Abstract
Rheumatoid factors are antibodies directed against the Fc region of immunoglobulin G. First detected in patients with rheumatoid arthritis 70 years ago, they can also be found in patients with other autoimmune and nonautoimmune conditions, as well as in healthy subjects. Rheumatoid factors form part of the workup for the differential diagnosis of arthropathies. In clinical practice, it is recommended to measure anti-cyclic citrullinated peptide antibodies and rheumatoid factors together because anti-cyclic citrullinated peptide antibodies alone are only moderately sensitive, and the combination of the two markers improves diagnostic accuracy, especially in the case of early rheumatoid arthritis. Furthermore, different rheumatoid factor isotypes alone or in combination can be helpful when managing rheumatoid arthritis patients, from the time of diagnosis until deciding on the choice of therapeutic strategy.
1. Introduction
Rheumatoid factors (RFs), a class of immunoglobulins (Igs) that have different isotypes and affinities, were first detected more than 70 years ago, but there is still much to discover about the mechanisms underlying their production, physiological role, and pathological effects [1].
Waaler described an antibody directed against serum gamma-globulins that promoted the agglutination of sheep red blood cells sensitised by subagglutinating doses of rabbit antibodies in 1940 [2], although it had actually been previously found in patients with liver cirrhosis and chronic bronchitis by Kurt Meyer in 1922. In 1948, Rose described these antibodies in patients with rheumatoid arthritis (RA) [3], and in 1952 they were finally christened RFs because of their association with RA [4].
However, although they owe their name to their first detection in RA patients, RFs are found in patients with other autoimmune and nonautoimmune diseases, as well as-in healthy subjects.
The aim of this review is to describe the clinical applications of testing for RFs.
2. Methods of Detection
Classic agglutination techniques were initially used because of the ability of IgMs to induce agglutination. The first RF detection assay was based on the fact that RF agglutinates sheep red blood cells sensitised with rabbit IgGs (i.e., the classic Waaler-Rose test) [2, 3], and this was followed by the development of other IgG carriers such as bentonite [5, 6] and latex particles [7, 8].
Automated techniques such as nephelometry and enzyme-linked immunosorbent assays gradually replaced the other semiquantitative methods because of their simplicity and greater reproducibility [9–12].
Multiplexed immunoassaying is an emerging high-throughput technique for the quantitative detection of multiple analytes from a single biological sample [13]. Although they have yet to be standardised and validated, multiplexed immunoassays can reduce analytical time and enhance accuracy. However, it is known that RFs can interfere with a number of laboratory immunoassays and lead to false positive results: for example, in patients with high RF levels, the analysis of vancomycin can be compromised if serum rather than plasma samples are used [14, 15].
RFs can also interfere with other laboratory tests, including those designed to detect anticardiolipin antibodies (especially if IgM levels are in the low positive range) [16], anti-β2GPI antibodies [17], anti-HCV antibodies [18], antirubella antibodies [19], thyroid assays [20, 21], and tests for carbohydrate antigen 19–9 [22] and various cytokines [23].
3. Rheumatoid Factors in Nonrheumatic Conditions
As shown in Table 1, RFs can be detected in patients with many nonrheumatic conditions. Infections and chronic diseases may be characterised by the presence of serum RFs, but unlike those detected in RA patients, the RFs produced during infections are usually transient and not detrimental. Given the ability of RFs to increase the clearance of immune complexes and the fact that RF-producing B cells may behave as antigen-presenting cells (APCs) and aid the immune response against the infectious antigens, it is likely that the net impact of RF production during infections is protective for the host [24, 26].
Table 1.
Rheumatoid factor frequency in different diseases and conditions.
| Disease | Frequency, % |
|---|---|
| Arthritis | |
| Rheumatoid arthritis | 70–90 |
| Juvenile idiopahtic arthritis | 5 |
| Psoriatic arthritis | <15 |
| Reactive arthritis | <5 |
| Other connective tissue diseases | |
| Primary Sjgren's syndrome | 75–95 |
| Mixed connective tissue disease | 50–60 |
| Systemic lupus erythematosus | 15–35 |
| Systemic sclerosis | 20–30 |
| Dermato-/polymyositis | 20 |
| Systemic vasculitides (panarteritis nodosa, Wegener's granulomatosis) |
5–20 |
| Infectious diseases | |
| Bacterial infections | |
| Subacute bacterial endocarditis | 40 |
| Chlamydia pneumoniae infection | |
| Klebsiella pneumoniae infection | |
| Syphilis primary-tertiary | 8–37 |
| Tuberculosis | 15 |
| Viral infections | |
| Coxsackie B virus infection | 15 |
| Dengue virus infection | 10 |
| EBV and CMV infections | 20 |
| Hepatitis A, B and C virus infection | 25 |
| HCV infection | 40–76 |
| Herpes virus infection | 10–15 |
| HIV infection | 10–20 |
| Measles | 8–15 |
| Parvovirus infection | 10 |
| Rubella | 15 |
| Parasitic | |
| Chagas | 15–25 |
| Malaria | 15–18 |
| Onchocerciasis | 10 |
| Toxoplasmosis | 10–12 |
| Other diseases | |
| Mixed cryoglobulinemia type II | 100* |
| Liver cirrhosis | 25 |
| Primary biliary cirrhosis | 45–70 |
| Malignancy | 5–25 |
| After multiple immunisations | 10–15 |
| Chronic sarcoidosis | 5–30 |
| Healthy 50-year olds | 5 |
| Healthy 70-year olds | 10–25 |
These natural RFs are generally low-affinity, polyreactive IgM antibodies produced by CD5-positive B cells [27, 28], and coexistence of RF-positive B cells and nonautoimmune IgG antigen in healthy subjects suggests the existence of tolerance mechanisms [26].
