ transport is not known (Günzel et al., 2006; Alix and Blanc-Potard, 2007).
Table 1.
Designations of the genes in the Brucella abortus 2308 genome sequence predicted to encode the individual components of the metal transporters depicted in Figs. 1 and 2.
| Gene product | Predicted function | Gene designation |
|---|---|---|
| DhbC | Biosynthesis of 2,3-DHBA | BAB2_0015 |
| DhbE | Biosynthesis of 2,3-DHBA | BAB2_0014 |
| DhbA | Biosynthesis of 2,3-DHBA | BAB2_0012 |
| DhbB | Biosynthesis of 2,3-DHBA/ Conversion of 2,3-DHBA to brucebactin | BAB2_0013 |
| EntD | Conversion of 2,3-DHBA to brucebactin | BAB2_0011 |
| VibH | Conversion of 2,3-DHBA to brucebactin | BAB2_0016 |
| Fiu | 2,3-DHBA/brucebactin transport | BAB2_0233 |
| FatB | 2,3-DHBA/brucebactin transport | BAB2_0564 |
| FatC | 2,3-DHBA/brucebactin transport | BAB2_0562 |
| FatD | 2,3-DHBA/brucebactin transport | BAB2_0563 |
| FatE | 2,3-DHBA/brucebactin transport | BAB2_0561 |
| Cir | 2,3-DHBA/brucebactin transport | BAB1_1367 |
| FepB | 2,3-DHBA/brucebactin transport | BAB1_1366 |
| FepC | 2,3-DHBA/brucebactin transport | BAB1_1364 |
| FepD | 2,3-DHBA/brucebactin transport | BAB1_1365 |
| BhuA | Heme transport | BAB2_1150 |
| BhuT | Heme transport | BAB2_0483 |
| BhuU | Heme transport | BAB2_0484 |
| BhuV | Heme transport | BAB2_0485 |
| BhuO | Heme degradation/Fe2+ release | BAB2_0677 |
| SfuA1 | Fe3+ transport | BAB2_0539 |
| SfuB1 | Fe3+ transport | BAB2_0538 |
| SfuC1 | Fe3+ transport | BAB2_0540 |
| SfuA2 | Fe3+ transport | BAB2_0519 |
| SfuB2 | Fe3+ transport | BAB2_0520 |
| SfuC2 | Fe3+ transport | BAB2_0521 |
| BfeA | Fe2+ transport | BAB2_0840 |
| BfeB | Fe2+ transport | BAB2_0839 |
| BfeC | Fe2+ transport | BAB2_0838 |
| BfeD | Fe2+ transport | BAB2_0837 |
| MntH | Mn2+ transport | BAB1_1460 |
| ZnuA | Zn2+ transport | BAB2_1079 |
| ZnuB | Zn2+ transport | BAB2_1081 |
| ZnuC | Zn2+ transport | BAB2_1080 |
| NikA | Ni2+ transport | BAB2_0433/0434a |
| NikB | Ni2+ transport | BAB2_0435 |
| NikC | Ni2+ transport | BAB2_0436 |
| NikD | Ni2+ transport | BAB2_0437 |
| NikE | Ni2+ transport | BAB2_0438 |
| NikK | Ni2+ transport | BAB1_1384 |
| NikL | Ni2+ transport | BAB1_1386 |
| NikM | Ni2+ transport | BAB1_1385 |
| NikO | Ni2+ transport | BAB1_1388 |
| NikQ | Ni2+ transport | BAB1_1387 |
| CbtA | Co2+ transport | BAB1_1329 |
| CbtB | Co2+ transport | BAB1_1330 |
| MgtB | Mg2+ transport | BAB2_0036 |
| MgtE | Mg2+ transport | BAB2_0360 |
| CorA | Mg2+ transport | BAB1_0583 |
| MgtC | Mg2+ transport (?)b | BAB2_0039 |
The region homologous to the nikA gene in other Brucella genome sequences is annotated as two adjacent pseudo-genes in the B. abortus 2308 genome sequence.
The precise role of the MgtC in magnesium transport in bacteria is unknown.
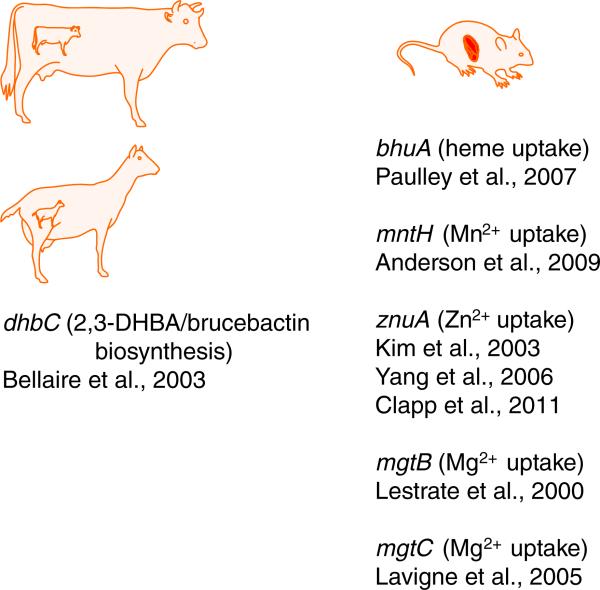
Iron, manganese, and magnesium are required for the optimal growth of Brucella strains in vitro (ZoBell and Meyer, 1932; McCullough et al., 1947; Sanders et al., 1953; Waring et al., 1953; Evenson and Gerhardt, 1955; Gerhardt, 1958), and phenotypic evaluations of defined mutants has shown that in addition to these three metals, efficient transport of zinc is also required for the virulence of these strains in experimentally infected animals (Fig. 3). The following sections will further describe the Brucella metal acquisition genes that have been experimentally linked to virulence.
Fig. 3.
