Abstract
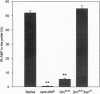
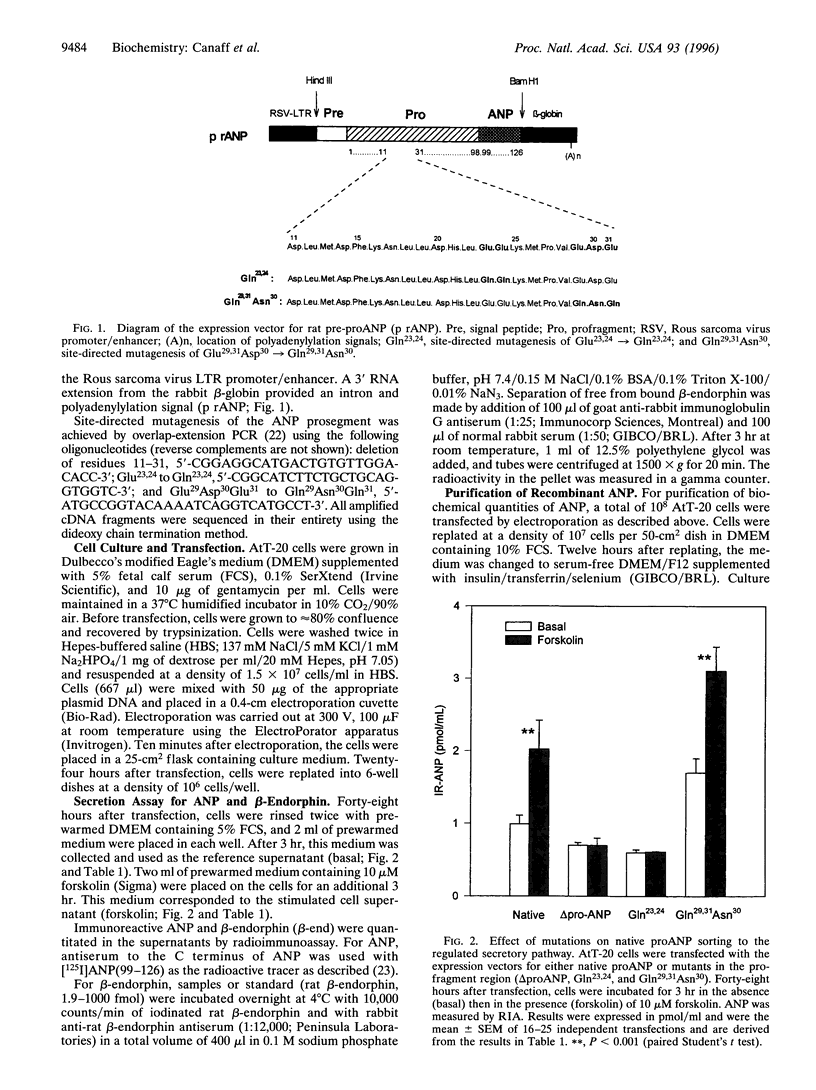
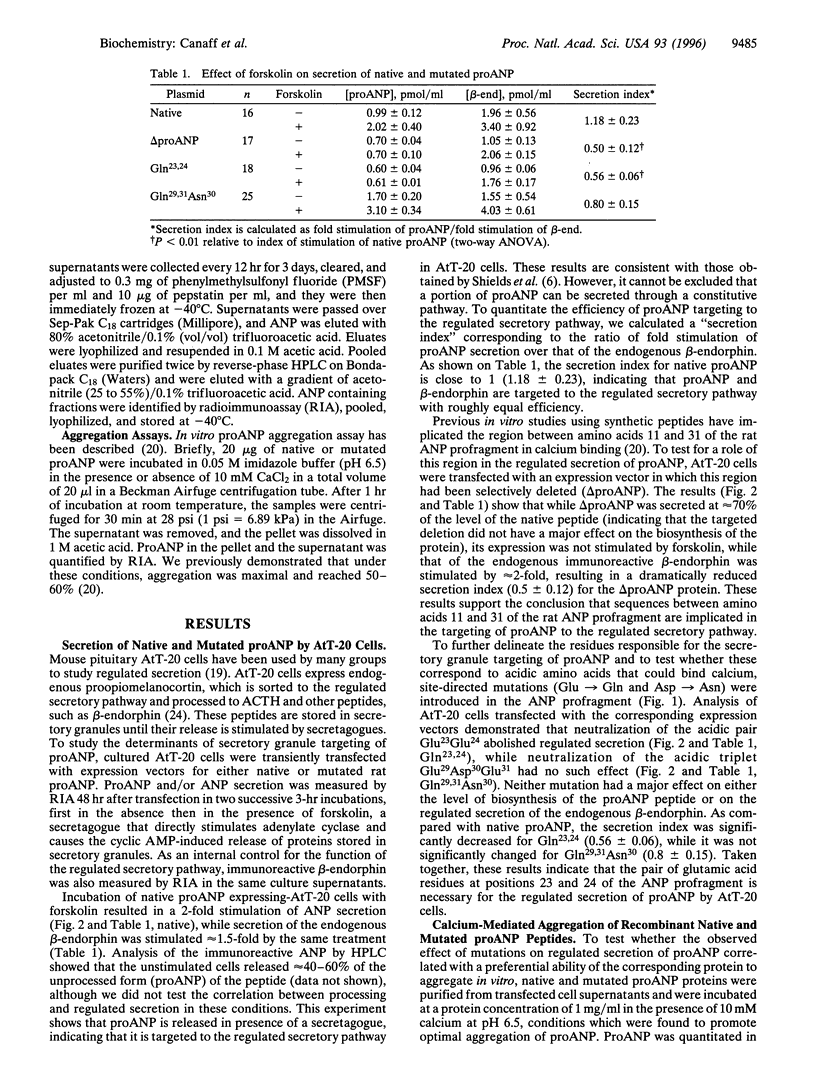
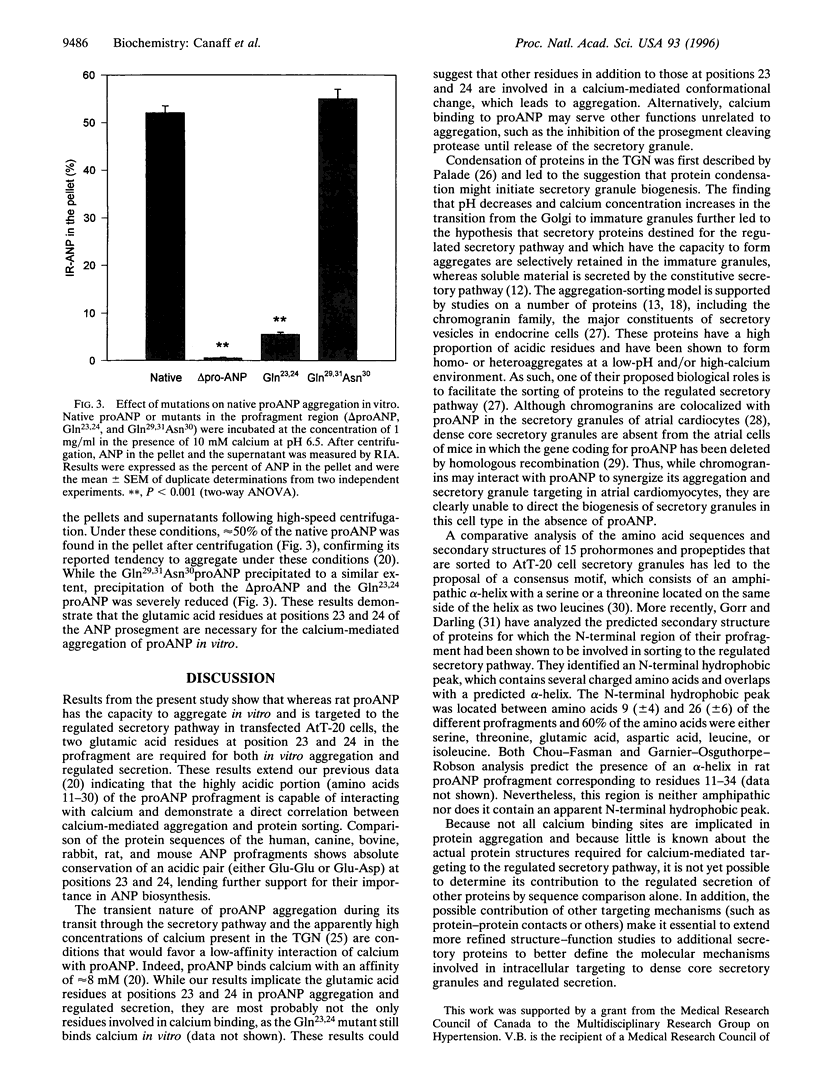
Atrial natriuretic peptide (ANP) is a 28-aa peptide hormone secreted predominantly from atrial cardiocytes. ANP is first synthesized in the form of a 126-aa precursor (proANP) which is targeted to dense core granules of the regulated secretory pathway. ProANP is stored until the cell receives a signal that triggers the processing and release of the mature peptide (regulated secretion). Various models have been proposed to explain the targeting of selected proteins to the regulated secretory pathway, including specific "sorting receptors" and calcium-mediated aggregation. As potential calcium binding regions had previously been reported in the profragment of ANP, the current study was undertaken in an effort to determine the relationship between the ability of ANP to enter the regulated secretory pathway and its calcium-mediated aggregation. Deletion and site-directed mutagenesis of selected regions of the prosegment demonstrates that acidic amino acids at positions 23 and 24 are critical for both regulated secretion of proANP from transfected AtT-20 cells and calcium-mediated aggregation of purified recombinant proANP in vitro. These results demonstrate that the ability of certain proteins to enter secretory granules is directly linked to their calcium-mediated aggregation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Arrandale J. M., Dannies P. S. Inhibition of rat prolactin (PRL) storage by coexpression of human PRL. Mol Endocrinol. 1994 Aug;8(8):1083–1090. doi: 10.1210/mend.8.8.7997234. [DOI] [PubMed] [Google Scholar]
- Chu W. N., Baxter J. D., Reudelhuber T. L. A targeting sequence for dense secretory granules resides in the active renin protein moiety of human preprorenin. Mol Endocrinol. 1990 Dec;4(12):1905–1913. doi: 10.1210/mend-4-12-1905. [DOI] [PubMed] [Google Scholar]