RFs can be found in 40–50% of patients with HCV infection, but their frequency can reach 76% [29]. Their production is probably due to chronic stimulation of the immune system by HCV, and, as HCV infection is highly prevalent in various countries (1.5–3% in southern Europe) and represents the first cause of increased serum RFs, HCV antibodies should be sought in all subjects with increased RF levels [29, 30] (Figure 1).
Figure 1.
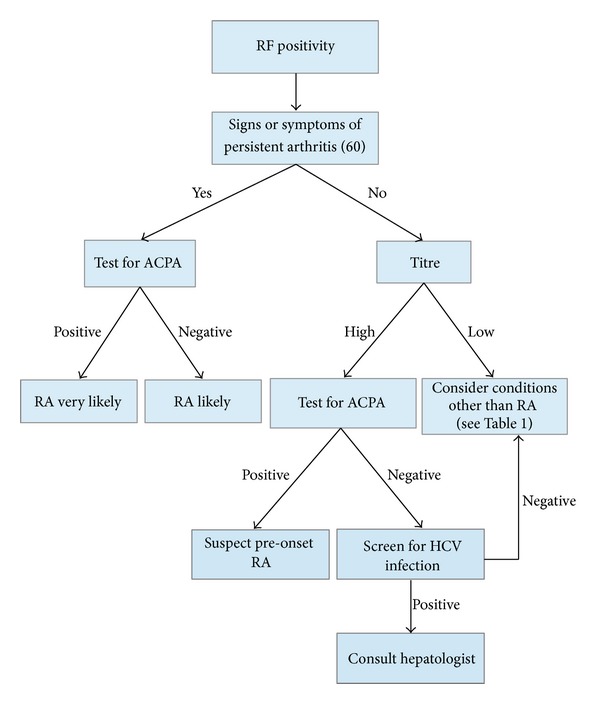
Proposed decision-making algorithm for patients who are rheumatoid factor positive at the first evaluation. RF: rheumatoid factor; RA: rheumatoid arthritis; ACPA: anti-cyclic citrullinated protein/peptide antibody.
4. RFs in Healthy Subjects
RF positivity has also been reported in the healthy population [31–33], and up to 4% of young Caucasians may be RF positive, with a similar distribution between the two genders. It is thought that genetic and environmental factors are responsible for the worldwide variability in distribution of RFs: for example, their highest prevalence (up to 30%) has been observed in North American Indians tribes [34–36]. The RFs found in healthy subjects are different from those present in RA patients as their titres are low/moderate and they are likely to be produced by CD5-expressing B cells as low-affinity, poly-reactive IgMs without any signs of maturation affinity [31]. The transient production of low-affinity IgM RFs may be induced by polyclonal B cell activators such as bacterial lipopolysaccharides and Epstein-Barr virus [28, 37], but it has been shown that high RF titres in healthy subjects predict the development of RA [38]. Furthermore, IgM RFs are sometimes observed in healthy elderly people, which suggests that they may be a consequence of the age-related immune deregulation (Figure 1) [39, 40].
5. RFs in Patients with Autoimmune Diseases
RFs are frequently detected in patients with systemic autoimmune diseases, such as systemic lupus erythematosus, mixed connective tissue disease, polymyositis, and dermatomyositis (Table 1) [24, 25].
Patients with Sjogren's syndrome (SS) [41] and those with type II and III mixed cryoglobulinemia (usually HCV related) [42] have the highest RF titres.
About 60% of the patients with primary SS are RF positive, with males having higher IgA RF levels than females [41]. It is also thought that the disease-related transformation of activated RF-positive B cell clones is involved in the pathogenesis of the lymphoproliferative disorders that develop in about 5% of SS patients [43]. Most SS patients have high titres of polyclonal RFs, whereas monoclonal RFs can be detected in patients with type II mixed cryoglobulinemia and, to a lesser extent, in SS patients with lymphoproliferative disorders [24, 34].
6. RFs and Rheumatoid Arthritis
Although RFs can be detected in patients with other connective tissue diseases, RF isotypes are helpful in the management of RA patients from the time of diagnosis until deciding on the choice of therapeutic strategy (Figures 1 and 2) [44, 45]. RF testing in RA patients has a sensitivity of 60% to 90% and a specificity of 85% [46, 47].
Figure 2.