Brucella genes experimentally linked to virulence in pregnant ruminants and mice. Virulence in pregnant ruminants is measured by bacterial colonization of the dam and fetus and fetal pathology (e.g. abortion or the birth of a weak kid or calf). Virulence in mice is measured by chronic colonization of the spleen.
Iron transport
Owing to its chemical versatility, iron serves as a co-factor for a wide range of proteins (Crichton, 2009). In fact, to the author's knowledge, bacteria in the genera Lactobacillus and Borrelia are the only organisms that have been documented to be able to live without this metal (Archibald, 1983; Posey and Gherardini, 2000). Presumably, a large and diverse group of Brucella proteins require iron for their activity. Some examples for which this requirement has been verified experimentally include catalase (Waring et al., 1953), aldolase (Gary et al., 1955), and CobG, an enzyme involved in cobalamin (vitamin B12) biosynthesis (Schroeder et al., 2009).
Siderophores
Siderophores are low molecular weight chelators that microbes release into their external environment to capture iron (Raymond and Dertz, 2004). Brucella strains produce two catechol siderophores when exposed to iron deprivation – 2,3-dihydroxybenzoic acid (2,3-DHBA) and the 2,3-DHBA-based molecule brucebactin (López-Goñi et al., 1992; González-Carreró et al., 2002). Owing to its instability in the laboratory, the precise structure of brucebactin is currently unknown. The biochemical features of the enzymes predicted to be encoded by the genes responsible for the production of these side-rophores, however, indicate that brucebactin is likely to be a monocatechol consisting of 2,3-DHBA linked to a polyamine or an amino acid (Bellaire et al., 2003a). Experimental evidence suggests that siderophore production plays a critical role in the virulence of Brucella strains in the gravid ruminant reproductive tract, but is not required for the persistence of these strains in host macrophages. A B. abortus dhbC mutant, which produces neither 2,3-DHBA nor brucebactin, for instance, does not cause abortion in pregnant goats (Bellaire et al., 2000) or cattle (Bellaire et al., 2003a) (Fig. 3). In contrast, this mutant and isogenic B. abortus mutants that produce 2,3-DHBA but cannot convert it to brucebactin display wild-type virulence in the mouse model of chronic infection (Bellaire et al., 1999; González-Carreró et al., 2002; Parent et al., 2002).
One possible explanation that has been put forth for the apparent differential requirement for siderophore production by B. abortus in the ruminant reproductive tract is linked to the capacity of this bacterium to utilize erythritol as its preferred carbon and energy source (Anderson and Smith, 1965; Meyer, 1967). Ruminant placental trophoblasts produce copious amounts of this four carbon sugar alcohol during the latter stages of pregnancy (Enright, 1990), and it has been proposed that the capacity of the brucellae to efficiently utilize this carbon source is linked to their virulence in pregnant ruminants (Smith et al., 1962). In vitro studies have shown that B. abortus 2308 displays a much greater need for iron when it is growing in the presence of erythritol than when it is growing with other readily utilizable carbon and energy sources (Bellaire et al., 2003b; Jain et al., 2011). Accordingly, it has been proposed that siderophore production plays an important role in supplying this strain with the iron it needs to fuel rapid and extensive bacterial replication in placental trophoblasts that leads to abortion (Bellaire et al., 2003b).
Not all B. abortus and B. melitensis strains produce catechol siderophores in response to iron deprivation in vitro (López-Goñi and Moriyón, 1995), and some of the siderophore biosynthesis genes in Brucella strains other than B. abortus 2308 are annotated as pseudo-genes in the genome sequences available in GenBank (Roop et al., 2011). Thus, it will be important to better define the link between siderophore production and erythritol metabolism in Brucella strains and perform definitive experiments to determine whether or not this link is responsible for the extreme attenuation displayed by the B. abortus dhbC mutant in pregnant ruminants. Likewise, it will also be important to determine whether or not siderophore production is required for the virulence of other Brucella strains in a variety of pregnant and non-pregnant natural hosts.
Utilization of heme as an iron source
Degradation of senescent and damaged erythrocytes and the recycling of the iron released from these cells is one of the major functions of mammalian macrophages (Bratosin et al., 1998). Ruminant placental trophoblasts also ingest maternal erythrocytes and degrade these cells to provide a source of iron to the developing fetus (Anderson et al., 1986). During both processes, a considerable amount of heme is released into these host phagocytes. Both B. abortus 2308 and B. melitensis 16M can use heme as an iron source in in vitro assays (Bellaire, 2001; Danese, 2001; Paulley et al., 2007). Heme transport is mediated by the TonB-dependent outer membrane protein, BhuA, and a periplasmic binding protein-dependent ABC-type transporter comprised of the proteins BhuT, U and V (Fig. 1), and the genes encoding these proteins appear to be well-conserved among Brucella strains (Roop et al., 2011). Brucella strains also possess a heme oxygenase (Puri and O'Brian, 2008). Presumably, this enzyme, which we have given the designation BhuO (Roop et al., 2011), allows the brucellae to use heme as an iron source by degrading the heme once it has been transported into the cytoplasm (Fig. 1). An isogenic bhuA mutant constructed from B. abortus 2308 displays significant attenuation in experimentally infected mice (Paulley et al., 2007) (Fig. 3), suggesting that the capacity to transport heme represents a critical virulence determinant. Whether or not heme utilization plays an important role in the virulence of other Brucella strains, or in natural hosts, remains to be experimentally determined.