- Chung K. N., Walter P., Aponte G. W., Moore H. P. Molecular sorting in the secretory pathway. Science. 1989 Jan 13;243(4888):192–197. doi: 10.1126/science.2911732. [DOI] [PubMed] [Google Scholar]
- Colomer V., Kicska G. A., Rindler M. J. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996 Jan 5;271(1):48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Genest J., Cantin M. The atrial natriuretic factor: its physiology and biochemistry. Rev Physiol Biochem Pharmacol. 1988;110:1–145. doi: 10.1007/BFb0027530. [DOI] [PubMed] [Google Scholar]
- Gorr S. U., Darling D. S. An N-terminal hydrophobic peak is the sorting signal of regulated secretory proteins. FEBS Lett. 1995 Mar 13;361(1):8–12. doi: 10.1016/0014-5793(95)00142-v. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Genest J., Thibault G., Garcia R., Larochelle P., Cusson J. R., Kichel O., Hamet P., De Léan A., Cantin M. Circulating forms and radioimmunoassay of atrial natriuretic factor. Endocrinol Metab Clin North Am. 1987 Mar;16(1):183–198. [PubMed] [Google Scholar]
- Halban P. A., Irminger J. C. Sorting and processing of secretory proteins. Biochem J. 1994 Apr 1;299(Pt 1):1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Natori S. Regulated secretion. Helper proteins for neuroendocrine secretion. Curr Biol. 1995 Mar 1;5(3):242–245. doi: 10.1016/s0960-9822(95)00049-2. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Tooze S. A. Biosynthetic protein transport in the secretory pathway. Curr Opin Cell Biol. 1989 Aug;1(4):648–654. doi: 10.1016/0955-0674(89)90029-x. [DOI] [PubMed] [Google Scholar]
- Irons C. E., Sei C. A., Glembotski C. C. Regulated secretion of atrial natriuretic factor from cultured ventricular myocytes. Am J Physiol. 1993 Jan;264(1 Pt 2):H282–H285. doi: 10.1152/ajpheart.1993.264.1.H282. [DOI] [PubMed] [Google Scholar]
