Abstract
The origin of the MHC class I-presented peptides are thought to be primarily from newly synthesized but defective proteins, termed DRiPs. Most of the data supporting this concept come from studies where inhibitors of protein synthesis were found to rapidly block antigen presentation even when cells contained a pool of mature protein. However, these data only indirectly address the origin of presented peptides and in most studies the contribution of mature functional protein to the class I peptide pool has not been directly quantified. In this report we address the efficiency and contribution of mature protein by using a tetracycline-inducible system to express antigen that is conditionally stabilized upon ligand binding. This system circumvents the use of general inhibitors of protein synthesis to control antigen expression. Moreover, by controlling antigen stabilization, we could investigate whether the degradation of mature antigen contributed to antigen presentation at early and/or late time points. We show that mature protein is the major contributor of peptides presented on class I for two distinct antigenic constructs. Furthermore our data show that the protein synthesis inhibitors used previously to test the contribution of defective proteins actually block antigen presentation in ways that are independent from blocking antigen synthesis. These data suggest that for the constructs we have analyzed, mature functional protein rather than DRiPs are the predominant source of MHC class I presented-peptides
Keywords: DRiPs, antigen presentation, MHC
Introduction
MHC class I molecules display peptides predominantly derived from a cell's endogenously synthesized proteins. This allows CD8+ T cells to monitor the genes expressed in cells and detect ones that are mutated or of viral origin. A majority of these MHC class I-presented peptides are generated from the hydrolysis of endogenously expressed proteins by proteasomes (1). Since the proteasome is responsible for degrading the majority of endogenously synthesized proteins (2), it was originally thought that class I-presented peptides were generated as the proteome was catabolized by the ubiquitin-proteasome pathway (3).
Within the proteome, different proteins are turned over at different rates, resulting in half-lives that vary from minutes to days. It was suggested that if turnover was the source of presented peptides, then the immune system would be slow to detect and respond to stable proteins with long lifetimes. However, it was noted that soon after infection the immune system recognizes and responds very rapidly to many stable viral proteins (4). This led to the idea that there might be a mechanism for rapidly generating and presenting peptides from otherwise stable proteins (5).
It was hypothesized that rapid presentation of peptides from all antigens could occur if there were mistakes during protein synthesis that led to the rapid degradation of a fraction of all newly synthesized proteins. Indeed it was known that the proteasome rapidly hydrolyzes abnormal proteins, including ones that have abnormal sequence (e.g. from puromycin incorporation or from non-natural amino acids like canavanine), or those that are misfolded, mislocalized, or prematurely terminated (6). It was initially proposed that such mistakes would come from inherent defects in translation. Such products were called Defective Ribosomal Products (DRiPs) (5). Key to the DRiP hypothesis is the idea that these immature and defective proteins are rapidly degraded by the proteasome within minutes of their synthesis (7). Although presentation from defective nascent proteins have been documented, the frequency of these errors under natural conditions and their contribution to peptides presented on class I remain controversial (8, 9).
Much of the strongest evidence that DRiPs contribute to antigen presentation came from experiments evaluating the effect of terminating protein synthesis on the generation of presented peptides. Upon cessation of antigen synthesis it was observed that antigen presentation very rapidly stopped even if cells contained a large pool of previously synthesized mature antigen (7, 10–14). This seemed to indicate that presented peptides were coming from newly synthesized antigens rather than older mature protein. It was estimated that the half-life of the newly synthesized antigen contributing to antigen presentation was <5 minutes, and that this was independent of the stability of the mature protein. These results were interpreted to indicate that newly synthesized antigen, rather than the proteome, was the dominant source of presented peptides (7, 10, 11, 15).
Other findings, such as the observation that antigen presentation coincides with the appearance of the viral protein in cells (16), have been interpreted to support the DRiPs model. However, most of the data in support of DRiPs being the dominant source of presented peptides are indirect. Likewise, there is little data that directly quantifies the efficiency of presentation from newly synthesized versus old antigen or from DRiPs versus functional protein. To address these issues we have used an antigen expression system that allows for the control of antigen expression and turnover without affecting other cellular components. In addition, we address the effects of inhibitors of protein synthesis on presentation of peptides from mature protein. Our data demonstrate that mature protein is the primary and highly efficient source of peptides presented on class I molecules for the 2 constructs we have analyzed. Furthermore, we show that inhibitors of protein synthesis previously used to conclude that DRiPs contribute to class I presentation, block MHC class I presentation by interfering with steps beyond antigen synthesis.
Materials and Methods
Cell Lines and Constructs
The hamster cell line E36 and human cell line HeLa expressing the murine class I MHC, H-2 Kb were described previously (hereafter E36 Kb and HeLa Kb) (17, 18). Also used in the present study is the murine lymphoma EL4 which endogenously expresses H-2 Kb. For the copepod GFP (copGFP) containing construct, full-length, and codon optimized, copGFP was amplified from pMaxGFP (Lonza) (primers), and then fused with a mutant form of FK506-binding protein 12 (FKBP) (19) and expressed in the vector pPTuner (Clontech). The immunodominant class I epitope from ovalbumin, SIINFEKL (S8L) and terminal HA-tag were incorporated into primers at the C-terminal end of the fusion. The final product was then cloned into the lentiviral vector pTRIPz (Open Biosystems) following digestions with AgeI and MluI. FKBP was removed from the above vector to form ΔcopGFP by overlapping PCR. For the EGFP construct, fluorescent protein was amplified from the pEGFP N1 vector (Clontech) and the S8L epitope was incorporated into overlapping primers used to fuse FKBP to EGFP. A single HA-tag was placed downstream of EGFP and cloned into the viral vector pTRIPz. A cartoon illustrating these two antigen constructs is shown in Fig1A and Fig2A. Cells expressing antigen constructs cloned into pTRIPz were selected with puromycin following lentiviral transduction. E36 Kb cells transfected with plasmids (pcDNA 3.1) carrying FKBP-S8L-EGFP without the Tet-inducible promoter were selected with hygromycin. Clones were generated in E36 Kb and Hela Kb cells by limiting dilution or colony lifted and screened for GFP expression and 25-D1.16 staining (described below) following culture in 1μg/ml Dox (Sigma) and 5μM Shield (CheminPharma). Transduced EL4 cells were sorted by flow cytometry for both EGFP expression and for presentation of S8L in the context of Kb using the 25-D1.16 antibody.
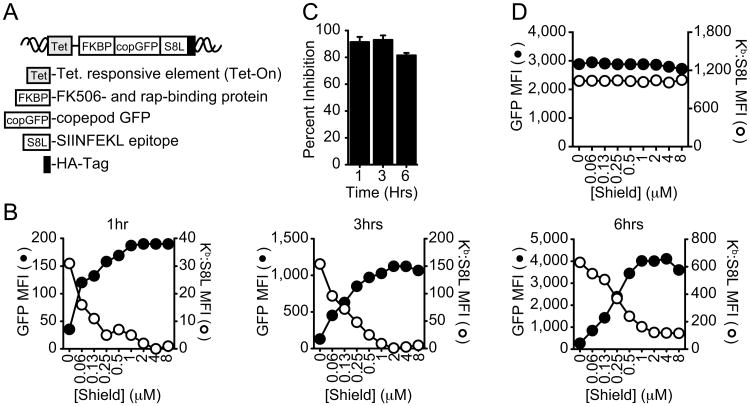
Figure 1. Loss of MHC class I antigen presentation upon antigen stabilization.
A, Illustration of the construct used to follow protein maturation and antigen presentation. B Kinetics of copGFP expression (black, GFP MFI) and SIINFEKL (S8L) presentation (white, Kb:S8L MFI) in E36 Kb cells at 1hr (left panel), 3hrs (middle panel) and 6hrs (right panel) following the induction of protein synthesis (Dox) and protein stabilization in the absence (0μM) or presence of increasing concentrations of Shield. Graphs shown are representative of at least 3 independent experiments. C, Quantitation of the percent antigen presentation inhibited after Dox induction in the presence of increasing concentrations of Shield at 1,3 and 6hrs after induction. Results shown are the mean ± SEM comparing untreated (Dox alone with 0.05% ethanol) to cells treated with 8μM Shield (n=3). D, Shield titration in E36 Kb cells expressing copGFP without FKBP (ΔcopGFP). GFP fluorescence (black) and Kb:S8L expression (white) are shown from a representative experiment (n>3).