Due to its potential toxicity, the heme that is not incorporated into cellular proteins in mammalian cells is actively routed to the endoplasmic reticulum (ER) where it can be degraded by heme oxygenase (Taketani, 2005). Cell biology studies have shown that the membrane-bound vacuoles within which the brucellae replicate in host macrophages (known as the replicative Brucella-containing vacuoles or rBCVs) are derived through extensive interactions of phagosomes with the host cell ER (Celli et al., 2003). The rBCVs initially interact with the ER exit sites, and eventually fuse with the ER (Celli et al., 2005). Extensive interactions of rBCVs with the host cell ER have also been observed microscopically in experimentally infected HeLa and Vero cells (Detilleux et al., 1990; Pizzaro-Cerdá et al., 1998) and in placental trophoblasts from experimentally infected ruminants (Anderson et al., 1986). Consequently, to gain a better understanding of the host–pathogen interactions in brucellosis, it will also be important to determine how the interactions of the rBCVs with the host cell ER influence the availability of heme as an iron source for Brucella strains during their intracellular residence in macrophages and placental trophoblasts.
Manganese transport
The ABC-type transporters exemplified by the SitABCD transporter of Salmonella and the proton-symporters of the MntH family are the most common types of manganese transporters that have been described in prokaryotes (Papp-Wallace and Maguire, 2006). Many bacteria possess both of these types of manganese transporters, but an analysis of the currently available Brucella genome sequences and phenotypic analysis of a B. abortus mntH mutant suggest that Brucella strains utilize MntH (Fig. 2) as their sole high affinity Mn2+ transporter (Anderson et al., 2009). A B. abortus mntH mutant is extremely attenuated in the mouse model of chronic infection (Fig. 3). The basis for this attenuation is presently unknown. The B. abortus mntH mutant possesses reduced Mn superoxide dismutase activity compared to the parental strain, but an isogenic sodA mutant exhibits only modest attenuation in mice (Martin et al., 2012), indicating that reduced SodA activity is not the basis for the severe attenuation exhibited by the mntH mutant. The B. abortus mntH mutant also exhibits aberrant expression of the genes encoding the Type IV secretion machinery (Anderson et al., 2009), and although the relationship between Mn2+ transport and virB expression has not been investigated, one plausible explanation for this relationship is that orthologs of the (p)ppGpp synthetase/hydrolase known as Rsh (Dozot et al., 2006), which is required for virB expression as well as induction of the stringent response in Brucella, are manganese-dependent enzymes (Papp-Wallace and Maguire, 2006).
Escherichia coli exhibits increased mntH expression in response to exposure to H2O2 (Anjem et al., 2009), and recent genetic and biochemical studies indicate that by elevating the intracellular ratio of Mn2+:Fe2+ this bacterium can substitute Mn2+ for Fe2+ in key metabolic enzymes such as ribulose-5-phosphate epimerase (Rpe), a major enzyme in the pentose-phosphate pathway (Sobota and Imlay, 2011). Unlike Fe2+, Mn2+ does not participate in Fenton chemistry, hence this substitution protects Rpe from H2O2-mediated damage. Exposure of B. abortus 2308 to H2O2 in vitro also results in increased mntH expression (E. Menscher, unpublished results), and an isogenic mntH mutant displays an increased sensitivity to exposure to H2O2 in in vitro assays compared to the parental 2308 strain (Anderson et al., 2009). Consequently, it will be important to determine whether Brucella strains have the same capacity to substitute Mn2+ for Fe2+ in metabolic enzymes as a mechanism for protecting these proteins from H2O2 mediated damage as has been demonstrated in E. coli.
Zinc transport
Zinc functions as a structural or enzymatic co-factor for a wide array of bacterial enzymes (Andreini et al., 2006). The Cu/Zn superoxide dismutase SodC represents an important virulence determinant for B. abortus 2308 (Tatum et al., 1992; Gee et al., 2005), and the Brucella carbonic anhydrases I and II and histidinol dehydrogenase are zinc-dependent enzymes that have been proposed to be good targets for the development of antimicrobials (Lopez et al., 2012). Two separate groups have independently shown that the znuA gene is essential for the wild-type virulence of B. abortus and B. melitensis strains in experimentally infected mice (Kim et al., 2004, Yang et al., 2006; Clapp et al., 2011) (Fig. 3). This gene encodes the periplasmic metal-binding component of an ABC-type high affinity zinc transporter, with ZnuB and ZnuC being the cytoplasmic permease and ATPase components of this transporter, respectively, (Fig. 2).
Magnesium transport
Magnesium is present in bacterial cells at high (i.e., mM) concentrations. It plays an important role in maintaining the structural integrity of ribosomes and cell membranes, and serves as a structural and enzymatic co-factor for a variety of cellular proteins (Moomaw and Maguire, 2008). Erythritol kinase, the enzyme that catalyzes the first step in the catabolism of erythritol in Brucella strains, for instance, requires Mg2+ for its activity (Sperry and Robertson, 1975).
Homologs of two genes associated with magnesium transport in other bacteria have been genetically linked to virulence in Brucella strains. MgtB is a bacterial P-type ATPase (Fig. 2) and the activity of this protein as a magnesium transporter has been best described in Salmonella (Smith et al., 1993). A B. melitensis mgtB mutant was isolated during a screen of signature-tagged transposon mutants derived from B. melitensis 16M for attenuation in experimentally infected mice (Lestrate et al., 2000). Interestingly, this mutant did not exhibit a growth defect when cultured in magnesium limited medium. This suggests that similar to other bacteria, and as depicted in Fig. 2, the brucellae possess multiple transport systems for magnesium. Although the precise role of MgtC in magnesium transport has not been established (Günzel et al., 2006; Alix and Blanc-Potard, 2007), a B. suis mgtC mutant does not grow well in a magnesium-restricted medium and displays significant attenuation in the murine macrophage-like J774 cell line (Lavigne et al., 2005). More importantly, this attenuation can be partially alleviated by supplementation of the cell culture medium with MgCl2.