- John S. W., Krege J. H., Oliver P. M., Hagaman J. R., Hodgin J. B., Pang S. C., Flynn T. G., Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995 Feb 3;267(5198):679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- Kizer J. S., Tropsha A. A motif found in propeptides and prohormones that may target them to secretory vesicles. Biochem Biophys Res Commun. 1991 Jan 31;174(2):586–592. doi: 10.1016/0006-291x(91)91457-n. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Coordinate synthesis of corticotropins and endorphins by mouse pituitary tumor cells. J Biol Chem. 1978 Feb 10;253(3):651–655. [PubMed] [Google Scholar]
- Moore H. H., Kelly R. B. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986 May 22;321(6068):443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rosa P., Weiss U., Pepperkok R., Ansorge W., Niehrs C., Stelzer E. H., Huttner W. B. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J Cell Biol. 1989 Jul;109(1):17–34. doi: 10.1083/jcb.109.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskoaho H. Atrial natriuretic peptide: synthesis, release, and metabolism. Pharmacol Rev. 1992 Dec;44(4):479–602. [PubMed] [Google Scholar]
- Sei C. A., Hand G. L., Murray S. F., Glembotski C. C. The cosecretional maturation of atrial natriuretic factor by primary atrial myocytes. Mol Endocrinol. 1992 Mar;6(3):309–319. doi: 10.1210/mend.6.3.1533896. [DOI] [PubMed] [Google Scholar]
- Shields P. P., Sprenkle A. B., Taylor E. W., Glembotski C. C. Rat pro-atrial natriuretic factor expression and post-translational processing in mouse corticotropic pituitary tumor cells. J Biol Chem. 1990 Jul 5;265(19):10905–10911. [PubMed] [Google Scholar]
- Shiono S., Suganuma N., Bo M., Boime I., Seibert K., Nakao K., Mukoyama M., Imura H., Needleman P. Post-translational processing and secretory pathway of human atriopeptin in rat pheochromocytoma cells. Biochem Biophys Res Commun. 1991 May 15;176(3):1232–1238. doi: 10.1016/0006-291x(91)90417-6. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Broderick R., Shuman H., Buhle E. L., Jr, Somlyo A. P. Atrial-specific granules in situ have high calcium content, are acidic, and maintain anion gradients. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6222–6226. doi: 10.1073/pnas.85.16.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Fricker L. D. Calcium- and pH-dependent aggregation of carboxypeptidase E. J Biol Chem. 1995 Apr 7;270(14):7963–7967. doi: 10.1074/jbc.270.14.7963. [DOI] [PubMed] [Google Scholar]
- Steiner H. J., Weiler R., Ludescher C., Schmid K. W., Winkler H. Chromogranins A and B are co-localized with atrial natriuretic peptides in secretory granules of rat heart. J Histochem Cytochem. 1990 Jun;38(6):845–850. doi: 10.1177/38.6.2139887. [DOI] [PubMed] [Google Scholar]
- Thibault G., Doubell A. F. Binding and aggregation of pro-atrial natriuretic factor by calcium. Am J Physiol. 1992 Apr;262(4 Pt 1):C907–C915. doi: 10.1152/ajpcell.1992.262.4.C907. [DOI] [PubMed] [Google Scholar]
- Thibault G., Garcia R., Gutkowska J., Bilodeau J., Lazure C., Seidah N. G., Chrétien M., Genest J., Cantin M. The propeptide Asn1-Tyr126 is the storage form of rat atrial natriuretic factor. Biochem J. 1987 Jan 1;241(1):265–272. doi: 10.1042/bj2410265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A. Biogenesis of secretory granules. Implications arising from the immature secretory granule in the regulated pathway of secretion. FEBS Lett. 1991 Jul 22;285(2):220–224. doi: 10.1016/0014-5793(91)80805-d. [DOI] [PubMed] [Google Scholar]
- Xu H., Shields D. Prosomatostatin processing in permeabilized cells. Endoproteolytic cleavage is mediated by a vacuolar ATPase that generates an acidic pH in the trans-Golgi network. J Biol Chem. 1994 Sep 9;269(36):22875–22881. [PubMed] [Google Scholar]
- Yoo S. H. pH- and Ca(2+)-dependent aggregation property of secretory vesicle matrix proteins and the potential role of chromogranins A and B in secretory vesicle biogenesis. J Biol Chem. 1996 Jan 19;271(3):1558–1565. [PubMed] [Google Scholar]