Abstract
The aim of this study was to evaluate the impact of the surgical excisional procedures for cervical intraepithelial neoplasia (CIN) treatment both on subsequent fertility (cervical factor) and pregnancy complication (risk of spontaneous preterm delivery). We retrospectively analyzed 236 fertile women who underwent conization for CIN. We included in the study 47 patients who carried on pregnancy and delivered a viable fetus. Patients were asked about postconization pregnancies, obstetrical outcomes, and a possible diagnosis of secondary infertility caused by cervical stenosis. We evaluated the depth of surgical excision, the timing between cervical conization and subsequent pregnancies, surgical technique, and maternal age at delivery. We recorded 47 deliveries, 10 cases of preterm delivery; 8 of them were spontaneous. The depth of surgical excision showed a statistically significant inverse correlation with gestational age at birth. The risk of spontaneous preterm delivery increased when conization depth exceeded a cut-off value of 1.5 cm. Our data do not demonstrated a relation between conization and infertility due to cervical stenosis.
1. Introduction
Cervical intraepithelial neoplasia (CIN) is defined as a series of intraepithelial changes which includes nuclear pleomorphism, loss of polarity, and presence of abnormal mitoses. It is confined to squamous epithelium, but it may shift from a benign to a malignant lesion [1]. The risk of cervical cancer in women older than 30 years with carcinoma in situ is estimated at 31% [2].
Although the great majority of all HPV-infections resolve spontaneously within the first 2 years, the subset of infection remained has a high-persistence potential [3]. Management guidelines therefore recommend treatment for women with moderate-to-severe dysplasia [4, 5].
Surgical techniques currently adopted by the majority of the practitioners consist in ablative or excisional approaches. The excisional approach usually performed by laser conisation or large loop excision of the transformation zone (LLETZ) or cold knife conisation, offers advantages over the ablative method both permitting the histological investigation of removed lesion and ensuring a greater excision of cervical transformation zone. Indeed, incomplete excisions or destruction of the transformation zone are an important indicator for patients at risk of treatment failure or disease recurrence [6].
Since the great majority of women with high-grade CIN are of reproductive age [7], it is important to not compromise future pregnancies by surgical interventions on the cervix, which could be related to one of the most important causes of neonatal morbidity and mortality: preterm delivery (PD) [8, 9].
Several risk factors for spontaneous preterm birth have been suggested, but causal associations have been difficult to prove until now. Previous studies reported relations between surgical procedures for CIN and PD [9–11]; nevertheless explanations for this relation remain unclear [12]. Probably, the depth effect of surgical excision might explain discrepancies in the recent literature on the association between PD and prior treatment of cervical precancer lesions since different centers may have applied deep or less deep excisions.
Furthermore, cervical stenosis following excisional treatment for CIN has been reported more frequently among women who had long cones removed. Cervical stenosis has several potential adverse effects, including cervical factor infertility [13].
The primary aim of our study was to identify whether surgical excisional procedures for cervical intraepithelial neoplasia treatment are associated with increased risk of spontaneous preterm delivery (PD). The secondary outcome regarded the relation between these procedures and cervical factor infertility.
2. Materials and Methods
We performed a retrospective study using data collected from clinical records of patients who underwent surgical treatment for CIN at the Department of Obstetrics, Gynecology and Neonatology, University of Parma, from January 2005 to December 2011. Overall, 408 patients were treated in this timeframe.
We included in the cohort only patients in reproductive age (13–45 years) at time of the surgical procedures. Twenty-eight patients were excluded from the study on the basis of fertility status; 144 patients were excluded because they refused to answer the questionnaire and 236 patients were included in the study group.
Data collected regarded demographic features, gynecological and obstetric history and details about cervical procedure (CIN grade, surgical technique, and histopathology report) are reported in Table 1. We examined histopathology reports and for each patient we looked for the dimensions reported into the macroscopic description of the tissue removed. The height of the cone was the only feature considered, because this was comparable with the depth of the excision.
Table 1.