Nickel transport
Urease is one of the few bacterial proteins that have been shown to require nickel as a co-factor (Li and Zamble, 2009). This enzyme is essential for the virulence of B. abortus 2308 and B. suis 1330 in mice when these strains are introduced via the oral route, but not when they are administered via the peritoneal route (Bandara et al., 2007; Sangari et al., 2007). B. abortus and B. suis urease mutants also exhibit wild-type virulence in mammalian cell cultures. The proposed explanation for these findings is that urease assists the brucellae in resisting the very low pH they encounter during passage through the stomach and gastrointestinal tract after ingestion, but is not required for intracellular survival in eukaryotic cells. Two nickel transporters, NikABCDE and NikKMLQO (Fig. 2) have been identified in Brucella (Jubier-Maurin et al., 2001; Sangari et al., 2010), but the role that these transporters play in virulence is unresolved. Although nikA expression is upregulated in B. suis 1330 during the intracellular replication of this strain in J774 cells, an isogenic nikA mutant derived from this strain displays wild-type virulence in the human monocytic cell line THP-1 (Jubier-Maurin et al., 2001). In order to gain a better understanding of the requirement for nickel transport by Brucella strains in the host, it will be important to assess the virulence properties of Brucella strains lacking either the NikABCDE or NikKMLQO transporter, or both, in cultured macrophages and in mice infected via both the intraperitoneal and oral routes. A comparison of the phenotypes displayed by these mutants with those exhibited by isogenic urease mutants in these experimental models will also be important for determining if Brucella strains require nickel for the proper function of enzymes other than urease.
Metalloregulators and metal storage/detoxification proteins p
As mentioned previously in this review, proteins that directly participate in metal homeostasis are essential for preventing toxicity due to the over-accumulation of these important micronutrients. Three transcriptional regulators that control the expression of Brucella metal acquisition genes have been characterized – Irr (Martínez et al., 2005, 2006), DhbR (Anderson et al., 2008) and Mur (Menscher et al., 2012). Irr is an iron-responsive transcriptional regulator that controls iron acquisition and iron metabolism genes; DhbR is an AraC-type transcriptional regulator that activates the transcription of the siderophore biosynthesis genes in B. abortus 2308 in response to Fe3+-siderophore levels in the external environment; and Mur regulates the expression of the gene encoding the Mn2+ transporter MntH in response to cellular Mn2+ levels. Brucella strains also produce bacterioferritin (Bfr), a protein that stores and detoxifies intracellular iron (Denoel et al., 1995; Almirón and Ugalde, 2010). To date, only Irr and Bfr have been examined for their roles in virulence. A B. abortus irr mutant is attenuated in the mouse model (Anderson et al., 2011), but neither B. abortus nor B. melitensis bfr mutants exhibit attenuation in cultured human primary explant macrophages (Denoel et al., 1997), J774 or HeLa cells (Almirón and Ugalde, 2010), or experimentally infected mice (Denoel et al., 1997). The reader is pointed to a computational study described by Rodionov et al. (2006) and a recent book chapter (Roop et al., 2011) for a more comprehensive consideration of the Brucella genes involved in metal homeostasis.
Conclusion
It seems clear that Brucella strains are well equipped to deal with the metal deprivation they encounter in their mammalian hosts. However, the contributions of many of the metal transporters shown in Figs. 1 and 2 to virulence remain to be determined. Considering the conserved strategies the α-proteobacteria employ to establish and maintain chronic infections in their eukaryotic hosts (Batut et al., 2004), it will be particularly interesting to determine what role CbtAB-mediated Co2+ transport plays in the virulence of Brucella strains. Cobalt-containing enzymes play a critical role in the capacity of Sinorhizobium meliloti, a close phylogenetic relative of the brucellae, to maintain a symbiotic relationship with its eukaryotic plant host (Taga and Walker, 2010).
A final point that bears consideration is that the vast majority of the studies that have evaluated the contributions of Brucella metal acquisition to virulence have been performed in the mouse model of chronic infection, which is used as a measure of the ability of these strains to survive and replicate in host macrophages. But as the studies with B. abortus siderophore biosynthesis mutants well demonstrate (Bellaire et al., 1999, 2000, 2003a; González-Carreró et al., 2002; Parent et al., 2002), the results obtained with the mouse model may not always predict how a mutant will behave in the natural host, especially in pregnant ruminants. The sources of iron (e.g. Fe2+, Fe3+, and heme or heme-containing proteins) and other metals available and the metabolic requirements of the intracellular brucellae for these metals may differ depending upon whether or not these bacteria are residing in macrophages or placental trophoblasts, and pregnancy may have an impact on these differences. Consequently, it will be important in future studies to assess the importance of metal acquisition genes to virulence in a variety of pregnant and non-pregnant natural and experimental hosts.
Acknowledgments
Research on Brucella metal acquisition systems in the laboratory of R.M.R. was funded by grants from the National Institutes of Allergy and Infectious Diseases (AI-63516) and the United States Department of Agriculture's Competitive Research Grants Program (95-01995, 98-02620 and 02-02215). The author is extremely grateful to the individuals, present and past, who have worked on these projects.