MHC Class I Antigen Presentation
Antigen presentation was monitored on a single cell level using the monoclonal antibody specific for Kb:SL8 complexes (25-D1.16) (20). Total GFP expression was monitored by antibody targeting the C-terminally fused HA epitope (Cell Signaling) by western blot and by flow cytometry for folded GFP expression. Flow cytometry data was collected on an LSR2 instrument using FACS DIVA software (Beckton Dickinson) or on a FAC Calibur using CellQuest software (Beckton Dickinson). Data were analyzed from live cell gates using FlowJo software (Tree Star). Geometric mean fluorescence intensities (MFI) of each stain were expressed as MFI following subtraction of background staining from control treated cells, as indicated. Alternatively, we measured S8L presentation to RF33.70-Luc cells expressing firefly luciferase under the control of the NFAT promoter (21). Display of new MHC:peptide complexes was stopped by treating cells with 5μg/ml BFA (Sigma) or by fixation with 4% PFA (w/v in PBS), prior to presentation to the T cell hybridoma RF33.70-Luc.
To assess the effects of Shield on antigen presentation during antigen synthesis, E36 Kb and HeLa Kb cells expressing antigen were treated with 0.1μg/ml Dox in RPMI [containing 2mM L-glutamine (Gibco), 1× antibiotics (Gibco) and 10% (v/v) FCS] and various concentrations of Shield for 1-6hrs. Cells were washed and trypsinized before staining for Kb:S8L expression as stated above. EL4 cells induced to express antigen prior to the addition of Shield were first cultured in the presence of Dox alone over time, and then subjected to acid stripping (0.132M Citric acid, 0.06M sodium phosphate, pH 3.0) followed by culture in the presence of 0.5μg/ml Dox and Shield, as described above. All staining was performed on ice to prevent further protein synthesis and antigen presentation. Data were analyzed as described above and plotted in Prism (GraphPad software). Percent inhibition of antigen presentation was determined by comparing 25-D1.16 MFI expressed in cells treated with Dox alone to those treated with 8-16μM Shield, as indicated.
For class I presentation of mature protein in the absence of protein synthesis, GFP expression in E36 Kb cells were induced with 0.1μg/ml Dox and 5μM Shield in RPMI for 24hrs. Cells were then washed with cold RPMI and then exposed to 5μM Shield alone (in the absence of Dox to stop new antigen synthesis) for an additional 24hrs. Cells were then subject to acid strip to remove preformed surface Kb:S8L complexes, as described above. Cells were further cultured in RPMI containing either 5μM Shield alone, 10μM MG132 (Enzo) alone or containing the carrier controls 0.02% Ethanol and 0.1% DMSO. Cells were analyzed for GFP expression and presentation of S8L (25-D1.16) as described above.
Efficiency of Class I Presentation
To determine the efficiency of antigen presentation from old protein compared to newly synthesized protein, E36 Kb cells expressing copGFP were cultured in the presence on 0.1μg/ml Dox, 5μM Shield and 10μM MG132 over time. The time required to generate equivalent copGFP protein (in MFI) during synthesis (in the presence of Dox) as compared to an old cohort of protein that had been stabilized with Shield in the absence of synthesis (in the absence of Dox) was noted. In parallel, we followed the generation of Kb:S8L complexes from newly synthesized protein by culturing cells in 0.1μg/ml Dox alone over time. Next, we quantified Kb:S8L complexes generated during antigen synthesis at the times where equivalent copGFP was expressed, as described above. We added the time required for newly formed Kb:S8L complexes to transit from the ER to the cell surface (30min) (Fig S2B). Antigen presentation in the absence of synthesis (from old protein) was compared to antigen presented during synthesis (Kb:S8L MFI) and was expressed as percent efficiency of class I presentation from mature protein.
An alternative way of measuring the presentation efficiency from newly synthesized protein compared to the efficiency from the turnover of mature protein in the absence of synthesis was as follows. E36 Kb cells expressing copGFP were induced with Dox (50, 100, or 150 ng/mL) in the presence or absence of 1μM Shield. Induction was started at 20min intervals by mixing acid-stripped uninduced cells kept on ice with media containing the drugs mentioned above. Cells were harvested after induction times of 180, 200, 220, 240, 260, 280, and 300min. Thus the time range covered was 120min. This short induction experiment allowed us to calculate the efficiency of presentation from rapidly degraded de novo synthesized protein over a 2hr period. The samples with Shield were used to calculate the amount of protein synthesized and degraded (GFP MFI), whereas the samples without Shield provided the corresponding S8L presentation levels (25-D1.16 MFI). The efficiency of presentation was assessed by plotting S8L presentation vs. GFP fluorescence for all time points and data points were fit by linear regression analysis using Prism (GraphPad). For mature protein in the absence of synthesis, antigen was induced and stabilized for 24hr and then cultured in Shield alone for an additional 24hrs as mentioned above. After this, cells were acid-stripped and kept on ice. Every 20min cells were transferred to media containing a low concentration of Shield (0.1μM) to allow for the linear degradation of the protein pool previously built up. The degradation times were 60, 80, 100, 120, 140, 160, and 180min, covering a range of 2hrs as in the short induction experiment described above. All cells were stained with 25-D1.16 to assess presentation of S8L. Cells were fixed in 4% PFA for 5min, washed with PBS and then analyzed in a FACS Calibur flow cytometer (BD biosciences). Using FlowJo software, GFP positive cells were gated and their geometrical MFI for GFP and 25-D1.16 were obtained. Linear regression lines were fitted using Prism. The plots comparing the presentation efficiencies from these experiments are shown in Fig S2C-F.
Loading of Exogenous Antigen
E36 Kb and EL4 cells were pretreated for 1hr with either 20μM (11.1 μg/ml) emetine (Calbiochem), 40μM (11.4μg/ml) CHX (Sigma), or a control solution (0.1% DMSO). Ovalbumin (Worthington Scientific) was introduced into E36 Kb and EL4 cells by hypertonic loading followed by osmotic lysis in hypotonic media (22). Alternatively, in this same experimental design, 2μg ovalbumin was loaded into cells using the PULsin protein delivery reagent (Polyplus Transfection Inc) in accordance to manufacturer instructions, in place of hypertonic loading. Following antigen loading, cells were then incubated for 90min in the presence or absence of the inhibitors described above to allow for antigen processing and presentation. Cell viability was assessed by trypan blue exclusion and was found to not be impacted by the treatment conditions described above. Cells were then washed extensively to remove inhibitors and treated with 5μg/ml BFA to prevent the egress of new peptide loaded class I molecules out of the ER. Protein synthesis inhibitor treatment never exceeded 2.5hrs in the experiments listed above.
Antigen presentation of exogenous peptides was performed as described previously (21). Briefly, E36 Kb and EL4 cells were pretreated for 1hr using the same inhibitor treatment listed above. Preformed class I was then removed by acid stripping or left untreated. Cells were then treated with inhibitors for an additional 90min followed by washing in cold PBS and fixation in 4% PFA. Fixed cells were left alone or exposed to increasing concentrations of S8L peptide, as indicated, for 30min at RT followed by extensive washing to remove unbound peptide.
Antigen presentation was detected by co-culturing antigen presenting cells overnight with the T cell hybridoma RF33-Luc, as described. Luciferase activity was determined using the Luciferase Assay System (Promega) as directed and read on a FLUOstar Optima plate reader (BMG Labtech). The efficiency of antigen loading was assessed by immunoblotting E36 Kb cell lysates for intracellular ovalbumin. For hypertonic loading, mock loaded cells were subject to the same conditions in the absence of antigen. As a control, cells were also cultured with ovalbumin in normal media. For PULsin treated cells, control samples were treated in the presence of ovalbumin without the PULsin reagent. None of the control conditions resulted in luciferase activity above background.