Patients' features.
| Term delivery (percentage) |
Spontaneous preterm delivery (percentage) | P | |
|---|---|---|---|
| Age | |||
| <20 | — | — | n.s.* |
| 20–30 | 11 (29.7) | 4 (50) | |
| 30–40 | 23 (62.2) | 4 (50) | |
| >40 | 3 (8.1) | — | |
| Ethnicity | |||
| Caucasian | 34 (91.9) | 8 (100) | n.s.* |
| Other | 3 (8.1) | — | |
| Socioeconomic status | |||
| Upper middle class | 27 (73) | 5 (62.5) | n.s.* |
| Lower middle class | 4 (10.8) | 2 (25) | |
| Other | 6 (16.2) | 1 (12.5) | |
| Parity | |||
| Nulliparous | 26 (70.3) | 8 (100) | n.s.* |
| Nonnulliparous | 11 (29.7) | — | |
| Conization-to-conception interval | |||
| <1 year | 19 (51.4) | 1 (12.5) | 0.04* |
| >1 year | 18 (48.6) | 7 (87.5) |
*Chi square test (χ2 test).
We interviewed patients to collect data about obstetrical history subsequent to cervical treatment: number of pregnancies, conization-to-conception interval, gestational age at delivery, and delivery modality. We also asked about diagnosis of secondary infertility caused by cervical stenosis.
On the basis of the information recorded we divided cases in two groups according to the gestational age at delivery: term delivery and preterm delivery (24–37 weeks). Only the first pregnancy after conization was taken into account. Statistics regarding features possibly related to PD (cone depth, timing between cervical surgery and pregnancy, and surgical technique) were performed with logistic regression. We used Student's t-test to assess statistical differences between the mean heights of excision and relative standard deviation, (SD) for the two groups. Subsequently, we calculated the odds ratio (OR) with 95% confidence interval for a 1.5 cm cutoff (mean height of excision). Following this, a linear regression curve was elaborated.
Statistical analyses were performed by SPSS software 19 for Windows, using parametric and nonparametric tests when appropriate. The normality of the distribution was assessed by the Kolmogorov-Smirnov. Differences between two means were assessed with the Student's t-test and associations between categorical variables were assessed with Pierson's chi2 or the Fisher exact test.
Differences were considered statistically significant at P < 0.05.
3. Results and Discussion
3.1. Results
Among the 236 patients included, 56 (23.7%) conceived after the surgical procedure. Five of them decided to have abortion; four patients had a first-trimester spontaneous miscarriage; we did not report second-trimester miscarriages. Forty-seven women carried on pregnancy and delivered a viable fetus. None of the patients included in our study had a previous PD nor common risk factors for PD.
Ten patients delivered preterm: mild preterm delivery (from 32 to <37 weeks) occurred in 9 cases, whereas severe preterm delivery (28 to <32 weeks) only in 1 case. The mean gestational age at delivery was 35.07 (DS = 2.58) weeks for PD and 39.35 (DS = 1.08) weeks for term deliveries. There were 2 cases of induced preterm delivery and 8 cases of spontaneous preterm delivery; in our statistics we considered only spontaneous PD. Premature preterm rupture of membranes (P-PROM) occurred in 5 patients (62.5%); vaginal delivery was recorded in 40% of patients who delivered preterm and in 64.9% of women who delivered at term.
Concerning the interval time between the conization and pregnancy, we reported that only 1 case (12.5%) of PD occurred in patient who conceived within 12 months after cervical surgery, while the remaining 7 cases (87.5%) of PD in patients who conceived after 1 year from surgical treatment (P = 0.04). Regarding term delivery, 19 patients (51.4%) conceived before 1 year from cervical surgery while 18 ones (48.6%) conceived after 1 year from cervical treatment (P = n.s) (Table 1).
The mean cone depth was, respectively, 1.42 (DS = 0.47) cm and 1.82 (DS = 0.66) cm in patients with term and spontaneous PD (Table 2). We found a statistically significant relation (P < 0.05) between depth of the cone and gestational age (Figure 1). Student's t-test showed a statistically significant difference (P < 0.05) between the mean depth of excision for the two study groups; we therefore demonstrated that the risk of preterm delivery is higher when cone depth exceeded a cutoff value of 1.5 cm (O.R. 7.143, 95% CI 1.37–37.228).