References
- Alix E, Blanc-Potard JB. MgtC: a key player in intramacrophage survival. Trends in Microbiology. 2007;15:252–256. doi: 10.1016/j.tim.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Almirón M, Ugalde RA. Iron homeostasis in Brucella abortus: the role of bacterioferritin. Journal of Microbiology. 2010;48:668–673. doi: 10.1007/s12275-010-0145-3. [DOI] [PubMed] [Google Scholar]
- Anderson ES, Paulley JT, Gaines JM, Valderas MW, Martin DW, Menscher E, Brown TD, Burns CS, Roop RM., II The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infection and Immunity. 2009;77:3466–3474. doi: 10.1128/IAI.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ES, Paulley JT, Martinson DA, Gaines JM, Steele KH, Roop RM., II The iron-responsive regulator Irr is required for the wild-type expression of the gene encoding the heme transporter BhuA in Brucella abortus 2308. Journal of Bacteriology. 2011;193:5359–5364. doi: 10.1128/JB.00372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ES, Paulley JT, Roop RM., II The AraC-like transcriptional regulator DhbR is required for maximum expression of the 2,3-dihydroxybenzoic acid biosynthesis genes in Brucella abortus 2308 in response to iron deprivation. Journal of Bacteriology. 2008;190:1838–1842. doi: 10.1128/JB.01551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GJ, Vulpe CD. Mammalian iron transport. Cellular and Molecular Life Sciences. 2009;66:3241–3261. doi: 10.1007/s00018-009-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JD, Smith H. The metabolism of erythritol by Brucella abortus. Journal of General Microbiology. 1965;38:109–124. doi: 10.1099/00221287-38-1-109. [DOI] [PubMed] [Google Scholar]
- Anderson TD, Cheville NF, Meador VP. Pathogenesis of placentitis in the goat inoculated with Brucella abortus. II. Ultrastructural studies. Veterinary Pathology. 1986;23:227–239. doi: 10.1177/030098588602300302. [DOI] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. Journal of Proteome Research. 2006;5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Molecular Microbiology. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiology Letters. 1983;19:29–32. [Google Scholar]
- Ariza J, Bosilkovski M, Cascio A, Colmenero JD, Corbel MJ, Falagas ME, Memish ZA, Roushan MRH, Rubinstein E, Sipsas NV, Solera J, Young EJ, Pappas G. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Medicine. 2007;4:e317. doi: 10.1371/journal.pmed.0040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara AB, Contreras A, Contreras-Rodriguez A, Martins AM, Dobrean V, Poff-Reichow S, Rajasekaran P, Sriranganathan N, Schurig G, Boyle SM. Brucella suis urease encoded by ure1 but not ure2 is necessary for intestinal infection of mice. BMC Microbiology. 2007;7:57. doi: 10.1186/1471-2180-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batut J, Andersson SGE, O'Callaghan D. The evolution of chronic infection strategies in the α-proteobacteria. Nature Reviews Microbiology. 2004;2:933–945. doi: 10.1038/nrmicro1044. [DOI] [PubMed] [Google Scholar]
- Bellaire BH. Doctoral dissertation. Louisiana State University Health Sciences Center; Shreveport, Louisiana, USA: 2001. Production of the siderophore 2,3-dihydroxybenzoic acid by Brucella abortus is regulated independent of Fur and is required for virulence in cattle. [Google Scholar]
- Bellaire BH, Baldwin CL, Elzer PH, Roop RM., II The siderophore 2,3-dihydroxybenzoic acid contributes to the virulence of Brucella abortus in ruminants. Abstracts of the 100th General Meeting of the American Society for Microbiology. 2000:44. Abstract B-17. [Google Scholar]
- Bellaire BH, Elzer PH, Baldwin CL, Roop RM., II The siderophore 2,3-dihydroxybenzoic acid is not required for virulence of Brucella abortus in BALB/c mice. Infection and Immunity. 1999;67:2615–2618. doi: 10.1128/iai.67.5.2615-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaire BH, Elzer PH, Baldwin CL, Roop RM., II Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro. Infection and Immunity. 2003b;71:2927–2932. doi: 10.1128/IAI.71.5.2927-2932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaire BH, Elzer PH, Hagius S, Walker J, Baldwin CL, Roop RM., II Genetic organization and iron-responsive regulation of the Brucella abortus 2,3-dihydroxybenzoic acid biosynthesis operon, a cluster of genes required for wild-type virulence in pregnant cattle. Infection and Immunity. 2003a;71:1794–1803. doi: 10.1128/IAI.71.4.1794-1803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco JM. Epididymite contagieuse du belier ou infection à Brucella ovis. In: Lefevre PC, Blancou J and Chermette R (eds) Principales Maladies Infectieuses et Parasitaires du Bétail. Paris: Lavoiser. 2003:905–917. [Google Scholar]
- Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Amiesen JC, Aminoff D, Montreuil J. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. Biochimie. 1998;80:173–195. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- Byrd TF, Horwitz MA. Interferon gamma-activated human monocytes down-regulate transferrin receptors and inhibit intracellular multiplication of Legionella pneumophila by limiting the availability of iron. Journal of Clinical Investigation. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J, de Chastellier C, Franchini DM, Pizarro-Cerdá J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. Journal of Experimental Medicine. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J, Salcedo SP, Gorvel JP. Brucella coopts the small GTPase Sar1 for intracellular replication. Proceedings of the National Academy of Sciences USA. 2005;102:1673–1678. doi: 10.1073/pnas.0406873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes and Infection. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Corbel MJ. Brucellosis: an overview. Emerging Infectious Diseases. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- Clapp B, Skyberg JA, Yang X, Thornburg T, Walters N, Pascual DW. Protective live oral brucellosis vaccines stimulate Th1 and Th17 cell responses. Infection and Immunity. 2011;79:4165–4174. doi: 10.1128/IAI.05080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton RR. Iron Metabolism – from Molecular Mechanisms to Clinical Consequences. 3rd edn. John Wiley & Sons; West Sussex, UK: 2009. [Google Scholar]
