Abstract
The objective of this study was to investigate the effect of long-term exercise training on concentrations of five hormones related to appetite and insulin resistance in overweight adolescents. Additionally, we were interested in the relationships of these hormones with each other and with anthropometric and/or cardiovascular disease marker changes. Participants were ≥ the 85th percentile for body mass index for age and sex and participated in an eight month supervised aerobic training program. Anthropometrics, cardiovascular fitness assessment, and fasting blood samples were taken pre- and post-training. Glucose, insulin, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, leptin, active ghrelin, total peptide YY (PYY), adiponectin, and resistin concentrations were measured. The participants increased their time to exhaustion on an incremental treadmill test and decreased both percent body fat and blood triglyceride concentrations. Total PYY concentration increased and resistin concentration decreased after long-term exercise training, which are favorable outcomes. Leptin concentrations were related to weight, percent body fat, waist circumference, and triglyceride concentrations pre- and post-training. The changes in resistin concentrations were related to the changes in triglyceride concentrations. We conclude that long-term exercise training has beneficial effects for overweight adolescents with respect to PYY and resistin, hormones related to appetite and insulin sensitivity.
Introduction
In the period of 1999 – 2002 in the United States 16% of children and adolescents overweight and 31% were at risk for overweight (1). Adolescents develop the same weight-associated diseases such as Type 2 diabetes and dyslipidmeia seen in adults (2, 3). The effect of aerobic exercise in the prevention of cardiovascular disease and enhanced glucose uptake is well-documented (4, 5). Exercise is therefore used as therapeutic treatment for both diabetes and cardiovascular disease. Aerobic fitness is inversely related to adiposity in children and there is currently evidence that exercise may prevent Type 2 diabetes (6, 7). If a regimen of exercise, along with healthy eating, is developed as a lifestyle in overweight children and adolescents, there is the potential that they will attain a healthy weight and the consequences of unhealthy weight such as development of Type 2 diabetes and cardiovascular disease will not occur.
Hormones that play a role in appetite and insulin sensitivity are of interest to the health and medical community because of their potential to be regulated in patients that are overweight and/or insulin resistant. Leptin, ghrelin, and peptide YY (PYY) are related to appetite. Leptin suppresses appetite, reduces food intake, and has metabolic actions related to lipid oxidation and storage (8, 9). Leptin production correlates with the size and lipid content of the individual fat cell (10); a relationship maintained with weight loss (11). Ghrelin levels increase immediately before a meal and fall within one hour after eating suggesting a role for ghrelin in meal initiation (12). PYY is secreted postprandially in proportion to caloric load in humans and inhibits appetite and food intake (13, 14). Obese humans have lower pre-meal PYY concentrations, have lower post-meal PYY concentrations, and consume more calories compared to lean controls when both are presented with comparable food choices (15). Adiponectin and resistin are related to insulin sensitivity. Adiponectin plays a role in reducing hyperglycemia and is associated with increased insulin sensitivity (16). Adiponectin is protective against the development of insulin resistance and dyslipidemia (17). Concentrations of adiponectin are lower in obese than non-obese subjects (18). Resistin has been reported to be one of the links between obesity and insulin resistance (19). Resistin has been reported to be positively related to insulin resistance in humans (20). Although the role of resistin in energy homeostasis is not completely defined, interest in this hormone remains high. The understanding of the concentrations of these hormones in overweight people and the role they may play in obesity and/or diabetes in children and adolescents is important. Just as important is an understanding of how life-style choices such as regular aerobic exercise impact these hormones’ concentrations so there is a better insight into their regulation.
We have examined five hormones related to appetite and insulin sensitivity in overweight adolescents and have determined the response of these hormones to long-term exercise training. We are not aware of any published papers that have made concomitant measures of leptin, adiponectin, resistin, active ghrelin, and PYY in overweight adolescents. There are also no published data on the response of these hormones in relationship with each other to a long-term exercise program. We hypothesized that eight months of exercise training would favorably alter the milieu of hormones related to appetite, adiposity, and insulin sensitivity: resistin and ghrelin would be decreased and leptin, adiponectin, and PYY would be increased with prolonged aerobic training. Furthermore, these alterations would be accompanied by favorable changes in insulin, glucose, and the traditional cardiovascular disease risk markers of blood lipids.