Real-Time PCR
E36 Kb cells expressing copGFP were cultured for 24hrs in the presence or absence of 0.1μg/ml Dox and 5μM Shield. Cells were then washed in PBS and cultured in the absence of Dox over time. RNA was harvested using the Qiagen RNeasy kit (Qiagen) with DNAse I (Qiagen). cDNA was then synthesized using the iScript cDNA synthesis kit (BioRad). Real-Time PCR was performed with the iQ SYBR Green Supermix (BioRad) containing 25ng of cDNA from each sample using the iCycler from BioRad. Specific primers used include; cop-GFP (TGATGGGCTACGGCTTCTAC and CCTCGTAGCGGTAGCTGAAG), HPRT (ACCAGTCAACAGGGGACATAAAAG and GTCTGCATTGTTTTGCCAGTGTC) and 18SRNA (CAGCCACCCGAGATTGAGCA and TAGTAGCGACGGGCGGTGTG). Samples were normalized to either 18S RNA or HPRT expression at each time point. Fold expression was determined by comparing Dox-induced samples to samples cultured without Dox.
Western blot
Samples were washed in PBS and lysed in cold RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% (v/v) Triton x-100, 1% (w/v) Sodium deoxycholate, 0.1 (w/v) % SDS, pH 7.4) with complete protease inhibitor cocktail (Roche). Quantitation of total protein was performed by bicinchoninic acid protein assay (Pierce). Proteins were separated on 10% or 12% NU-PAGE gels before transfer. Membranes were probed for expression of copGFP (anti-HA, Cell Signaling), or ovalbumin (MP Biomedicals). GAPDH antibodies (Millipore) were probed on the same membranes as the antigen to confirm equivalent total protein loaded.
Results
Inhibition of antigen presentation by ligand-induced protein stabilization
In order to address the contribution of mature proteins to class I presentation, we developed an experimental system which allowed us to; 1) selectively and reversibly control antigen synthesis without affecting synthesis of other cellular proteins, 2) measure the fraction of mature protein and its turnover, 3) quantitatively distinguish presentation from defective protein versus mature functional protein, and 4) to determine the efficiency of presentation from antigens at various times after synthesis. Our approach took advantage of a mutant form of the FK506 binding protein-12 (denoted FKBP hereafter) that was rapidly degraded by the proteasome even when expressed as a fusion protein. Importantly, rapid degradation of the mature FKBP fusion could be blocked by the addition of a fully reversible cell-permeable ligand, called Shield (19). In order to bind Shield these constructs must be properly folded and therefore by definition are not DRiPs (11, 19). Shield was shown to selectively stabilize FKBP fusions without affecting other cellular processes (19, 23, 24), and has been used by others to study antigen presentation (11, 12, 25). We fused FKBP to the copepod green fluorescent protein (copGFP). This allowed for us to follow mature protein by monitoring GFP fluorescence, a process requiring precise folding for the formation of the fluorophore (26). In order to monitor class I antigen presentation, we introduced the T cell epitope SIINFEKL (S8L) from ovalbumin at the C-terminus of the fusion protein followed by a terminal HA-tag that was used to monitor antigen expression regardless of protein conformation (Fig 1A). Finally, the presence of an inducible transcriptional activator (Tet) allowed for specific and reversible activation of antigen transcription in response to doxycycline (Dox) (Fig S1A).
We transduced E36 cells, expressing the murine class I molecule H-2 Kb, with lentivirus carrying the copGFP fusion construct (fusion protein referred to as copGFP hereafter) and isolated clones with Dox-inducible GFP expression. To assess the effects of protein stabilization on antigen presentation, we induced the expression of the copGFP fusion with Dox and titrated Shield for 1hr, a time when DRiPs might be expected to be a more dominant source of presented peptides. As shown in Fig 1B (left panel), increasing the concentration of Shield resulted in a concomitant increase in protein stabilization as shown by the increase in copGFP fluorescence (Fig 1B, black) and immunoreactive protein levels (Fig S1B). These data fit well with previous work demonstrating protein stabilization of FKBP fusions with Shield (19). Importantly, protein stabilization was accompanied by a dose-dependent inhibition of class I presentation, as assessed by staining cells with an antibody (25-D1.16) recognizing S8L bound to the murine class I molecule H-2 Kb (Fig 1B, white). We examined whether inhibition continued over 3hrs (Fig 1B, middle panel) and 6hrs (Fig 1B, right panel) following induction of protein synthesis. Multiple experiments revealed that the inhibition of class I presentation at 1hr was 90.3% (±5.2% SEM) and 93.0% (±3.3%SEM) at 3hrs, with slightly lower inhibition occurring at the later time points (Fig 1C).
Given the striking inhibition of class I presentation in the presence of Shield, we sought to confirm that Shield was specifically affecting our FKBP-copGFP fusion and not other processes required for antigen presentation. To do this we made an identical construct shown in Fig 1A, except lacking the FKBP domain (ΔcopGFP) and induced expression of this variant construct in the presence of increasing concentrations of Shield. As expected, addition of Shield had no effect on GFP fluorescence from this construct lacking the Shield-binding domain (Fig 1D, black). Likewise, antigen presentation was also unaffected by the addition of Shield (Fig 1D, white), indicating that the effects observed in Fig 1B were only due to the stabilization of FKBP with Shield and not a pleotropic effect of this agent. Consistent with this conclusion we also found that Dox and Shield did not induce cellular stress, as assayed by the induced splicing of the transcription factor XBP-1 (27) (Fig S1C), affect cell viability/metabolic activity, as measured by MTS assay (Fig S1F), or inhibit expression of class I molecules (Fig S1E). Our data confirmed what other groups have already shown concerning the target specificity and lack of cellular toxicity from these agents (19, 23, 24, 28). Together, these data show that when mature protein is selectively stabilized by Shield, antigen presentation is markedly inhibited demonstrating a predominant contribution of this pool of functional (ligand-binding) protein to class I presentation.
Source of protein and epitope location do not affect presentation from mature protein
The observation that mature protein is the primary source of MHC class I-presented peptides is at variance with an earlier study that used a very similar experimental approach. In this report, Dolan et al. showed that stabilizing mature protein in EL4 cells reduced antigen presentation by a much smaller degree (≤36%) (11). One difference in this earlier study was the use of EGFP rather than copGFP as the source of antigen. These are very different proteins with the copepod (Pontellina plumata) sequence having only 19% amino acid identity with the jellyfish (Aequorea victoria) GFP (29). Other differences include the incorporation of the peptide S8L at the opposite end of the fluorescent protein and, very different sequence flanking the epitope (Fig 2A). These differences raised the possibility that the contribution of DRiPs versus functional mature protein to class I presentation could vary based on the protein and/or epitope location. To test this possibility, we generated a new construct that was essentially identical to the one used by Dolan (Fig 2A, EGFP) (11). E36 Kb clones were isolated as described for the copGFP expressing cells. Surprisingly, we observed a similar effect of Shield on protein stabilization and antigen presentation that we observed for copGFP. As shown in Fig 2B, E36 Kb cells expressing EGFP displayed a pronounced increase in protein stabilization (black) and a corresponding inhibition of antigen presentation (white) with increasing concentrations of Shield (83.6 ±4.2% SEM). These data further demonstrate that the source of antigen and location of epitope does not influence the contribution of mature protein to class I presentation, at least for these two constructs.
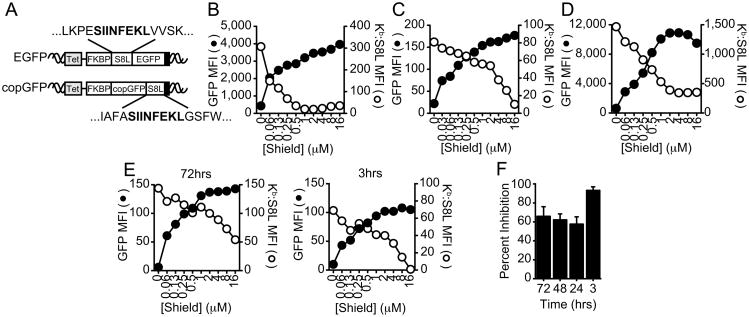
Figure 2. Effects of Shield are independent of the expressed protein, location of the epitope, or cell type expressing the antigen.