Table 2.
Cytopathological findings and surgical procedures in women with PD and term delivery.
| Term delivery (percentage) |
Spontaneous preterm delivery (percentage) | P | |
|---|---|---|---|
| PAP-test | |||
| LSIL | 11 (29.7) | 3 (37.5) | n.s.* |
| HSIL | 13 (35.1) | 1 (12.5) | |
| ASCUS | 7 (18.9) | 1 (12.5) | |
| UNKNOWN | 6 (16.3) | 3 (37.5) | |
| Histological diagnosis | |||
| CIN 1 | 2 (5) | 1 (12.5) | n.s.* |
| CIN2 | 18 (48.6) | 4 (50) | |
| CIN 3 | 17 (46.4) | 3 (37.5) | |
| Surgical technique | |||
| LEEP | 31 (83.8) | 6 (75) | n.s.* |
| Cold knife | 6 (16.2) | 2 (25) | |
| Mean (±SD) height of cone (cm) | 1.42 (SD = 0.47) | 1.82 (SD = 0.66) | 0.022** |
*Chi square test (χ2 test).
**t-test.
Figure 1.
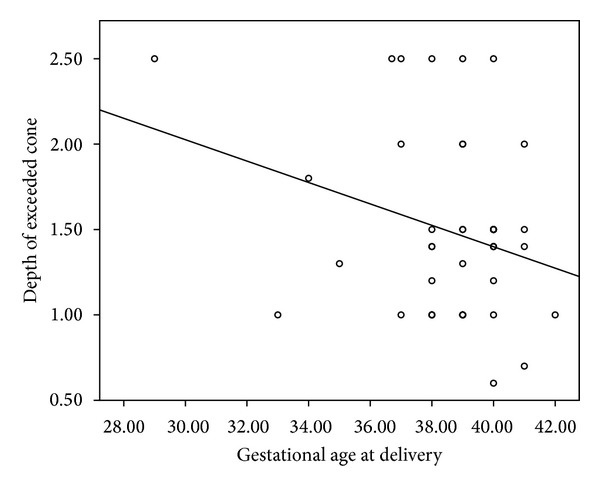
Linear regression curve shows the linear reverse correlation between gestational age at delivery (weeks) and height of exceeded cone (cm) (P < 0.05).
Concerning the relationship between surgical procedures for CIN and cervical factor infertility we observed that, among the 180 patients interviewed who did not get pregnant, 16 (8.8%) underwent hysterectomy and 5 (2.7%) underwent physiological menopause (i.e., absence of menses for at least 1 year with FSH serum value more than 30 IUs). Three patients (1.7%) declared secondary infertility caused by cervical stenosis (Table 3). In all cases there were a postsurgical hemorrhagic complication, resolved with suturing; in two of three cases the surgical procedure were performed with cold knife. On the basis of our data we could not find a relation between cervical stenosis and depth of excision.
Table 3.
Infertile patients because of cervical stenosis.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Histological diagnosis | CIN 3 | CIN 3 | CIN 3 |
| Age | 42 | 24 | 26 |
| Surgical technique | Cold knife | LEEP | Cold knife |
| Cone height (mm) | — | 20 | — |
| Parity | 1 | 0 | 1 |
| Complicarions of surgical treatment | Hemorrhagic complication | Hemorrhagic complication | Hemorrhagic complication |
3.2. Discussion
Our results showed that, after surgical treatment for CIN, particularly when the excision exceeded 15 mm in depth, the risk of PD is higher in women with deep versus less deep cones.
Sadler et al. [14] already demonstrated that for excisions of 17 mm or more the risk of pPROM but not of PD was higher (RR 3.6, 95% CI 1.8–7.5), whereas Samson et al. and Sjøborg et al., independently from depth of excision, after cervical laser conisation or loop electrosurgical excision procedure found an increased risk of both pPROM and PD [15, 16].