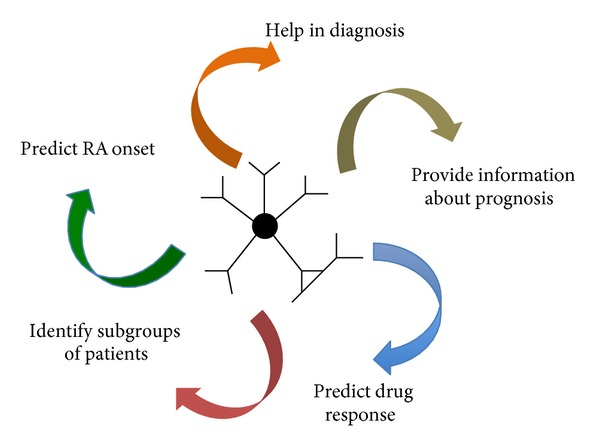
Role of rheumatoid factors in the management of rheumatoid arthritis patients.
A number of hypotheses have been postulated in order to explain the possible key role of RFs in RA, including their capacity to increase the elimination of immune complexes by macrophages [48], the improved cytotoxicity of antiviral antibodies [49], and the increased elimination of parasites [1]. It has also been suggested that RFs potentiate the presentation of antigens to T cells by means of the dendritic cell uptake of immune complexes with exogenous antigens and by means of RF B cells, which seem to be more efficient APCs than other B cells [50] (Figure 3). Finally, it is possible that the rapid secretion of large amounts of low-affinity RFs prevents the activation of higher-affinity RF B cells and additional B cells [51–53].
Figure 3.
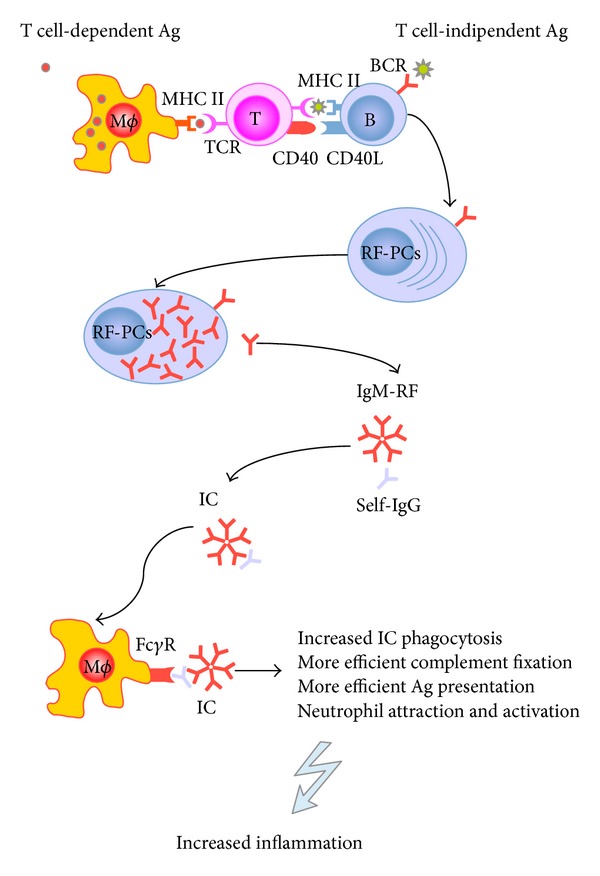
The immunological role of rheumatoid factors (RFs) in rheumatoid arthritis. RFs may be produced both in a T cell-dependent or T cell-independent pathway. Macrophages and B cells may act as antigen-presenting cells and efficiently present antigens to T cells. Ag: antigen; BCR: B cell receptor; TCR: T cell receptor; RF-PCs: rheumatoid factor plasma cells; IC: immune complex.
Defining RFs as anti-IgG or anti-gamma-globulins is inaccurate because it restricts RF reactivity to the IgG Fc fragment. IgM RFs are the most frequently detected isotype, but IgG, IgA, IgE, and IgD RFs can also be observed [54].
It has been shown that three RF isotypes (IgM, IgA, and IgG) are detected in up to 52% of RA patients but in fewer than 5% of patients with other connective tissue diseases. Moreover, the presence of IgA and IgG RF isotypes in absence of IgM-RF is more prevalent in patients with connective tissue diseases than in RA patients, whereas an increase in both IgM and IgA RFs is almost exclusively observed in patients with RA [55, 56]. IgM-RF specificity increases considerably at high titres [57].
6.1. The Role of RFs in the Diagnosis of Rheumatoid Arthritis
It has long been recognised that RFs play a pivotal role in the differential diagnosis of polyarthritis because they make it possible to identify RA patients [58]. For this reason, RF testing has been one of the classification criteria for RA since 1987 [59] and, although many years have passed since their identification, their crucial role in classifying RA has been confirmed by the updated criteria [60].
However, in order to increase the specificity of the latest RA classification criteria, anti-cyclic citrullinated protein/peptide antibody (ACPA) testing has been added. A meta-analysis [46] has shown that the pooled sensitivities of ACPA and RF are similar, but ACPA positivity is more specific for RA than IgM RF, IgG RF, or IgA RF positivity [61] and more specific for early RA than IgM RF [62]. On the other hand, sensitivity is reduced because positivity for both ACPA and RF is a more stringent criterion than positivity for either alone [46]; combining ACPA and RF positivity is more permissive in terms of sensitivity because the antibodies complement each other, especially for early RA [63–66].