A, Illustration of constructs used to test whether the origin of protein or location of epitope alters class I presentation from mature protein. B, E36 Kb cells expressing EGFP were induced with 0.1μg/ml Dox and stabilized with increasing concentrations of Shield for 3hrs as described in Fig 1B. C, EL4 cells expressing EGFP were induced with 0.25μg/ml Dox for 3hrs or HeLa Kb cells expressing copGFP (D) were induced with 0.1μg/ml Dox for 3hrs with an increasing concentration of Shield. E, EL4 cells expressing EGFP were induced with 0.5μg/ml Dox alone over time. Preformed MHC class I molecules were removed by acid stripping, followed by culture with Dox and increasing concentrations of Shield for the final 3hrs. Shield titrations in cells pretreated for 72hrs with Dox (left panel) or without pretreatment (right panel) are shown. F, Statistics from 3 independent experiments performed at 3, 24, 48 and 72hrs, as described in E, are shown (mean ± SEM). GFP fluorescence (black) and S8L presentation (white) are shown in B-E from representative experiments (n≥3).
Next, we assessed whether cell type or species influenced the contribution of mature protein to antigen presentation in this experimental system. We transduced the murine lymphoma cell EL4 with an inducible construct expressing the EGFP fusion described previously (same cell and antigen as used by Dolan et al.) (11) and assessed whether increasing concentrations of Shield inhibited antigen presentation. Surprisingly, and in contrast to the previous report, antigen presentation of SIINFEKL in EL4 cells was strongly inhibited (92.2%±3.9% SEM) (Fig 2C). Similar results were observed in human cells (HeLa, 80.5%±3.2%) expressing murine class I MHC Kb and the antigen construct copGFP (Fig 2D). Therefore, for the constructs we have analyzed, mature protein makes a major contribution to antigen presentation in both human and rodent cells regardless of the protein source or location of the epitope.
Another difference between our experiments and those of Dolan et al. is that we added Shield at the same time that antigen synthesis was initiated while Dolan et al. added Shield just after acid stripping cells that had been continuously synthesizing antigen. To investigate whether continuous antigen expression for one to several days might account for the differences shown here and those reported previously, we induced antigen synthesis for up to 72hrs, then acid stripped the cells and added both Dox and Shield. Under these conditions we observed less Shield-induced inhibition of antigen presentation (Fig 2E), similar to the findings of Dolan et al. These data suggest that over time there is an accumulation of some antigen that cannot be stabilized by Shield. However, since this Shield-resistant component is not seen upon the initiation of synthesis but takes >6 hours to accumulate, it is by definition almost certainly not arising from DRiPs (Fig 2F).
Does old fully mature protein contribute to MHC class I presentation in the absence of new antigen synthesis?
Thus far, our data has shown that stabilization of a mature protein during protein synthesis (with Dox) results in the inhibition of class I antigen presentation. However, as stated above, previous experiments from several groups have shown that blocking antigen synthesis rapidly inhibits MHC class I antigen presentation despite the presence of a pool of previously synthesized antigen (7, 10–14). These data have been offered as support for the importance of DRiPs to antigen presentation and in any case have suggested that MHC class I-presented peptides come predominantly from newly synthesized antigen rather than older protein. Our experimental system allowed us to examine this proposition in the absence of protein synthesis inhibitors because we could stabilize a cohort of protein in the presence of Shield and then examine whether it could contribute to antigen presentation long after it was synthesized. To do this we induced and stabilized copGFP by culturing cells in the presence of Dox and Shield for 24hrs in order to build up a pool of fully mature protein. Next, we removed Dox to stop further synthesis of the antigen and continued to culture cells in the presence or absence of Shield. Fig 3A shows the dramatic loss of copGFP fluorescence following the removal of Dox and Shield (white), but not in the continued presence of Shield alone (black), indicating that Shield stabilized the mature protein for an extended period of time after synthesis was terminated. Although nearly all of the protein was lost within 6hrs (with a half-life of 1.2hrs) following the removal of Dox and Shield, remaining mRNA transcripts may further contribute to protein expression (30). Therefore, we measured the lifetime of copGFP mRNA following the removal of Dox by quantitative RT-PCR. Fig 3B shows that mRNA was degraded to background levels by 18hrs after Dox had been withdrawn, indicating that protein must be stabilized for at least 18hrs to have a cohort of fully mature protein without any newly synthesized antigen molecules.
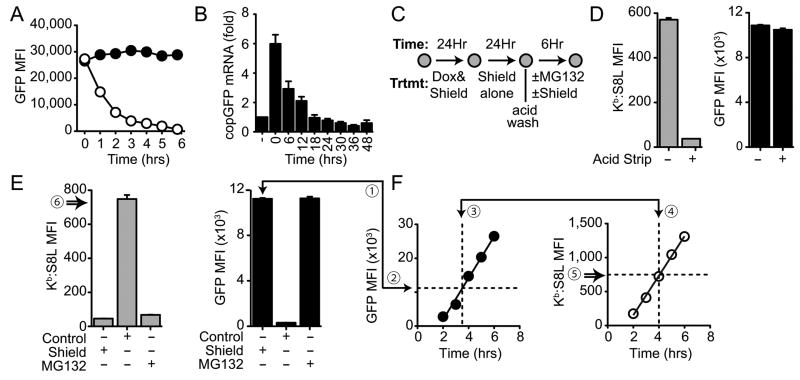
Figure 3. Antigen presentation from old protein is as efficient as newly synthesized protein.
A, E36 Kb cells expressing copGFP were cultured for 24hrs with 0.1μg/ml Dox and 5μM Shield. Dox and Shield were removed by cold PBS wash and then the culture continued in the presence of 5μM Shield alone (black) or in control media (0.02% ethanol) (white) for 6hrs. Cells were harvested at the given times to assess GFP fluorescence by flow cytometry. B, E36 Kb cells expressing copGFP were cultured as described in A. Cells were harvested at the given times and copGFP mRNA expression was assessed by QPCR, as described in the methods. Samples were normalized to 18S RNA or HPRT expression and graphed as fold over the untreated control (-). Values shown are means (±SEM) for 3 independent experiments. C, Experimental scheme used to analyze the role of mature protein contributing to class I presentation in the absence of antigen synthesis. D, Buildup of total fluorescence (GFP MFI, black) and antigen presentation (Kb:S8L MFI, gray) over 48hrs before (-) or after (+) the removal of preformed class I:peptide complexes by acid stripping. E, Class I presentation in the absence of synthesis. Cells were cultured for an additional 6hrs following acid strip in the presence of 5μM Shield alone (Shield), 10μM MG132 alone (MG132) or 0.1% DMSO and 0.1% ethanol control (Control). Changes in GFP fluorescence (GFP MFI, black) and class I presentation (Kb:S8L MFI, gray) from mature protein are shown as the mean of triplicates (±SEM) from one of 3 independent experiments. F, Efficiency of antigen presentation from mature protein. The kinetics of new protein expression was determine by culturing E36 Kb cells in the presence of 0.1μg/ml Dox, 5μM Shield and 10μM MG132 (F, left). The kinetics of presentation was determined by culturing cells in 0.1μg/ml Dox alone (F, right) over 6hrs. Protein stabilized in the absence of protein synthesis (E ①) was compared to new protein generated during protein synthesis (F ②). The time required to generate equivalent levels of protein (F ③) (plus 30 min for transport of complexes from the ER (F ④) was used to determine the amount of Kb:S8L complexes formed in this time (F ⑤). Efficiency of presentation from mature protein was calculated by dividing Kb:S8L complexes (MFI) formed from old protein (E ⑥) in the absence of synthesis by those formed during synthesis (F ⑤). Data shown are one representative experiment from three. Refer to Table 1 for the remaining experimental data.