Recent studies found an increased risk of PD after LEEP; Noehr et al. [17] evaluated this condition either in singleton or twin pregnancies even after adjustment for several confounding factors [18]. Jakobsson et al. [19] reported an increased risk among women without previous preterm birth, emphasizing that the surgical procedure is a stronger risk factor than maternal obstetrical history.
In our study we found only three cases of PD before 34 weeks, of whom 1 before 32 weeks, therefore we could not demonstrate an increased risk of extremely preterm birth, as reported by Armarnik et al. [20]. We did not find a relation (P > 0.05) between short conization-to-conception interval and preterm birth, although Himes and Simhan [21] showed that conception within 2-3 months after CIN surgical treatments may be associated with an increased risk of PD. In our study 5 patients (10.6% of all deliveries) who had a term delivery conceived in 2-3 months after the surgical treatment.
Several studies examined the effects of cone size on the risk of PD [9, 13, 14, 18]. Acharya et al. [22] found a 4-fold increase risk of PD when the loop size exceeded 25 mm (RR 4.0, 95% CI 1.0–16.0); Kyrgiou et al. [10] and Simoens et al. [23] found a cutoff value of 10 mm (O.R., resp., 2.61, 95% CI 1.28–5.34 and 4.55, 95% CI 1.32–15.65). Jakobsson et al. [24, 25] reported an estimated 20% increase in PD per millimeter of cone size excised (O.R. 1.5, 95% CI 1.0–1.4), whereas Noehr et al. [26] estimated a 6% increase risk per millimeter of excision (O.R. 1.96, 95% CI 1.03–1.09). In 2012, Khalid et al. [27] showed a 3-fold increase in the risk of PD when the specimen exceeded 12 mm (RR = 2.98; 95% CI 1.27–7.01) and 6 cm³ (RR = 3.00; 95% CI 1.45–5.92). Our data fixed this cutoff value at 15 mm in depth, since in our previous clinical practice, this value seemed the best in terms of both oncological outcome and subsequent reproductive outcome.
Our study aimed to find a correlation between surgical procedures for CIN and infertility due to cervical factor. On the basis of our data we could not demonstrate a relation between conization and infertility due to cervical stenosis; there were only 3 cases of cervical stenosis (1.3% of all patients included); a percentage lower than that was reported in many studies as complication of surgical treatment (cold knife 3–37%; LEEP 4–9%) [28, 29]. In two of three cases we could not assess the macroscopic dimensions of cone specimen into histopathology reports, whereas in the remainder case the cone measured 20 mm; Baldauf et al. [28] found a cutoff value for increased risk of cervical stenosis after conization of 20 mm (RR 2.96, 95% CI 1.63–5.38) and concluded that cervical stenosis is related to aggressive excision with cold knife and laser conization involving the endocervix. All three patients with secondary cervical stenosis experienced postoperative hemorrhage and need of suturing or cauterization of the surgical wound; Monteiro et al. [30] demonstrated that postoperative hemorrhagic complications are associated with cervical stenosis.
Certainly, in the era of HPV vaccination, the achievement of herd immunity would reduce more and more the number of women surgically treated and, consequently, the number of pregnancy complication such as PD linked to previous cervical precancerous lesion treatment [31].
Our study is affected by a weakness and potential source of bias related to the fact that certain data were obtained by interview and not from medical records (for instance, by linkage with maternity files) and the rather low participation rate.
4. Conclusions
Our results suggest that surgical procedures for CIN increase the risk of spontaneous PD when the depth of the cone specimen exceeds 15 mm (O.R. 7.143, 95% CI 1.37–37.228).
To avoid the malignant transformation of CIN and reduce the future pregnancies complications we therefore recommend to perform cone excision with a depth not more than 15 mm in women in reproductive age.
Conflict of Interests
All authors declare no conflict of interests.