Furthermore, although the cut-off value of each commercial kit is slightly different, it has been suggested that the best ACPA cut-off value should be ≥40 U/mL, which leads to a positive likelihood ratio of 5.49 and a negative likelihood ratio of 0.50 [46, 67].
It has also been shown that RFs are useful in predicting the development of RA, as the detection of IgM, IgA, and IgG RFs may predate its onset by years [38, 68], and it has been reported that their appearance in serum is sequential before diagnosis: first IgM RF, then IgA RF, and finally IgG RF [57, 69].
6.2. Prognostic and Therapeutic Relevance in Rheumatoid Arthritis
The detection of IgM RFs is also helpful as a prognostic index, and some studies have shown that immunosuppressive treatment can decrease serum RF levels. However, the clinical usefulness of RFs in monitoring disease activity [70] and treatment response is limited [71].
It has been shown that a progressive decrease in the RF levels parallels the decrease of clinical activity in patients treated with traditional disease modifying antirheumatic drugs [72] or biologic agents such as infliximab [73–75], etanercept [76], adalimumab [77], rituximab [78, 79], and abatacept or tocilizumab [80, 81].
There are conflicting published data concerning the potential role of RFs in predicting responses to antitumor necrosis factor alpha (TNF-α): some studies have found that RF positivity before therapy is insufficient to predict a response [82–85], whereas others have found that it predicts a negative response [86, 87]. In particular, it has been reported that high pretreatment levels of IgA RF are associated with a poor clinical response to TNF-α inhibitors [88].
High serum levels of RF are predictors of more severe disease forms and B cell-depleting therapy can have a beneficial effect: RF-positive RA patients have a better response to rituximab than those who are RF negative [89–92].
7. Conclusions
It has been demonstrated that low-affinity RFs appear to be key player in immune responses to many infectious organisms, and high-affinity RFs indicate more severe and persistent disease in patients with RA. RFs are probably the result of the immune response to inflammation (depending on genetic background) and may have regulatory effects on Ig production by controlling B cell activation.
References
- 1.Dörner T, Egerer K, Feist E, Burmester GR. Rheumatoid factor revisited. Current Opinion in Rheumatology. 2004;16(3):246–253. doi: 10.1097/00002281-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Waaler E. On the occurrence of a factor in human serum activating the specific agglutination of sheep blood corpuscles. Acta Pathologica Microbiologica Scandinavica. 1940;17(2):172–188. doi: 10.1111/j.1600-0463.2007.apm_682a.x. [DOI] [PubMed] [Google Scholar]
- 3.Rose HM, Ragan C, et al. Differential agglutination of normal and sensitized sheep erythrocytes by sera of patients with rheumatoid arthritis. Proceedings of the Society for Experimental Biology and Medicine. 1948;68(1):1–6. doi: 10.3181/00379727-68-16375. [DOI] [PubMed] [Google Scholar]
- 4.Pike RM, Sulkin SE, Coggeshall HC. Serological reactions in rheumatoid arthritis; factors affecting the agglutination of sensitized sheep erythrocytes in rheumatid-arthritis serum. Journal of Immunology. 1949;63(4):441–446. [PubMed] [Google Scholar]
- 5.Ball J, Bloch KJ, Burch TA, Kellgren JH, Lawrence JS, Tsigalidou V. Comparative studies of serologic tests for rheumatoid disease. II. A comparison of the bentonite flocculation test and the sensitized sheep cell agglutination test. Arthritis and Rheumatism. 1962;5:61–69. doi: 10.1002/art.1780050108. [DOI] [PubMed] [Google Scholar]
- 6.Ball J, De Graaff R, Valkenburg HA, Boerma FW. Comparative studies of serologic tests for rheumatoid disease. I. A comparison of a latex test and two erythrocyte agglutination tests in a random population sample. Arthritis and Rheumatism. 1962;5(1):55–60. doi: 10.1002/art.1780050107. [DOI] [PubMed] [Google Scholar]
- 7.Plotz CM, Singer JM. The latex fixation test. I. Application to the serologic diagnosis of rheumatoid arthritis. The American Journal of Medicine. 1956;21(6):888–892. [PubMed] [Google Scholar]
- 8.Plotz CM, Singer JM. The latex fixation test. II. Results in rheumatoid arthritis. The American Journal of Medicine. 1956;21(6):893–896. [PubMed] [Google Scholar]
- 9.Ailus K, Melamies L, Tuomi T, Palosuo T, Aho K. Measuring rheumatoid factor in nonrheumatoid subjects: immunoturbidimetric assay, latex slide test, and enzyme-linked immunosorbent assay compared. Clinical Chemistry. 1991;37(10, part 1):1766–1769. [PubMed] [Google Scholar]
- 10.Wolfe F, Cathey MA, Roberts FK. The latex test revisited: rheumatoid factor testing in 8,287 rheumatic disease patients. Arthritis and Rheumatism. 1991;34(8):951–960. doi: 10.1002/art.1780340804. [DOI] [PubMed] [Google Scholar]