Methods and Procedures
Subjects
Subjects were recruited by an ad placed in the local newspaper, through pediatricians, and from a web-based email announcement system that is available to East Carolina University employees. Participation was voluntary and to be included in the study the subjects had to meet the following criteria: (1) 12 – 18 years of age, (2) body mass index (BMI) above the 85th percentile for age and sex, (3) physician approval to participate in the project, (4) no medical conditions that would prevent participation in a vigorous aerobic exercise program, and (5) signed informed consent by parent/guardian and assent by children under the age of 18. All participants reported being sedentary prior to participation. Three subjects dropped out of the study due to conflicts with school work and the inability of their parents to get them to the exercise facility. The data from these subjects was not included because it was not complete. Attendance for the study was over 90%.
Study protocol
The study protocol was approved by the East Carolina University and Medical Center Institutional Review Board. Anthropometric measurements, cardiorespiratory fitness, and blood samples were taken pre- and post-exercise training. Exercise training duration was 32 weeks. Subjects were assigned to a mentor which was an upperclass undergraduate exercise and sport science major. The subjects met with their mentors three times per week for approximately one hour. The role of the mentor was to encourage the subject to attend the sessions, provide motivation, and ensure the subject was exercising at the appropriate intensity. Typical exercise sessions consisted of a five min warm up of light stretching, 45 min of aerobic training at 60–85% of measured peak oxygen uptake (VO2), and five to 10 min of cool down activity. In order to keep subjects motivated, they chose which piece of equipment for aerobic exercise they wanted to use. Available to them was a treadmill, Stairmaster, elliptical trainer, rowing machine, stationary cycle, Dance Dance Revolution, or outdoors play activity. Polar heart rate monitors were worn by the subjects during every exercise session. Heart rate (HR), duration, intensity, and the pieces of equipment used at each session were recorded. When HR responses were reduced more than five beats per minute on average as compared to a previous week at a given workload, exercise intensity was increased for subsequent workouts to bring the exercising heart rate back up to the heart rate target that equated to 70% of VO2peak.
Anthropometrics
Anthropometric measurements of height, weight, percent body fat, and circumference measurements were taken pre- and post-exercise training. Percent body fat was determined using the skinfold method. Body density was predicted with the generalized skinfold equation of Jackson and Pollock (21). Body density was transformed to percent body fat with the Siri equation (22). Skinfolds were obtained using Harpendon skinfold calipers. All measurements were made on the right side of the body at the following sites: chest (pectoral), midaxillary, triceps, subscapular, abdominal, suprailiac, thigh, and medial calf. Skinfold measurements were taken sequentially in the same order twice and recorded to the nearest 0.2mm. Measurements were repeated if the two values differed by more than 2mm. The coefficient of variation (CV) for repeat measures of percent body fat on a given day was 0.44%. Circumference measurements were taken with a Guilick Tape Measure to the closest 0.1 mm. Measurements were made at the following sites: neck, minimum waist, umbilicus, maximal hip, arm, thigh, and mid-calf.
Cardiorespiratory fitness
Cardiorespiratory fitness was determined pre- and post-exercise training. Subjects completed a maximal treadmill test to exhaustion to determine VO2peak. Subjects did a three min warm up at a speed pre-determined before the test began. This speed was determined in a treadmill familiarization session at least one day before the test to ensure the speed was one the subject could sustain for the duration of the test. Elevation was increased by 2% every minute after the warm-up. Speed was increased to a faster walk or slow jog depending on the subject’s comfort level with the treadmill. Expired gasses were collected every 20 seconds by a Parvo Medics TrueMax 2400 metabolic cart, Quinton Q-Stress system, and Quinton TM55 treadmill. Heart rate and rate of perceived exertion (RPE) were recorded during the final 15 seconds of every minute. Successful tests were determined by meeting one or more of the following criteria: respiratory exchange ratio (RER) ≥ 1.0, rating of perceived exertion (RPE) ≥ 17, predicted maximal heart rate ten beats from age predicted maximum (220 - age).
Assays
Twelve-hour fasting blood samples from the antecubital vein were taken pre- and post-exercise training between 7:00 a.m. and 9:30 a.m. The samples were analyzed for glucose, insulin, total cholesterol, HDL cholesterol, triglycerides, leptin, resistin, adiponectin, active ghrelin, and total PYY. Glucose, insulin, cholesterol, HDL cholesterol, and triglycerides were measured by a certified analytical laboratory (Lab Corps of America, Greenville, NC). LDL cholesterol was predicted using the Friedwald equation (LDL = cholesterol - HDL - (triglycerides/5) (23). Plasma was prepared for hormone measurements. A portion of plasma was acidified to protect active (octanoylated) ghrelin. Samples were frozen at −70°C for later use. Leptin, resistin, adiponectin, and active ghrelin were measured using ELISA kits from LINCO Research, Inc. (St. Charles, MO, USA) and total PYY was measured using an ELISA kit from Diagnostic Systems Laboratories, Inc. (Webster, TX, USA). The intraassay CVs were 2.6% for leptin, 3.7% for adiponectin, 2.7% for resistin, 9.2% for active ghrelin, and 2.3% for total PYY.