With this information, we designed experiments aimed at determining the ability of an old cohort of mature protein to contribute to MHC class I presentation (Fig 3C). E36 Kb cells were induced to express copGFP and to stabilize its expression with Shield for 24hrs. Next, Dox was removed to selectively stop transcription of the antigen construct and the culture continued in the presence of Shield to allow the Dox-induced mRNA to degrade while the “old” mature protein was stabilized for an additional 24hrs. In order to exclusively address the role of the mature protein in antigen presentation, we first removed pre-formed surface class I-peptide complexes that were generated during the initial buildup and stabilization of antigen. This treatment had no effect on stabilized GFP expression (Fig 3D, right panel), but was efficient at removing the preformed complexes (Fig 3D, left panel). Next, we either continued to stabilize protein by culturing cells in Shield alone (Shield), withdrew Shield and allowed the antigen to degrade (DMSO), or cultured cells in the presence of proteasomal inhibitor (MG132) to inhibit antigen degradation by the proteasome for an additional 6hrs. As shown in Fig 3E, treatment with Shield alone continued to stabilize mature protein (black, right panel). Under these conditions the stabilized mature protein did not contribute to class I presentation (compare Fig 3D with Fig 3E, gray), as expected. In contrast, when Shield was removed and replaced with control media (DMSO), the antigen was degraded and Kb:S8L complexes were rapidly generated (Fig 3E, left panel and Fig S2A). As predicted, the dramatic loss of copGFP fluorescence occurred due to proteasomal degradation, because in the presence of MG132 protein turnover and presentation of the antigen was completely inhibited (Fig 3E, MG132). Overall these data show that long after new antigen synthesis (24hrs following Dox removal), mature protein can contribute peptides to class I presentation.
Presentation of mature protein is a highly efficient source of antigenic peptides
We next sought to determine whether there was any difference in the efficiency of generating presented peptides from newly synthesized protein versus older mature protein in the absence of protein synthesis, as shown in Fig 3E. To do this we first followed new copGFP expression over time by measuring fluorescence in the presence of Dox, Shield and MG132 (to prevent degradation) (Fig 3F, left panel). In parallel to the kinetics of protein expression, we measured the amount of Kb:S8L complexes generated from the newly synthesized protein when they were allowed to degrade in the absence of Shield (Fig 3F, right panel). As described in detail in the materials and methods, the time required to achieve equivalent protein from the newly synthesized protein compared to the old cohort of antigen was used to determine the efficiency of class I presentation from these pools of proteins. Our data demonstrates that class I presentation from antigen processed and presented during synthesis (Fig 3F, 758 Kb:S8L MFI) was as efficient as presentation from old mature antigen in the absence of synthesis (Fig 3E, 749 Kb:S8L MFI). The data presented in Supplemental Table 1 details the efficiency of antigen presentation from old mature protein compared to newly synthesized protein (97.5 ± 2.9% SEM, n=3).
We also assessed the efficiency of antigen presentation from old and newly synthesized proteins by comparing the rate at which Kb:S8L complexes appeared on the cell surface as the copGFP protein was degraded over a 2hr period (see material and methods for full details). The results clearly show the presentation efficiencies are essentially no different, indicating that mature protein is as efficient of a substrate for class I presentation as rapidly degraded, newly synthesized protein for the antigens tested here (Fig S2E and Fig S2F).
Antigen independent effects of protein synthesis inhibitors on antigen presentation
Our data are at variance with previous reports suggesting that newly synthesized defective proteins are the main contributor to class I presentation (7, 10, 11, 15), and that the contribution of mature protein to class I presentation is inefficient (11, 13). Many reports supporting the role of DRiPs as a primary source of class I-presented peptides have relied on protein synthesis inhibitors to form these conclusions (7, 10–14). In these reports it was found that inhibitors of protein synthesis quickly blocked antigen presentation despite the presence of a pool of mature antigen in the cell, implying that presented peptides only came from newly synthesized protein rather than old mature protein. However, this conclusion assumed that blocking protein synthesis did not inhibit other cellular processes necessary for antigen presentation apart from antigen synthesis. Therefore we examined whether inhibiting protein synthesis had any effect on antigen presentation in situations where the antigen was pre-existing (mature) and did not require continued synthesis. We reasoned that if these inhibitors are only affecting antigen synthesis, as previously believed, then they should not block class I presentation from fully mature protein, as shown in Fig 3E.
To test this, we subjected E36 Kb cells to increasing amounts of the irreversible inhibitor of protein synthesis emetine, or the reversible inhibitor cycloheximide (CHX), in order to determine the dose necessary for complete inhibition of antigen synthesis. As expected, emetine concentrations as low as 20μM (11.1μg/ml) and CHX concentrations as low as 40μM (11.4μg/ml) were sufficient to completely block copGFP expression (Fig S3A) without inducing general cellular (Fig S1C, right panel and Fig S1D, right panel). These conditions and results are similar to those published previously (31, 32). Next we expressed a cohort of Shield-stabilized mature protein in the absence of new antigen synthesis as described in Fig 3C (Fig 4A). Cells with stabilized antigen were then treated with inhibitors of protein synthesis or a carrier control (0.04% DMSO) for 1hr in the continued presence of Shield. Following removal of class I:peptide conjugates by acid stripping, the cells were further cultured in control medium (DMSO), or in the presence of protein synthesis inhibitors and Shield was withdrawn to allow the cohort of “old” mature protein to degrade. For reasons that are currently unclear, we consistently observed an increase in protein levels with cells cultured in the presence of emetine (black) compared to control treated cells (white) (Fig 4B, left panel). However importantly, protein synthesis inhibitors blocked class I presentation by 60-80% (Fig 4B, middle panel), despite the fact preexisting mature protein was the only source of antigen.
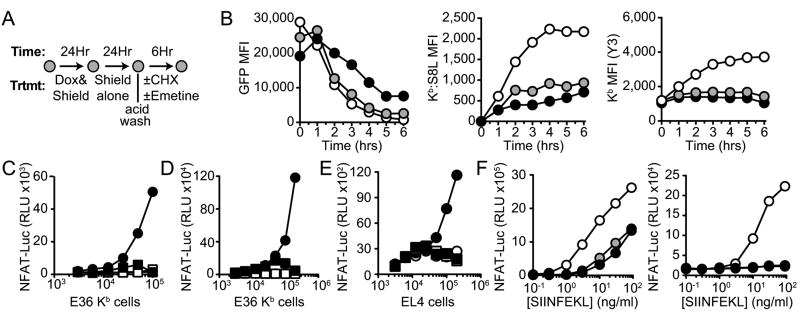
Figure 4. Antigen independent effects of protein synthesis inhibitors.
A, Experimental design used to analyze the effects of protein synthesis inhibitors on the presentation of mature proteins in the absence of antigen synthesis. B, E36 Kb cells were pretreated for 2hrs with control media (DMSO, white), 40μM CHX (gray) or 20μM emetine (black), as indicated. Cells were acid stripped and then cultured again in control media (0.04% DMSO), 40μM CHX, or 20μM emetine for 6hrs. GFP expression (left panel), Kb:S8L expression (center panel) and total class I levels (right panel) were assessed by flow cytometry from samples taken every 60min. Data shown are representative of one of 3 independent experiments. C, E36 Kb cells pretreated with 20μM emetine (squares) or 0.02% water control (circles) were subjected to hypertonic loading in the presence of mature ovalbumin (black) or control (white). Full-length ovalbumin was then released into the cytosol by osmotic lysis of endosomes, as described in the materials and methods. Antigen loaded E36 Kb cells were diluted 2-fold and co-cultured with the T cell hybridoma RF33-Luc for 18hrs and NFAT-luciferase activity was assessed by luminescence (RLU) (n=5). D, E36 Kb cells were pretreated a described above and then loaded with ovalbumin using the PULsin protein delivery reagent, as described. Symbols are the same as described in C (n=3). E, EL4 cells were treated with 40μM CHX (squares) or 0.1% DMSO (circles) for 1hr prior to hypertonic loading of ovalbumin (black) as described for E36 Kb cells in C (n=3). F, E36 Kb cells (left) and EL4 cells (right) were pretreated for 60min with 0.1% DMSO (white), 40μM CHX (gray) or 20μM emetine (black). Cells were subjected to acid stripping to removed preformed MHC class I and followed by an additional 90min treatment in the presence of the inhibitors listed above. Cells were then washed and fixed with 4% PFA, pulsed with S8L peptide titrated by 3-fold serial dilutions, as shown. Unbound peptide was removed by extensive washing and then cells were co-cultured with RF33-Luc overnight (n=3).