- Danese I. Doctoral dissertation. Facultes Universitaires Notre-Dame de la Paix; Namur: 2001. Contribution à l'étude de l'assimilation du fer chez Brucella melitensis 16M. [Google Scholar]
- Dawson CE, Stubblefield EJ, Perrett LL, King AC, Whatmore AM, Bashiruddin JB, Stack JA, MacMillan AP. Phenotypic and molecular characterization of Brucella isolates from marine mammals. BMC Microbiology. 2008;8:224. doi: 10.1186/1471-2180-8-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoel PA, Crawford RM, Zygmunt MS, Tibor A, Weynants VE, Godfroid F, Hoover DL, Letesson JJ. Survival of a bacterioferritin deletion mutant of Brucella melitensis 16M in human monocyte-derived macrophages. Infection and Immunity. 1997;65:4337–4340. doi: 10.1128/iai.65.10.4337-4340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoel PA, Zygmunt MS, Weynants V, Tibor A, Lichtfouse B, Briffeuil P, Limet JN, Letesson JJ. Cloning and sequencing of the bacterioferritin gene of Brucella melitensis 16M strain. FEBS Letters. 1995;361:238–242. doi: 10.1016/0014-5793(95)00189-g. [DOI] [PubMed] [Google Scholar]
- Detilleux PG, Deyoe BL, Cheville NF. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Veterinary Pathology. 1990;27:317–328. doi: 10.1177/030098589002700503. [DOI] [PubMed] [Google Scholar]
- Dozot M, Boigegrain RA, Delrue RM, Hallez R, Ouahrani-Bettache S, Danese I, Letesson JJ, De Bolle X, Köhler S. The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cellular Microbiology. 2006;8:1791–1802. doi: 10.1111/j.1462-5822.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- Enright FM. The pathogenesis and pathobiology of Brucella infections in domestic animals. In: Nielsen KH, Duncan JR, editors. Animal Brucellosis. CRC Press; Boca Raton, FL: 1990. pp. 301–320. [Google Scholar]
- Evenson MA, Gerhardt P. Nutrition of brucellae: utilization of iron, magnesium and manganese for growth. Proceedings of the Society for Experimental Biology and Medicine. 1955;89:678–680. doi: 10.3181/00379727-89-21914. [DOI] [PubMed] [Google Scholar]
- Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, Pavlin JA, Christopher GW, Eitzen EM. Clinical recognition and management of patients exposed to biological warfare agents. Journal of the American Medical Association. 1997;278:399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- Gary ND, Kupferberg LL, Graf LH. Demonstration of an iron-activated aldolase in sonic extracts of Brucella suis. Journal of Bacteriology. 1955;69:478–479. doi: 10.1128/jb.69.4.478-479.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Valderas MW, Kovach ME, Grippe VL, Robertson GT, Ng W-L, Richardson JM, Winkler ME, Roop RM., II The Brucella abortus Cu,Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infection and Immunity. 2005;73:2873–2880. doi: 10.1128/IAI.73.5.2873-2880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P. The nutrition of brucellae. Bacteriological Reviews. 1958;22:81–98. doi: 10.1128/br.22.2.81-98.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carreró MI, Sangari FJ, Agüero J, García-Lobo JM. Brucella abortus 2308 produces brucebactin, a highly efficient catecholic siderophore. Microbiology. 2002;148:353–360. doi: 10.1099/00221287-148-2-353. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Iron in biological systems. In: Bullen JJ, Griffiths E, editors. Iron and Infection. Molecular, Physiological and Clinical Aspects. 2nd edn. John Wiley & Sons; New York: 1999. pp. 1–25. [Google Scholar]
- Günzel D, Kucharski LM, Kehres DG, Romero MF, Maguire ME. The MgtC virulence factor of Salmonella enterica serovar Typhimurium activates Na+,K+-ATPase. Journal of Bacteriology. 2006;188:5586–5594. doi: 10.1128/JB.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Rodriquez AC, Kimsawatde G, Seleem MN, Boyle SM, Sriranganathan N. Effect of entF deletion on iron acquisition and erythritol metabolism by Brucella abortus 2308. FEMS Microbiology Letters. 2011;316:1–6. doi: 10.1111/j.1574-6968.2010.02186.x. [DOI] [PubMed] [Google Scholar]
- Jubier-Maurin V, Rodrique A, Ouahrani-Bettache S, Layssac M, Mandrand-Berthelos MA, Köhler S, Liautard JP. Identification of the nik gene cluster of Brucella suis: regulation and contribution to urease activity. Journal of Bacteriology. 2001;183:426–434. doi: 10.1128/JB.183.2.426-434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Current Opinion in Chemical Biology. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Watanabe K, Shirahata T, Watarai M. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. Journal of Veterinary Medical Science. 2004;66:1059–1063. doi: 10.1292/jvms.66.1059. [DOI] [PubMed] [Google Scholar]
- Lavigne JP, O'Callaghan D, Blanc-Potard AB. Requirement of MgtC for Brucella suis intramacrophagic growth: a potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infection and Immunity. 2005;73:3160–3163. doi: 10.1128/IAI.73.5.3160-3163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestrate P, Delrue RM, Danese I, Didembourg C, Taminiau B, Mertens P, De Bolle X, Tibor A, Tang CM, Letesson JJ. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Molecular Micro-biology. 2000;38:543–551. doi: 10.1046/j.1365-2958.2000.02150.x. [DOI] [PubMed] [Google Scholar]
- LeVier K, Phillips RW, Grippe VK, Roop RM, II, Walker GC. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science. 2000;287:2492–2493. doi: 10.1126/science.287.5462.2492. [DOI] [PubMed] [Google Scholar]
- Li Y, Zamble DR. Nickel homeostasis and nickel regulation: an overview. Chemical Reviews. 2009;109:4617–4643. doi: 10.1021/cr900010n. [DOI] [PubMed] [Google Scholar]
- Lopez M, Köhler S, Winum JY. Zinc metalloenzymes as new targets against the bacterial pathogen Brucella. Journal of Inorganic Biochemistry. 2012 doi: 10.1016/j.jinorgbio.2011.10.019. (in press) [DOI] [PubMed] [Google Scholar]