References
- 1.Mitchell MF, Hittelman WN, Hong WK, Lotan R, Schottenfeld D. The natural history of cervical intraepithelial neoplasia: an argument for intermediate endpoint biomarkers. Cancer Epidemiology Biomarkers and Prevention. 1994;3(7):619–626. [PubMed] [Google Scholar]
- 2.McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. The Lancet Oncology. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 3.Saccardi C, Gizzo S, Noventa M, et al. High-riskhuman papillomavirus DNA test: could it be useful in low-grade cervical lesion triage? five-year follow-Up. doi: 10.1177/1933719113492214. Reproductive Sciences. In press. [DOI] [PubMed] [Google Scholar]
- 4.Wright TC, Jr., Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. American Journal of Obstetrics and Gynecology. 2007;197(4):340–345. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Jordan J, Arbyn M, Martin-Hirsch P, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology. 2008;19(6):342–354. doi: 10.1111/j.1365-2303.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Hirsch PP, Paraskevaidis E, Bryant A, Dickinson HO, Keep SL. Surgery for cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews. 2010;6 doi: 10.1002/14651858.CD001318.pub2.CD001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paraskevaidis E, Kitchener HC, Miller ID, Mann E, Jandial L, Fisher PM. A population-based study of microinvasive disease of the cervix—a colposcopic and cytologic analysis. Gynecologic Oncology. 1992;45(1):9–12. doi: 10.1016/0090-8258(92)90483-y. [DOI] [PubMed] [Google Scholar]
- 8.Norman JE, Morris C, Chalmers J. The effect of changing patterns of obstetric care in Scotland (1980–2004) on rates of preterm birth and its neonatal consequences: perinatal database study. PLoS Medicine. 2009;6(9) doi: 10.1371/journal.pmed.1000153.e1000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. British Medical Journal. 2008;337:p. a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. The Lancet. 2006;367(9509):489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 11.van Hentenryck M, Noel JC, Simon P. Obstetric and neonatal outcome after surgical treatment of cervical dysplasia. European Journal of Obstetrics Gynecology and Reproductive Biology. 2012;162(1):16–20. doi: 10.1016/j.ejogrb.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Kyrgiou M, Arbyn M, Martin-Hirsch P, Paraskevaidis E. Increased risk of preterm birth after treatment for CIN. British Medical Journal. 2012;4345 doi: 10.1136/bmj.e5847.e5847 [DOI] [PubMed] [Google Scholar]
- 13.Luesley DM, McCrum A, Terry PB. Complications of cone biopsy related to the dimensions of the cone and the influence of prior colposcopic assessment. British Journal of Obstetrics and Gynaecology. 1985;92(2):158–164. doi: 10.1111/j.1471-0528.1985.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 14.Sadler L, Saftlas A, Wang W, Exeter M, Whittaker J, McCowan L. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. Journal of the American Medical Association. 2004;291(17):2100–2106. doi: 10.1001/jama.291.17.2100. [DOI] [PubMed] [Google Scholar]
- 15.Samson S-LA, Bentley JR, Fahey TJ, McKay DJ, Gill GH. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstetrics and Gynecology. 2005;105(2):325–332. doi: 10.1097/01.AOG.0000151991.09124.bb. [DOI] [PubMed] [Google Scholar]
- 16.Sjøborg KD, Vistad I, Myhr SS, et al. Pregnancy outcome after cervical cone excision: a case-control study. Acta Obstetricia et Gynecologica Scandinavica. 2007;86(4):423–428. doi: 10.1080/11038120701208158. [DOI] [PubMed] [Google Scholar]