We extended these studies to another situation where the presented antigen was not synthesized by the antigen presenting cell, but in this case introduced by loading exogenous antigen into the cytosol (22). E36 Kb cells not expressing the antigen were first pretreated in the presence of emetine or a carrier control (0.04% water) for 1hr followed by incubation with a hypertonic solution containing mature ovalbumin. Loaded antigen was then released into the cytosol by culturing cells in hypotonic media (22). Antigen loaded cells were cultured for an additional 90min in the presence or absence of emetine to allow for processing of mature protein. Presentation of new peptides was stopped by the addition of BFA and cells were cultured for 18hrs with a T cell hybridoma specific for Kb:S8L (RF33-Luc) (21). As shown in Fig 4C, E36 Kb cells treated with ovalbumin in the presence of emetine (black square) showed a near complete inhibition of antigen presentation compared to those exposed to antigen in the absence of emetine (black circle). To rule out the possibility that emetine treatment impaired osmotic loading of the antigen into these cells, we lysed cells following antigen loading and performed western blots. Fig S3B shows that loading was not affected by inhibitor treatment, but that protein levels were somehow stabilized in the presence of emetine. These data are reminiscent of the increase in protein levels observed previously when emetine was present (Fig 4B, left panel). We used another method to introduce full-length protein into E36 Kb cells (PULsin protein delivery reagent), but at a lower protein concentration, and observed the same inhibition in the presence of emetine as observed before (Fig 4D). The affects of protein synthesis inhibitors on class I presentation were not specific to emetine or E36 cells, as we observed the same results when EL4 cells were treated with CHX (Fig 4E, Fig S3C).
Protein synthesis is necessary for all components of the antigen processing and presentation machinery and inhibitors of synthesis will have greater affect on those proteins that are limiting or that have shorter half-lives. Therefore, we addressed whether the protein synthesis inhibitors could inhibit levels of MHC I on the cell surface or the ability of the cells to present exogenous peptide. We found that the class I levels modestly decrease over time (Fig S3D) while the ability to present exogenous peptides (Fig S3E) was largely unchanged after inhibition of protein synthesis. However, if preformed class I was removed by acid stripping prior to treatment with synthesis inhibitors, recovery of surface MHC class I levels were markedly reduced (Fig 4B, right panel) as was the ability to present exogenous peptide (Fig 4F).
Together these data demonstrate that inhibitors of protein synthesis affect antigen presentation by a mechanism(s) distinct from just inhibition of antigen synthesis, and further highlight the difficulty in interpreting the contribution of DRiPs to class I presentation when inhibitors of protein synthesis are used.
Discussion
The data presented in this report address three major questions. First, is the primary source of peptides presented on class I MHC molecules derived predominantly from newly synthesized defective protein (DRiPs) or functionally mature proteins? Second, is newly synthesized antigen a more efficient source of presented peptides than ‘old’ mature protein? Lastly, we address whether inhibitors of protein synthesis, a widely used tool in experiments that support the DRiPs hypothesis, also affect class I presentation of mature proteins.
To address these questions we utilized a tet-induced antigen expression system encoding a reversible destabilizing domain. Due to the specificity of Shield for folded FKBP fusions, our system allowed mature protein to be stabilized without altering the formation and proteolysis of DRiPs. Our data demonstrated that Shield-stabilized protein was the primary source presented peptides from newly synthesized antigen. Is it possible that these Shield-stabilized constructs are actually DRiPs rather than mature protein? By definition, DRiPs are not functional, but instead represent defective proteins that never achieve a mature conformation (33). In contrast, the Shield-stabilized antigens bind ligand and are fluorescent, and therefore are not defective (11). Moreover, DRiPs are rapidly poly-ubiquitinated, a feature that was first used to identify and quantify these abnormal proteins (7). In contrast we and others detect no substantial ubiquitin laddering of the Shield stabilized constructs (34). In fact, proponents of the DRiPs hypothesis agree that such Shield-stabilized proteins are not DRiPs (11, 25).
Although our data clearly demonstrate that mature proteins are the more abundant contributors to the class I peptide pool for the two antigens we have analyzed, these results contrast with other recently published reports that utilized similar antigen constructs. Dolan et al. showed that most (60-70%) of the peptides feeding into the class I presentation pathway originated from an FKBP-antigen fusion that could not bind Shield and be stabilized (11). Given this observation and the fact that presentation from these constructs were rapidly blocked when protein synthesis was inhibited by CHX, it was suggested that DRiPs were the major source of the presented peptides. However, our data demonstrate that the concentration of Shield used in the Dolan study (2-5μM) does not fully stabilize the antigen. In fact, our data shows that as much as 16μM of Shield was necessary to fully stabilize the FKBP-EGFP fusion proteins, as indicated by the continual increase in GFP fluorescence and decrease in antigen presentation as the concentration of Shield increased. In addition, Dolan et al. used cells that had been continuously synthesizing antigen before exposure to acid stripping and Shield. Our data suggests that with extended antigen synthesis cells may accumulate a Shield-resistant form of antigen; this is not seen for > 6 hours after antigen synthesis is initiated. Moreover, we generalized our findings to two different FKBP antigens (with >80% difference in amino acid sequence) and in different antigen presenting cells, including ones from rodents and humans.
In addition to addressing the dominant source of presented peptides from our newly synthesized antigen constructs, we also investigated whether, and to what extent, “old” mature protein (“retirees”) was a source of peptides when it was degraded. We found that the presented peptides from our constructs were as efficiently generated from “old” mature protein as newly synthesized ones. As we show here, these results are likely due to the fact that the majority of peptides being presented during synthesis were generated from mature protein. These findings are different from what was originally described by others. Dolan and colleagues initially reported that the contribution of peptides from mature protein was small as compared to neosynthesized antigen (11). This suggested that peptides generated from newly synthesized DRiPs might have preferential access to the MHC class I pathway, and led to the idea that the processing and presentation of DRiPs was somehow compartmentalized (35). However, a subsequent paper from the same group and using the same expression system, reported that endogenously expressed mature protein was actually more efficient at contributing peptides to class I synthesis than newly synthesized protein (12). The basis for these discrepant results was not addressed, although the later findings are more similar to the ones we report here. We calculated the efficiency of antigen presentation without using protein synthesis inhibitors, while other studies have used these agents; it is likely that artifacts derived from the use of these inhibitors (see below) may have contributed to some of the discrepancies mentioned above.
Although our findings demonstrate a major contribution from mature protein, these results may be limited to our antigen constructs and it is possible that DRiPs and/or newly synthesized antigens are a more important source of presented peptides from other proteins or from other systems of expression (e.g. from viruses). However, our data are consistent with another recently published paper from our lab wherein where mature antigen was a dominant source of presented peptides for another two antigen constructs (17) and with older data showing that mature protein introduced directly into the cytosol was efficiently presented on class I molecules (3, 22, 36). Also consistent with our findings, an earlier study utilizing SILAC to follow the kinetics of class I presented peptides in a human cancer cell line showed that the majority of the class I peptides analyzed continued to be generated and presented for 6 or more hours post synthesis, a time period wherein DRiPs should have been long gone (37).