- López-Goñi I, Moriyón I. Production of 2,3-dihydroxybenzoic acid by Brucella species. Current Micro-biology. 1995;31:291–293. [Google Scholar]
- López-Goñi I, Moriyón I, Neilands JB. Identification of 2,3-dihydrobenzoic acid as a Brucella abortus siderophore. Infection and Immunity. 1992;60:4496–4503. doi: 10.1128/iai.60.11.4496-4503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero NE, Corazza R, Almuzara MN, Reynes E, Escobar GI, Boeri E, Ayala SM. Human Brucella canis outbreak linked to infection in dogs. Epidemiology and Infection. 2010;138:280–285. doi: 10.1017/S0950268809990525. [DOI] [PubMed] [Google Scholar]
- Martin DW, Baumgartner JE, Gee JM, Anderson ES, Roop RM., II SodA is a major metabolic antioxidant in Brucella abortus 2308 that plays a significant, but limited, role in the virulence of this strain in the mouse model. Microbiology. 2012 2012 May 3; doi: 10.1099/mic.0.059584-0. published online doi:10.1099/mic.0.059584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez J, Ugalde RA, Almirón M. Dimeric Brucella abortus Irr protein controls its own expression and binds heme. Microbiology. 2005;151:3427–3433. doi: 10.1099/mic.0.28213-0. [DOI] [PubMed] [Google Scholar]
- Martínez J, Ugalde RA, Almirón M. Irr regulates brucebactin and 2,3-dihydroxybenzoic acid biosynthesis, and is implicated in the oxidative stress resistance and intracellular survival of Brucella abortus. Microbiology. 2006;152:2591–2598. doi: 10.1099/mic.0.28782-0. [DOI] [PubMed] [Google Scholar]
- McCullough WG, Mills RC, Herbst EJ, Roessler WG, Brewer CR. Studies on the nutritional requirements of Brucella suis. Journal of Bacteriology. 1947;53:5–15. [PMC free article] [PubMed] [Google Scholar]
- Menscher EA, Caswell CC, Anderson ES, Roop RM., II Mur regulates the gene encoding the manganese transporter MntH in Brucella abortus 2308. Journal of Bacteriology. 2012;194:561–566. doi: 10.1128/JB.05296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ME. Metabolic characterization of the genus Brucella. VI. Growth stimulation by i-erythritol compared with strain virulence for guinea pigs. Journal of Bacteriology. 1967;93:996–1000. doi: 10.1128/jb.93.3.996-1000.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moomaw AS, Maguire ME. The unique nature of Mg2+ channels. Physiology (Bethesda) 2008;23:275–285. doi: 10.1152/physiol.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, Stackenbrandt E, Dorsch M, Wolters J, Busch M, Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. Journal of Bacteriology. 1990;172:3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron – a metal at the host-pathogen interface. Cellular Microbiology. 2010;12:1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella Typhimurium. Cellular Microbiology. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Nicoletti P, Lenk RP, Popescu MC, Swenson CE. Efficacy of various treatment regimens, using liposomal streptomycin in cows with brucellosis. American Journal of Veterinary Research. 1989;50:1004–1007. [PubMed] [Google Scholar]
- Nymo IH, Tryland N, Godfroid J. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Veterinary Research. 2011;42:93. doi: 10.1186/1297-9716-42-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Molecular Micro-biology. 1999;33:2110–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D, Whatmore AM. Brucella genomics as we enter the multi-genomeera. Briefings in Functional Genomics. 2011;10:334–341. doi: 10.1093/bfgp/elr026. [DOI] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annual Review of Microbiology. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Pappas G, Panagopoulou P, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infectious Diseases. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- Parent MA, Bellaire BH, Murphy EA, Roop RM, II, Elzer PH, Baldwin CL. Brucella abortus siderophore 2,3-dihydroxybenzoic acid (2,3-DHBA) facilitates intracellular survival of the bacteria. Microbial Pathogenesis. 2002;32:239–248. doi: 10.1006/mpat.2002.0500. [DOI] [PubMed] [Google Scholar]
- Paulley JT, Anderson ES, Roop RM., II Brucella abortus requires the heme transporter BhuA for maintenance of chronic infection in BALB/c mice. Infection and Immunity. 2007;75:5248–5254. doi: 10.1128/IAI.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzaro-Cerdá J, Méresse S, Parton RG, van der Goot G, Sola-Landa A, López-Goñi I, Moreno E, Gorvel JP. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infection and Immunity. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- Puri S, O'Brian MR. The hmuQ and hmuD genes from Bradyrhizobium japonicum encode heme-degrading enzymes. Journal of Bacteriology. 2008;188:6476–6482. doi: 10.1128/JB.00737-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond KN, Dertz EA. Biochemical and physical properties of siderophores. In: Crosa JH, Mey AR, Payne SM, editors. Iron Transport in Bacteria. ASM Press; Washington: 2004. pp. 3–17. [Google Scholar]
- Rodionov DA, Gelfand MS, Todd JD, Curson ARJ, Johnston AWB. Computational reconstruction of iron-and manganese-responsive transcriptional networks in α-proteobacteria. PLoS Computational Biology. 2006;2:1568–1585. doi: 10.1371/journal.pcbi.0020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop RM, II, Anderson E, Ojeda J, Martinson D, Menscher E, Martin DW. Metal acquisition by Brucella strains. In: López-Goñi I, O'Callaghan D, editors. Brucella: Molecular Microbiology and Genetics. Caister Academic Press; Norfolk: 2011. pp. 179–199. [Google Scholar]