- 11.Larkin JG, Sturrock RD, Stimson WH. A rapid enzyme immunoassay for the detection of IgM rheumatoid factor. A comparison of ’sero-negative’ and ’sero-positive’ rheumatoid patients. Journal of Clinical and Laboratory Immunology. 1986;20(4):207–209. [PubMed] [Google Scholar]
- 12.Ulvestad E, Wilfred LL, Kristoffersen EK. Measurement of IgM rheumatoid factor by ELISA. Scandinavian Journal of Rheumatology. 2001;30(6):366–366. doi: 10.1080/030097401317148598. [DOI] [PubMed] [Google Scholar]
- 13.Abreu I, Laroche P, Bastos A, et al. Multiplexed immunoassay for detection of rheumatoid factors by FIDISTM technology. Annals of the New York Academy of Sciences. 2005;1050:357–363. doi: 10.1196/annals.1313.038. [DOI] [PubMed] [Google Scholar]
- 14.LeGatt DF, Blakney GB, Higgins TN, et al. The effect of paraproteins and rheumatoid factor on four commercial immunoassays for vancomycin: implications for laboratorians and other health care professionals. Therapeutic Drug Monitoring. 2012;34(3):306–311. doi: 10.1097/FTD.0b013e318257335f. [DOI] [PubMed] [Google Scholar]
- 15.Surnamebartels GM, Surnameribel-Madsen G. Cytokine measurements and possible interference from heterophilic antibodies—Problems and solutions experienced with rheumatoid factor. Methods. 2013;61(1):18–22. doi: 10.1016/j.ymeth.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) Journal of Thrombosis and Haemostasis. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 17.Lakos G. Interference in antiphospholipid antibody assays. Seminars in Thrombosis and Hemostasis. 2012;38(4):353–359. doi: 10.1055/s-0032-1304714. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson DL, Harris AG, Neal KR, Irving WL. The presence of rheumatoid factor in sera from anti-HCV positive blood donors interferes with the detection of HCV-specific IgM. Journal of Hepatology. 1996;25(5):621–626. doi: 10.1016/s0168-8278(96)80229-7. [DOI] [PubMed] [Google Scholar]
- 19.Meurman OH, Ziola BR. IgM-class rheumatoid factor interference in the solid-phase radioimmunoassay of rubella-specific IgM antibodies. Journal of Clinical Pathology. 1978;31(5):483–487. doi: 10.1136/jcp.31.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Després N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clinical Chemistry. 1998;44(3):440–454. [PubMed] [Google Scholar]
- 21.Ramos-Levi AM, Montanez MC, Ortega I, Cobo MJ, Calle-Pascual AL. A case of biochemical assay discrepancy: interference with measurement of thyroid-stimulating hormone due to rheumatoid factor. Endocrinología y Nutrición. 2013;60(6):342–345. doi: 10.1016/j.endonu.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Berth M, Bosmans E, Everaert J, et al. Rheumatoid factor interference in the determination of carbohydrate antigen 19-9 (CA 19-9) Clinical Chemistry and Laboratory Medicine. 2006;44(9):1137–1139. doi: 10.1515/CCLM.2006.205. [DOI] [PubMed] [Google Scholar]
- 23.Bartels E, Falbe I, Andersen E, Danneskiold-Samsøe B, Bliddal H, Ribel-Madsen S. Rheumatoid factor and its interference with cytokine measurements: problems and solutions. Arthritis. 2011;2011:7 pages. doi: 10.1155/2011/741071.741071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newkirk MM. Rheumatoid factors: host resistance or autoimmunity? Clinical Immunology. 2002;104(1):1–13. doi: 10.1006/clim.2002.5210. [DOI] [PubMed] [Google Scholar]
- 25.Shmerling RH, Delbanco TL. The rheumatoid factor: an analysis of clinical utility. American Journal of Medicine. 1991;91(5):528–534. doi: 10.1016/0002-9343(91)90190-9. [DOI] [PubMed] [Google Scholar]
- 26.Westwood OMR, Nelson PN, Hay FC. Rheumatoid factors: what’s new? Rheumatology. 2006;45(4):379–385. doi: 10.1093/rheumatology/kei228. [DOI] [PubMed] [Google Scholar]
- 27.Dresser DW, Popham AM. Induction of an IgM anti (bovine) IgG response in mice by bacterial lipopolysaccharide. Nature. 1976;264(5586):552–554. doi: 10.1038/264552a0. [DOI] [PubMed] [Google Scholar]
- 28.Slaughter L, Carson DA, Jensen FC. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. Journal of Experimental Medicine. 1978;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palazzi C, Buskila D, D’Angelo S, D’Amico E, Olivieri I. Autoantibodies in patients with chronic hepatitis C virus infection: pitfalls for the diagnosis of rheumatic diseases. Autoimmunity Reviews. 2011;11(9):659–663. doi: 10.1016/j.autrev.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Charles ED, Orloff MI, Nishiuchi E, Marukian S, Rice CM, Dustin LB. Somatic hypermutations confer rheumatoid factor activity in hepatitis C virus-associated mixed cryoglobulinemia. Arthritis and Rheumatism. 2013;65(9):243–240. doi: 10.1002/art.38041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Børretzen M, Chapman C, Natvig JB, Thompson KM. Differences in mutational patterns between rheumatoid factors in health and disease are related to variable heavy chain family and germ-line gene usage. European Journal of Immunology. 1997;27(3):735–741. doi: 10.1002/eji.1830270323. [DOI] [PubMed] [Google Scholar]