The majority of data supporting DRiPs come from experiments wherein inhibitors of protein synthesis were shown to rapidly block presentation despite the presence of substantial pools of mature protein. However, as shown here, these are complex experiments as protein synthesis is required for class I presentation in ways other than just antigen synthesis. This is perhaps not surprising because all components of the class I pathway need to be synthesized by the cell and there may also be other as yet unidentified short-lived proteins that are needed for the pathway; some of these components may become limiting if they are not replenished by synthesis. Moreover, blocking protein synthesis has long been known to have indirect effects on other cellular processes (38) and it is possible that these indirect affects also alter class I antigen presentation. For example, several genetic studies in S. cerevisiae showed that CHX affected ubiquitin-modifiying proteins and components of the proteasome after short incubation times (39–41). These results may partially explain why we observed stabilization of antigen levels following treatment with emetine. Moreover, the necessity of protein synthesis for class I presentation may explain why some virus (e.g. vaccinia virus, EBV) target protein synthesis as a mechanism of evasion.
In at least one previous study, control experiments were performed to assess the antigen independent effects of protein synthesis inhibitors on class I presentation. Qian et al. treated cells with the reversible proteasome inhibitor MG132 to build up DRiPs (and other forms of antigen) and then found that when they removed MG132 to allow antigen degradation, CHX did not completely block antigen presentation (13). This was interpreted to indicate that CHX had minimal effects on peptide turnover and class I presentation. However, their data showed that when MG132 was removed CHX inhibited approximately 50% of class I presentation. These data, like ours and those from earlier studies (42–45), point to the problems associated with using protein synthesis inhibitors to study the contribution of newly synthesized proteins to antigen presentation.
In addition to the inhibitor experiments described above, a few experiments have used Tet-regulated systems to study the contribution of antigen neosynthesis to antigen presentation. This approach circumvents the problems with pleotropic effects associated with general inhibitors of protein synthesis. For LCMV (nucleoprotein) and EBV (EBNA1) viral antigens it was reported that when Tet was removed and antigen synthesis was terminated, the presentation of some peptides decreased at times when there was still a substantial pool of mature protein (46, 47). Similarly, while this manuscript was in preparation, it was reported for a tet-inducible FKBP-EBV antigen system that mature antigen did not contribute to antigen presentation (25). It is possible that these antigens are ones where the contribution from DRiPs is more substantial. However, an important limitation of these experiments is that the mechanism by which the pools of long-lived mature protein were degraded was not reported. In fact in other studies, at least 2 long-lived EBV derived proteins, including EBNA1, were found to be degraded by autophagy (48, 49), while other studies have shown that several other EBV antigens are degraded by an alternative ubiquitin-proteasome-independent pathway (50, 51). This is not surprising because autophagy is a pathway that degrades a number of stable cytosolic proteins. Importantly, autophagy degrades proteins in vacuoles that are likely inaccessible to the proteasome and MHC class I antigen presentation pathway. Therefore to interpret these experiments it is essential to determine how the mature antigens are degraded and this issue was not addressed in any of these Tet –regulated experiments.
Overall the data presented here do not argue against the concept that defects in protein synthesis, protein folding or targeting occur, or that the degradation of these mistakes does not somehow contribute to antigen presentation. However, our findings argue that the magnitude of this contribution is small in the experimental systems we have examined. Whether this is true more generally will need to be determined. In our system, Shield inhibits the presentation of newly synthesized antigen by 80-95%. It is possible that the portion of this response that isn't inhibited by Shield comes from DRiPs that could not be stabilized by Shield. However, it is also possible that the noncovalent nature by which Shield binds to FKBP allows for transient destabilization rendering a fraction of the fusion proteins susceptible to proteasomal processing and finally presentation.
The DRiP model was proposed to explain how stable proteins would get rapidly presented. It was suggested that antigen presentation from proteins with long half-lives would be significantly delayed and that the few peptides that might be generated would be outcompeted by all of the peptides generated from endogenous antigens. However, it has long been appreciated that the kinetics of degradation for mature antigens with either short or long half-lives begins immediately after synthesis, following first order rate kinetics (52–54). In other words there is no delay in this process. With the efficient presentation of peptides from a few mature proteins as shown here and elsewhere (12, 55) and the sensitivity of CD8+ T cells to recognize a small fraction of MHC:peptide complexes (less than 10 complexes per cell) (56), peptides from proteins expressed at low copy should be sufficiently presented for recognition by CD8+ T cells. Moreover, there is no evidence that there is competition among antigenic peptides under physiological conditions. Therefore, rapid presentation of viral antigens may not come just from the degradation of DRiPs.
Acknowledgments
Funding: These studies were supported by funding from the NIH (AI20248) to KLR and core resources from the University of Massachusetts Diabetes and Endocrinology Research Center (DK32520) were also used. JC was supported by training grant AI007272-25.
Abbreviations
- CHX
cycloheximide
- FKBP
FK506 binding protein
- Dox
doxycycline
- PFA
paraformaldehyde
- S8L
SIINFEKL
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HPRT
hypoxanthine phosphoribosyltransferase
- EBV
Epstein Barr Virus
- LCMV
Lymphocytic Choriomeningitis virus
- BFA
brefeldin A
- Tet
tetracycline
References
- 1.Rock KL, Goldberg AL. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 3.Grant EP, Michalek MT, Goldberg AL, Rock KL. Rate of antigen degradation by the ubiquitin-proteasome pathway influences MHC class I presentation. J Immunol. 1995;155:3750–3758. [PubMed] [Google Scholar]
- 4.Esquivel F, Yewdell J, Bennink J. RMA/S cells present endogenously synthesized cytosolic proteins to class I-restricted cytotoxic T lymphocytes. J Exp Med. 1992;175:163–168. doi: 10.1084/jem.175.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yewdell JW, Antón LC, Bennink JR. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol. 1996;157:1823–1826. [PubMed] [Google Scholar]
- 6.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 7.Schubert U, Antón LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 8.Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science. 2005;310:1960–1963. doi: 10.1126/science.1121925. [DOI] [PubMed] [Google Scholar]
- 9.Golovina TN, Morrison SE, Eisenlohr LC. The impact of misfolding versus targeted degradation on the efficiency of the MHC class I-restricted antigen processing. J Immunol. 2005;174:2763–2769. doi: 10.4049/jimmunol.174.5.2763. [DOI] [PubMed] [Google Scholar]
- 10.Reits EA, Vos JC, Grommé M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–778. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 11.Dolan BP, Li L, Veltri CA, Ireland CM, Bennink JR, Yewdell JW. Distinct pathways generate peptides from defective ribosomal products for CD8+ T cell immunosurveillance. J Immunol. 2011;186:2065–2072. doi: 10.4049/jimmunol.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolan BP, Sharma AA, Gibbs JS, Cunningham TJ, Bennink JR, Yewdell JW. MHC class I antigen processing distinguishes endogenous antigens based on their translation from cellular vs. viral mRNA. Proc Natl Acad Sci USA. 2012;109:7025–7030. doi: 10.1073/pnas.1112387109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian SB, Reits E, Neefjes J, Deslich JM, Bennink JR, Yewdell JW. Tight linkage between translation and MHC class I peptide ligand generation implies specialized antigen processing for defective ribosomal products. J Immunol. 2006;177:227–233. doi: 10.4049/jimmunol.177.1.227. [DOI] [PubMed] [Google Scholar]