- Roop RM, II, Gaines JM, Anderson ES, Caswell CC, Martin DW. Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Medical Microbiology and Immunology. 2009;198:221–238. doi: 10.1007/s00430-009-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TH, Higuchi K, Brewer CR. Studies on the nutrition of Brucella melitensis. Journal of Bacteriology. 1953;66:294–299. doi: 10.1128/jb.66.3.294-299.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangari FJ, Cayón AM, Seoane A, García-Lobo JM. Brucella abortus ure2 region contains an acid-activated urea transporter and a nickel transport system. BMC Microbiology. 2010;10:107. doi: 10.1186/1471-2180-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangari FJ, Seoane A, Rodríguez MC, Agüero J, García-Lobo JM. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infection and Immunity. 2007;75:774–780. doi: 10.1128/IAI.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz HC, Hubalek Z, Sedláček I, Vergnaud G, Tomaso H, Al Dahouk S, Melzer F, Kämpfer P, Nuebauer H, Cloeckaert A, Marquart M, Zygmunt MS, Whatmore AM, Falsen E, Bahn P, Göllner C, Pfeffer M, Huber B, Busse HJ, Knöckler K. Brucella microti sp. nov., isolated from the common vole Microtus arvalis. International Journal of Systematic and Evolutionary Microbiology. 2008;58:375–382. doi: 10.1099/ijs.0.65356-0. [DOI] [PubMed] [Google Scholar]
- Scholz HC, Knöckler K, Göllner C, Bahn P, Vergnaud G, Tomaso H, Al Dahouk S, Kämpfer P, Cloeckaert A, Marquart M, Zygmunt MS, Whatmore AM, Pfeffer M, Huber B, Busse HJ, De BK. Brucella inopinata sp. nov., isolated from a breast implant infection. International Journal of Systematic and Evolutionary Microbiology. 2010;60:801–808. doi: 10.1099/ijs.0.011148-0. [DOI] [PubMed] [Google Scholar]
- Schroeder S, Lawrence AD, Biedendieck R, Rose RS, Deery E, Graham RM, McLean KJ, Munro AW, Rigby SE, Warren MJ. Demonstration that CobG, the monooxygenase associated with the ring contraction process of the aerobic cobalamin (vitamin B12) biosynthetic pathway, contains an Fe-S center and a mononuclear non-heme iron center. Journal of Biological Chemistry. 2009;284:4796–4805. doi: 10.1074/jbc.M807184200. [DOI] [PubMed] [Google Scholar]
- Sieira R, Comerci DJ, Sánchez DO, Ugalde RA. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular replication. Journal of Bacteriology. 2000;182:4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota J, Imlay JA. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide, but can be protected by manganese. Proceedings of the National Academy of Sciences USA. 2011;108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola-Landa A, Pizarro-Cerdá J, Grilló MJ, Moriyón I, Blasco JM, Gorvel JP, López-Goñi I. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Molecular Microbiology. 1998;29:125–138. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- Smith DL, Tao T, Maguire ME. Membrane topology of a P-type ATPase. The MgtB magnesium transport protein of Salmonella typhimurium. Journal of Biological Chemistry. 1993;268:22469–22479. [PubMed] [Google Scholar]
- Smith H, Williams AE, Pearce JH, Keppie J, Harris-Smith PW, Fitzgeorge RB, Witt K. Foetal erythritol: a cause of the localization of Brucella abortus in bovine contagious abortion. Nature. 1962;193:47–49. doi: 10.1038/193047a0. [DOI] [PubMed] [Google Scholar]
- Sohn AH, Probert WS, Glaser CA, Gupta N, Bollen AW, Wong JD, Grace EM, McDonald WC. Human neurobrucellosis with intracebral granuloma caused by a marine mammal Brucella spp. Emerging Infectious Diseases. 2003;9:485–488. doi: 10.3201/eid0904.020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JF, Robertson DC. Erythritol catabolism by Brucella abortus. Journal of Bacteriology. 1975;121:619–630. doi: 10.1128/jb.121.2.619-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoenner HG, Lackman DB. A new species of Brucella isolated from the desert wood rat, Neotoma lepida Thomas. American Journal of Veterinary Research. 1957;18:947–951. [PubMed] [Google Scholar]
- Summers AO. Damage control: defenses against toxic metals and metalloids. Current Opinion in Microbiology. 2009;12:138–144. doi: 10.1016/j.mib.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Taga ME, Walker GC. Sinorhizobium meliloti requires a cobalamin-dependent ribonucleotide reductase for symbiosis with its plant host. Molecular Plant-Microbe Interactions. 2010;23:1643–1654. doi: 10.1094/MPMI-07-10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S. Acquisition, mobilization and utilization of cellular iron and heme; endless findings and growing evidence of tight regulation. Tohoku Journal of Experimental Medicine. 2005;205:297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- Tatum FM, Detilleux PG, Sacks JM, Halling SM. Construction of Cu-Zn superoxide dismutase deletions mutants of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infection and Immunity. 1992;60:2863–2869. doi: 10.1128/iai.60.7.2863-2869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderas MW, Roop RM., II . Brucella and bioterrorism. In: Anderson B, Friedman H, Bendinelli M, editors. Microorganisms and Bioterrorism. Springer; New York: 2006. pp. 139–153. [Google Scholar]
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nature Reviews Microbiology. 2009;6:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- Wanke MM. Canine brucellosis. Animal Reproduction Science. 2004;82–83:195–207. doi: 10.1016/j.anireprosci.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Waring WS, Elberg SS, Schneider P, Green W. The role of iron in the biology of Brucella suis. I. Growth and nutrition. Journal of Bacteriology. 1953;66:82–91. doi: 10.1128/jb.66.1.82-91.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Acquisition of iron and other nutrients in vivo. In: Roth JA, Bolin CA, Brogden KA, Minion FC, Wannemuehler MJ, editors. Virulence Mechanisms of Bacterial Pathogens. 2nd edn. ASM Press; Washington: 1995. pp. 79–93. [Google Scholar]
- Weiss G. Modification of iron regulation by the inflammatory response. Best Practices in Research in Clinical Haematology. 2005;18:183–201. doi: 10.1016/j.beha.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Yang X, Becker T, Walters N, Pascual DW. Deletion of znuA virulence factor attenuates Brucella abortus and confers protection against wild-type challenge. Infection and Immunity. 2006;74:3874–3879. doi: 10.1128/IAI.01957-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infection and Immunity. 2004;72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZoBell CE, Meyer KF. Metabolism studies on the Brucella group. VIII. Nutrient requirements in synthetic mediums. Journal of Infectious Diseases. 1932;51:344–360. [Google Scholar]