- 32.Simard JF, Holmqvist M. Rheumatoid factor positivity in the general population. British Medical Journal. 2012;345(article e5841) doi: 10.1136/bmj.e5841. [DOI] [PubMed] [Google Scholar]
- 33.Tasliyurt T, Kisacik B, Kaya SU, et al. The frequency of antibodies against cyclic citrullinated peptides and rheumatoid factor in healthy population: a field study of rheumatoid arthritis from northern turkey. Rheumatology International. 2013;33(4):939–942. doi: 10.1007/s00296-012-2458-5. [DOI] [PubMed] [Google Scholar]
- 34.Newkirk MM. Rheumatoid factors: what do they tell us? Journal of Rheumatology. 2002;29(10):2034–2040. [PubMed] [Google Scholar]
- 35.Jacobsson LTH, Knowler WC, Pillemer S, et al. Rheumatoid arthritis and mortality. A longitudinal study in Pima Indians. Arthritis and Rheumatism. 1993;36(8):1045–1053. doi: 10.1002/art.1780360804. [DOI] [PubMed] [Google Scholar]
- 36.Korpilähde T, Heliövaara M, Kaipiainen-Seppänen O, Knekt P, Aho K. Regional differences in Finland in the prevalence of rheumatoid factor in the presence and absence of arthritis. Annals of the Rheumatic Diseases. 2003;62(4):353–355. doi: 10.1136/ard.62.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dresser DW. Most IgM-producing cells in the mouse secrete auto-antibodies (rheumatoid factor) Nature. 1978;274(5670):480–483. doi: 10.1038/274480a0. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen SF, Bojesen SE, Schnohr P, Nordestgaard BG. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. Britich Medical Journal. 2012;345(article e5244) doi: 10.1136/bmj.e5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Schaardenburg D, Lagaay AM, Breedveld FC, Hijmans W, Vandenbroucke JP. Rheumatoid arthritis in a population of persons aged 85 years and over. British Journal of Rheumatology. 1993;32(2):104–109. doi: 10.1093/rheumatology/32.2.104. [DOI] [PubMed] [Google Scholar]
- 40.Ursum J, Bos WH, Van de Stadt RJ, Dijkmans BAC, Van Schaardenburg D. Different properties of ACPA and IgM-RF derived from a large dataset: further evidence of two distinct autoantibody systems. Arthritis Research and Therapy. 2009;11(3, article R75) doi: 10.1186/ar2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Díaz-López C, Geli C, Corominas H, et al. Are there clinical or serological differences between male and female patients with primary Sjögren’s syndrome? Journal of Rheumatology. 2004;31(7):1352–1355. [PubMed] [Google Scholar]
- 42.Sansonno D, Lauletta G, Nisi L, et al. Non-enveloped HCV core protein as constitutive antigen of cold-precipitable immune complexes in type II mixed cryoglobulinaemia. Clinical and Experimental Immunology. 2003;133(2):275–282. doi: 10.1046/j.1365-2249.2003.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masaki Y, Sugai S. Lymphoproliferative disorders in Sjögren’s syndrome. Autoimmunity Reviews. 2004;3(3):175–182. doi: 10.1016/S1568-9972(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 44.Swedler W, Wallman J, Froelich CJ, Teodorescu M. Routine measurement of IgM, IgG, and IgA rheumatoid factors: high sensitivity, specificity, and predictive value for rheumatoid arthritis. Journal of Rheumatology. 1997;24(6):1037–1044. [PubMed] [Google Scholar]
- 45.Aletaha D, Alasti F, Smolen JS. Rheumatoid factor determines structural progression of rheumatoid arthritis dependent and independent of disease activity. Annals of the Rheumatic Diseases. 2013;72(6):875–880. doi: 10.1136/annrheumdis-2012-201517. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Annals of Internal Medicine. 2007;146(11):797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 47.Nell VPK, Machold KP, Stamm TA, et al. Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Annals of the Rheumatic Diseases. 2005;64(12):1731–1736. doi: 10.1136/ard.2005.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zvaifler NJ. The immunopathology of joint inflammation in rheumatoid arthritis. Advances in Immunology. 1973;16(C):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- 49.Haberman AM, William J, Euler C, Shlomchik MJ. Rheumatoid factors in health and disease: structure, function, induction and regulation. Current Directions in Autoimmunity. 2003;6:169–195. doi: 10.1159/000066861. [DOI] [PubMed] [Google Scholar]
- 50.Stewart JJ, Agosto H, Litwin S, et al. A solution to the rheumatoid factor paradox: pathologic rheumatoid factors can be tolerized by competition with natural rheumatoid factors. Journal of Immunology. 1997;159(4):1728–1738. [PubMed] [Google Scholar]