- 14.Tellam J, Fogg MH, Rist M, Connolly G, Tscharke D, Webb N, Heslop L, Wang F, Khanna R. Influence of translation efficiency of homologous viral proteins on the endogenous presentation of CD8+ T cell epitopes. J Exp Med. 2007;204:525–532. doi: 10.1084/jem.20062508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apcher S, Daskalogianni C, Lejeune F, Manoury B, Imhoos G, Heslop L, Fåhraeus R. Major source of antigenic peptides for the MHC class I pathway is produced during the pioneer round of mRNA translation. Proc Natl Acad Sci USA. 2011;108:11572–11577. doi: 10.1073/pnas.1104104108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croft NP, Smith SA, Wong YC, Tan CT, Dudek NL, Flesch IEA, Lin LCW, Tscharke DC, Purcell AW. Kinetics of antigen expression and epitope presentation during virus infection. PLoS Pathog. 2013;9:e1003129. doi: 10.1371/journal.ppat.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farfán-Arribas DJ, Stern LJ, Rock KL. Using intein catalysis to probe the origin of major histocompatibility complex class I-presented peptides. Proc Natl Acad Sci USA. 2012;109:16998–17003. doi: 10.1073/pnas.1210271109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8-9 residues. Nat Immunol. 2002;3:1177–1184. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- 19.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AGL, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 21.Kincaid EZ, Che JW, York I, Escobar H, Reyes-Vargas E, Delgado JC, Welsh RM, Karow ML, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rock KL. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat Immunol. 2012;13:129–135. doi: 10.1038/ni.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 23.Maynard-Smith LA, Chen LC, Banaszynski LA, Ooi AGL, Wandless TJ. A directed approach for engineering conditional protein stability using biologically silent small molecules. J Biol Chem. 2007;282:24866–24872. doi: 10.1074/jbc.M703902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruett-Miller SM, Reading DW, Porter SN, Porteus MH. Attenuation of Zinc Finger Nuclease Toxicity by Small-Molecule Regulation of Protein Levels. PLoS Genet. 2009;5:e1000376. doi: 10.1371/journal.pgen.1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiebiger BM, Moosmann A, Behrends U, Mautner J. Mature proteins derived from Epstein-Barr virus fail to feed into the MHC class I antigenic pool. Eur J Immunol. 2012 doi: 10.1002/eji.201242627. [DOI] [PubMed] [Google Scholar]
- 26.Waldo GS, Standish BM, Berendzen J, Terwilliger TC. Rapid protein-folding assay using green fluorescent protein. Nat Biotechnol. 1999;17:691–695. doi: 10.1038/10904. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 28.Chang WYC, Clements D, Johnson SR. Effect of doxycycline on proliferation, MMP production, and adhesion in LAM-related cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L393–400. doi: 10.1152/ajplung.00437.2009. [DOI] [PubMed] [Google Scholar]
- 29.Wilmann PG, Battad J, Petersen J, Wilce MCJ, Dove S, Devenish RJ, Prescott M, Rossjohn J. The 2.1A crystal structure of copGFP, a representative member of the copepod clade within the green fluorescent protein superfamily. J Mol Biol. 2006;359:890–900. doi: 10.1016/j.jmb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 31.Tscherne JS, Pestka S. Inhibition of protein synthesis in intact HeLa cells. Antimicrob Agents Chemother. 1975;8:479–487. doi: 10.1128/aac.8.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel MR, Sisler HD. Inhibition of Protein Synthesis in vitro by Cycloheximide. 1963;200:675–676. doi: 10.1038/200675a0. Published online: 16 November 1963. [DOI] [PubMed] [Google Scholar]
- 33.Yewdell JW. DRiPs solidify: progress in understanding endogenous MHC class I antigen processing. Trends Immunol. 2011;32:548–558. doi: 10.1016/j.it.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egeler EL, Urner LM, Rakhit R, Liu CW, Wandless TJ. Ligand-switchable substrates for a ubiquitin-proteasome system. J Biol Chem. 2011;286:31328–31336. doi: 10.1074/jbc.M111.264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolan BP, Knowlton JJ, David A, Bennink JR, Yewdell JW. RNA polymerase II inhibitors dissociate antigenic peptide generation from normal viral protein synthesis: a role for nuclear translation in defective ribosomal product synthesis? J Immunol. 2010;185:6728–6733. doi: 10.4049/jimmunol.1002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada CY, Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982;29:33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- 37.Milner E, Barnea E, Beer I, Admon A. The turnover kinetics of major histocompatibility complex peptides of human cancer cells. Mol Cell Proteomics. 2006;5:357–365. doi: 10.1074/mcp.M500241-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg AL, St John AC. Intracellular Protein Degradation in Mammalian and Bacterial Cells: Part 2. Annual Review of Biochemistry. 1976;45:747–804. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- 39.Amshoff C, Jäck HM, Haas IG. Cycloheximide, a new tool to dissect specific steps in ER-associated degradation of different substrates. Biol Chem. 1999;380:669–677. doi: 10.1515/BC.1999.083. [DOI] [PubMed] [Google Scholar]
- 40.Gerlinger UM, Gückel R, Hoffmann M, Wolf DH, Hilt W. Yeast Cycloheximide-resistant crl Mutants Are Proteasome Mutants Defective in Protein Degradation. Mol Biol Cell. 1997;8:2487–2499. doi: 10.1091/mbc.8.12.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanna J, Leggett DS, Finley D. Ubiquitin depletion as a key mediator of toxicity by translational inhibitors. Mol Cell Biol. 2003;23:9251–9261. doi: 10.1128/MCB.23.24.9251-9261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990;249:918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 43.Donohue KB, Grant JM, Tewalt EF, Palmer DC, Theoret MR, Restifo NP, Norbury CC. Cross-priming utilizes antigen not available to the direct presentation pathway. Immunology. 2006;119:63–73. doi: 10.1111/j.1365-2567.2006.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roscoe DM, Ishikawa K, Lyles DS. Role of de novo protein synthesis in target cells recognized by cytotoxic T lymphocytes specific for vesicular stomatitis virus. J Virol. 1991;65:6856–6861. doi: 10.1128/jvi.65.12.6856-6861.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang XL, Fan Z, Colleton BA, Buchli R, Li H, Hildebrand WH, Rinaldo CR. Processing and Presentation of Exogenous HLA Class I Peptides by Dendritic Cells from Human Immunodeficiency Virus Type 1-Infected Persons. J Virol. 2005;79:3052–3062. doi: 10.1128/JVI.79.5.3052-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardinaud S, Starck SR, Chandra P, Shastri N. The Synthesis of Truncated Polypeptides for Immune Surveillance and Viral Evasion. PLoS ONE. 2010;5:e8692. doi: 10.1371/journal.pone.0008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan S, de Giuli R, Schmidtke G, Bruns M, Buchmeier M, van den Broek M, Groettrup M. Cutting Edge: Neosynthesis Is Required for the Presentation of a T Cell Epitope from a Long-Lived Viral Protein. J Immunol. 2001;167:4801–4804. doi: 10.4049/jimmunol.167.9.4801. [DOI] [PubMed] [Google Scholar]
- 48.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Münz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 49.Lee DY, Sugden B. The latent membrane protein 1 oncogene modifies B-cell physiology by regulating autophagy. Oncogene. 2008;27:2833–2842. doi: 10.1038/sj.onc.1210946. [DOI] [PubMed] [Google Scholar]
- 50.Landais E, Saulquin X, Bonneville M, Houssaint E. Long-term MHC class II presentation of the EBV lytic protein BHRF1 by EBV latently infected b cells following capture of BHRF1 antigen. J Immunol. 2005;175:7939–7946. doi: 10.4049/jimmunol.175.12.7939. [DOI] [PubMed] [Google Scholar]
- 51.Taylor GS, Long HM, Haigh TA, Larsen M, Brooks J, Rickinson AB. A role for intercellular antigen transfer in the recognition of EBV-transformed B cell lines by EBV nuclear antigen-specific CD4+ T cells. J Immunol. 2006;177:3746–3756. doi: 10.4049/jimmunol.177.6.3746. [DOI] [PubMed] [Google Scholar]
- 52.Devreotes PN, Fambrough DM. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975;65:335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 54.Claydon AJ, Thom MD, Hurst JL, Beynon RJ. Protein turnover: measurement of proteome dynamics by whole animal metabolic labelling with stable isotope labelled amino acids. Proteomics. 2012;12:1194–1206. doi: 10.1002/pmic.201100556. [DOI] [PubMed] [Google Scholar]
- 55.Villanueva MS, Fischer P, Feen K, Pamer EG. Efficiency of MHC class I antigen processing: a quantitative analysis. Immunity. 1994;1:479–489. doi: 10.1016/1074-7613(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 56.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]