- 17.Noehr B, Jensen A, Frederiksen K, Tabor A, Kjaer SK. Loop electrosurgical excision of the cervix and subsequent risk for spontaneous preterm delivery: a population-based study of singleton deliveries during a 9-year period. American Journal of Obstetrics and Gynecology. 2009;201(1):33.e1–33.e6. doi: 10.1016/j.ajog.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Noehr B, Jensen A, Frederiksen K, Tabor A, Kjaer SK. Loop electrosurgical excision of the cervix and risk for spontaneous preterm delivery in twin pregnancies. Obstetrics and Gynecology. 2009;114(3):511–515. doi: 10.1097/AOG.0b013e3181b1377b. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsson M, Gissler M, Paavonen J, Tapper A-M. Loop electrosurgical excision procedure and the risk for preterm birth. Obstetrics and Gynecology. 2009;114(3):504–510. doi: 10.1097/AOG.0b013e3181b052de. [DOI] [PubMed] [Google Scholar]
- 20.Armarnik S, Sheiner E, Piura B, Meirovitz M, Zlotnik A, Levy A. Obstetric outcome following cervical conization. Archives of Gynecology and Obstetrics. 2011;283(4):765–769. doi: 10.1007/s00404-011-1848-3. [DOI] [PubMed] [Google Scholar]
- 21.Himes KP, Simhan HN. Time from cervical conization to pregnancy and preterm birth. Obstetrics and Gynecology. 2007;109(2, part 1):314–319. doi: 10.1097/01.AOG.0000251497.55065.74. [DOI] [PubMed] [Google Scholar]
- 22.Acharya G, Kjeldberg I, Hansen SM, Sørheim N, Jacobsen BK, Maltau JM. Pregnancy outcome after loop electrosurgical excision procedure for the management of cervical intraepithelial neoplasia. Archives of Gynecology and Obstetrics. 2005;272(2):109–112. doi: 10.1007/s00404-005-0727-1. [DOI] [PubMed] [Google Scholar]
- 23.Simoens C, Goffin F, Simon P, Barlow P, Antoine J, Foidart JM. Arbyn M. Adverse obstetrical outcomes after treatment of precancerous cervical lesions: a Belgian multicentre study. An International Journal of Obstetrics & Gynaecology. 2012;119(10):1247–1255. doi: 10.1111/j.1471-0528.2012.03429.x. [DOI] [PubMed] [Google Scholar]
- 24.Jakobsson M, Gissler M, Sainio S, Paavonen J, Tapper A-M. Preterm delivery after surgical treatment for cervical intraepithelial neoplasia. Obstetrics and Gynecology. 2007;109(2, part 1):309–313. doi: 10.1097/01.AOG.0000253239.87040.23. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsson M, Gissler M, Sainio S, Paavonen J, Tapper AM. Erratum in: Preterm delivery after surgical treatment for cervical intraepithelial neoplasia. Obstetrics and Gynecology. 2008;112(4) doi: 10.1097/01.AOG.0000253239.87040.23. [DOI] [PubMed] [Google Scholar]
- 26.Noehr B, Jensen A, Frederiksen K, Tabor A, Kjaer SK. Depth of cervical cone removed by loop electrosurgical excision procedure and subsequent risk of spontaneous preterm delivery. Obstetrics and Gynecology. 2009;114(6):1232–1238. doi: 10.1097/AOG.0b013e3181bf1ef2. [DOI] [PubMed] [Google Scholar]
- 27.Khalid S, Dimitriou E, Conroy R, et al. The thickness and volume of LLETZ specimens can predict the relative risk of pregnancy-related morbidity. An International Journal of Obstetrics and Gynaecology. 2012;119(6):685–691. doi: 10.1111/j.1471-0528.2011.03252.x. [DOI] [PubMed] [Google Scholar]
- 28.Baldauf J-J, Dreyfus M, Ritter J, Meyer P, Philippe E. Risk of cervical stenosis after large loop excision or laser conization. Obstetrics and Gynecology. 1996;88(6):933–938. doi: 10.1016/S0029-7844(96)00331-6. [DOI] [PubMed] [Google Scholar]
- 29.Larsson G, Gullberg B, Grundsell H. A comparison of complications of laser and cold knife conization. Obstetrics and Gynecology. 1983;62(2):213–217. [PubMed] [Google Scholar]
- 30.Monteiro ACS, Russomano FB, de Camargo MJ, da Silva KS, Veiga FR, Oliveira RG. Cervical stenosis following electrosurgical conization. Sao Paulo Medical Journal. 2008;126(4):209–214. doi: 10.1590/S1516-31802008000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gizzo S, Noventa M, Nardelli GB. Gardasil administration to hr-HPV-positive women and their partners. Trends in Pharmacological Sciences. 2013;34(9):479–480. doi: 10.1016/j.tips.2013.07.001. [DOI] [PubMed] [Google Scholar]