- 51.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 52.Sweet RA, Cullen JL, Shlomchik MJ. Rheumatoid factor B cell memory leads to rapid, switched antibody-forming cell responses. Journal of Immunology. 2013;190(5):1974–1981. doi: 10.4049/jimmunol.1202816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burastero SE, Casali P, Wilder RL, Notkins AL. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. Journal of Experimental Medicine. 1988;168(6):1979–1992. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schroeder HW, Jr., Cavacini L. Structure and function of immunoglobulins. Journal of Allergy and Clinical Immunology. 2010;125(2):S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jónsson T, Steinsson K, Jónsson H, Geirsson ÁJ, Thorsteinsson J, Valdimarsson H. Combined elevation of IgM and IgA rheumatoid factor has high diagnostic specificity for rheumatoid arthritis. Rheumatology International. 1998;18(3):119–122. doi: 10.1007/s002960050069. [DOI] [PubMed] [Google Scholar]
- 56.Jónsson T, Valdimarsson H. What about IgA rheumatoid factor in rheumatoid arthritis? Annals of the Rheumatic Diseases. 1998;57(1):63–64. doi: 10.1136/ard.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deane KD, O’Donnell CI, Hueber W, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis and Rheumatism. 2010;62(11):3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller A, Mahtani KR, Waterfield MA, Timms A, Misbah SA, Luqmani RA. Is rheumatoid factor useful in primary care? A retrospective cross-sectional study. Clinical Rheumatology. 2013;32(7):1089–1093. doi: 10.1007/s10067-013-2236-0. [DOI] [PubMed] [Google Scholar]
- 59.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 60.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and Rheumatism. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 61.Bas S, Genevay S, Meyer O, Gabay C. Anti-cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology. 2003;42(5):677–680. doi: 10.1093/rheumatology/keg184. [DOI] [PubMed] [Google Scholar]
- 62.Rantapää-Dahlqvist S, De Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis and Rheumatism. 2003;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 63.Gilliam BE, Moore TL. The role of anti-cyclic citrullinated peptide (CCP) antibodies in early detection of rheumatoid arthritis: an overview of the INOVA Diagnostics, Inc. QUANTA Lite CCP assays. Expert Opinion on Medical Diagnostics. 2012;6(4):359–369. doi: 10.1517/17530059.2012.694423. [DOI] [PubMed] [Google Scholar]
- 64.Manivelavan D, Vijayasamundeeswari CK. Anti-cyclic citrullinated peptide antibody: an early diagnostic and prognostic biomarker of rheumatoid arthritis. Journal of Clinical and Diagnostic Research. 2012;6(8):1393–1396. doi: 10.7860/JCDR/2012/4692.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peoples C, Valiyil R, Davis RB, Shmerling RH. Clinical use of anti-cyclic citrullinated Peptide antibody testing. Journal of Clinical Rheumatology. 2013;19(6):351–352. doi: 10.1097/RHU.0b013e3182a22418. [DOI] [PubMed] [Google Scholar]
- 66.Demoruelle MK, Parish MC, Derber LA, et al. Anti-cyclic citrullinated peptide assays differ in subjects at elevated risk for rheumatoid arthritis and subjects with established disease. Arthritis and Rheumatism. 2013;65(9):2243–2252. doi: 10.1002/art.38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Debaugnies F, Servais G, Badot V, Noubouossie D, Willems D, Corazza F. Anti-cyclic citrullinated peptide antibodies: a comparison of different assays for the diagnosis of rheumatoid arthritis. Scandinavian Journal of Rheumatology. 2013;42(2):108–114. doi: 10.3109/03009742.2012.723746. [DOI] [PubMed] [Google Scholar]
- 68.Rantapää-Dahlqvist S. What happens before the onset of rheumatoid arthritis? Current Opinion in Rheumatology. 2009;21(3):272–278. doi: 10.1097/BOR.0b013e32832a2e44. [DOI] [PubMed] [Google Scholar]
- 69.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheumatic Disease Clinics of North America. 2010;36(2):213–241. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tillmann T, Krishnadas R, Cavanagh J, Petrides K. Possible rheumatoid arthritis subtypes in terms of rheumatoid factor, depression, diagnostic delay and emotional expression: an exploratory case-control study. Arthritis Research and Therapy. 2013;15(2, article R45) doi: 10.1186/ar4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barra L, Bykerk V, Pope JE, et al. Anticitrullinated protein antibodies and rheumatoid factor fluctuate in early inflammatory arthritis and do not predict clinical outcomes. Journal of Rheumatology. 2013;40(8):1259–1267. doi: 10.3899/jrheum.120736. [DOI] [PubMed] [Google Scholar]
- 72.Mikuls TR, O’Dell JR, Stoner JA, et al. Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti-cyclic citrullinated peptide antibody. Arthritis and Rheumatism. 2004;50(12):3776–3782. doi: 10.1002/art.20659. [DOI] [PubMed] [Google Scholar]
- 73.Bobbio-Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment. Arthritis research & therapy. 2004;6(3):R264–272. doi: 10.1186/ar1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Rycke L, Verhelst X, Kruithof E, et al. Rheumatoid factor, but not anti-cyclic citrullinated peptide antibodies, is modulated by infliximab treatment in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2005;64(2):299–302. doi: 10.1136/ard.2004.023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caramaschi P, Biasi D, Tonolli E, et al. Antibodies against cyclic citrullinated peptides in patients affected by rheumatoid arthritis before and after infliximab treatment. Rheumatology International. 2005;26(1):58–62. doi: 10.1007/s00296-004-0571-9. [DOI] [PubMed] [Google Scholar]
- 76.Chen HA, Lin KC, Chen CH, et al. The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;65(1):35–39. doi: 10.1136/ard.2005.038851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atzeni F, Sarzi-Puttini P, Dell’ Acqua D, et al. Adalimumab clinical efficacy is associated with rheumatoid factor and anti-cyclic citrullinated peptide antibody titer reduction: a one-year prospective study. Arthritis Research & Therapy. 2006;8(1, article R3) doi: 10.1186/ar1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cambridge G, Leandro MJ, Edwards JCW, et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis and Rheumatism. 2003;48(8):2146–2154. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 79.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis and Rheumatism. 2006;54(9):2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 80.Maneiro RJ, Salgado E, Carmona L, Gomez-Reino JJ. Rheumatoid factor as predictor of response to abatacept, rituximab and tocilizumab in rheumatoid arthritis: systematic review and meta-analysis. Seminars in Arthritis and Rheumatism. 2013;43(1):9–17. doi: 10.1016/j.semarthrit.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Faillace C, de Carvalho JF. Rheumatoid factor appearance after tocilizumab treatment seems to predict bad therapeutical response in rheumatoid arthritis. Rheumatology International. 2013:1909–1910. doi: 10.1007/s00296-012-2409-1. [DOI] [PubMed] [Google Scholar]
- 82.Hyrich KL, Watson KD, Silman AJ, Symmons DPM. Predictors of response to anti-TNF-α therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology. 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 83.Bos WH, Bartelds GM, Wolbink GJ, et al. Differential response of the rheumatoid factor and anticitrullinated protein antibodies during adalimumab treatment in patients with rheumatoid arthritis. Journal of Rheumatology. 2008;35(10):1972–1977. [PubMed] [Google Scholar]
- 84.Salgado E, Maneiro JR, Carmona L, Gomez-Reino J. nd response to TNF antagonists in rheumatoid arthritis: systematic review and meta-analysis of observational studies. Joint Bone Spine. 2013 doi: 10.1016/j.jbspin.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Salgado E, Maneiro JR, Carmona L, Gomez-Reino JJ. Safety profile of protein kinase inhibitors in rheumatoid arthritis: systematic review and meta-analysis. Annals of the Rheumatic Diseases. 2013 doi: 10.1136/annrheumdis-2012-203116. [DOI] [PubMed] [Google Scholar]
- 86.Klaasen R, Cantaert T, Wijbrandts CA, et al. The value of rheumatoid factor and anti-citrullinated protein antibodies as predictors of response to infliximab in rheumatoid arthritis: an exploratory study. Rheumatology. 2011;50(8):1487–1493. doi: 10.1093/rheumatology/ker010. [DOI] [PubMed] [Google Scholar]
- 87.Potter C, Hyrich KL, Tracey A, et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2009;68(1):69–74. doi: 10.1136/ard.2007.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bobbio-Pallavicini F, Caporali R, Alpini C, Moratti R, Montecucco C. Predictive value of antibodies to citrullinated peptides and rheumatoid factors in anti-TNF-α treated patients. Annals of the New York Academy of Sciences. 2007;1109:287–295. doi: 10.1196/annals.1398.034. [DOI] [PubMed] [Google Scholar]
- 89.Edwards JCW, Szczepański L, Szechiński J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. The New England Journal of Medicine. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 90.Quartuccio L, Fabris M, Salvin S, et al. Rheumatoid factor positivity rather than anti-CCP positivity, a lower disability and a lower number of anti-TNF agents failed are associated with response to rituximab in rheumatoid arthritis. Rheumatology. 2009;48(12):1557–1559. doi: 10.1093/rheumatology/kep314. [DOI] [PubMed] [Google Scholar]
- 91.Isaacs JD, Cohen SB, Emery P, et al. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Annals of the Rheumatic Diseases. 2013;72(3):329–336. doi: 10.1136/annrheumdis-2011-201117. [DOI] [PubMed] [Google Scholar]
- 92.Jones JD, Shyu I, Newkirk MM, Rigby WF. A rheumatoid factor paradox: inhibition of rituximab effector function. Arthritis Research and Therapy. 2013;15(1, article R20) doi: 10.1186/ar4152. [DOI] [PMC free article] [PubMed] [Google Scholar]