Statistical analysis
Values are presented as mean ± standard deviation. Pre-training and post-training values were determined with the Student’s t Test for paired comparisons. The relationships between variables were tested with linear regression analysis with the statistical significance set at p ≤0.05. These analyses were performed using Microsoft Office Excel 2007. 95% confidence intervals were determined with R, version 2.5.1, from The R Foundation for Statistical Computing (http://www.r-project.org/).
Results
Subject characteristics
Table 1 shows the characteristics of the 12 subjects that participated in the study. With long-term exercise training, subjects lost fat mass seen as a decrease in percent body fat of 2.2% although weight, waist circumference, and BMI did not change. There was significant improvement in time to exhaustion by one min on the treadmill although the increase in VO2peak, either as ml/kg·min or ml/kg lean body mass · min, was not significant. Absolute VO2 increased 6.5% but did not reach significance (p = 0.059). The subjects were not diabetic and had normal fasting glucose and insulin concentrations. Fasting glucose and insulin concentrations did not change with long-term exercise training. Height did not change during the duration of the study.
Table 1. Subject characteristics.
12 subjects consisted of 7 females and 5 males with a mean age of 15.3 ± 0.5 years
| Pre-training mean ± SD |
Post-training mean ± SD |
Significance | 95% CI | |
|---|---|---|---|---|
| Height (cm) | 169.2 ± 4.6 | 169.9 ± 4.6 | p = 0. 058 | −0.03, 1.62 |
| Weight (kg) | 90.9 ± 15.6 | 92.8 ± 17.8 | p = 0.298 | −4.22, 12.52 |
| Body mass index (BMI) | 31.8 ± 5.2 | 32.1 ± 5.6 | p = 0.588 | −0.89, 1.49 |
| Percent body fat (%) | 29.7 ± 6.1 | 27.5 ± 8.3 | p = 0.006 | −3.63, −0.76 |
| Waist circumference (cm) | 95.8 ± 9.6 | 93.5 ± 10.7 | p = 0.093 | −5.11, 0.46 |
| Time to exhaustion (s) | 507 ± 83 | 567 ± 90 | p = 0.0002 | 35.4, 84.55 |
| Peak VO2 (ml/kg ·min) | 35.2 ± 9.9 | 36.8 ± 10.8 | p = 0.215 | −1.09, 4.32 |
| Peak Vo2 (ml/kg lbm ·min) | 49.4 ± 11.3 | 49.9 ± 9.9 | p = 0.752 | −3.95, 2.94 |
| VO2 (l/min) | 3.072 ± 0.585 | 3.272 ± 0.595 | p = 0.059 | −0.408, 0.009 |
| Glucose (mg/dL) | 89 ± 6 | 90 ± 6 | p = 0.654 | −3.15, 4.81 |
| Insulin (uU/mL) | 13.7 ± 5.9 | 14.5 ± 7.8 | p = 0.757 | −5.06, 6.76 |
CI = Confidence interval
lbm = lean body mass
Cardiovascular disease risk factors
Table 2 presents the circulating, fasting lipid concentrations for total cholesterol (TC), HDL cholesterol, LDL cholesterol, and serum triglycerides pre- and post-training. Triglyceride concentration was decreased 23% with long-term exercise training while concentrations of TC, HDL cholesterol, and LDL cholesterol did not change. The HDL:TC ratio was 3.7 and 3.5 pre- and post-training, respectively.
Table 2.
Cardiovascular disease risk factors and plasma hormone concentrations
| Pre-training mean ± SD |
Post-training mean ± SD |
Significance | 95% CI | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 166 ± 32 | 159 ± 28 | p = 0.243 | −17.62, 4.96 |
| HDL cholesterol (mg/dL) | 45 ± 7 | 46 ± 8 | p = 0.147 | −0.72, 4.22 |
| LDL cholesterol (mg/dL) | 100 ± 31 | 96 ± 29 | p = 0.269 | −11.52, 3.55 |
| Serum triglycerides (mg/dL) | 104 ± 53 | 80 ± 34 | p = 0.013 | −42.32, −6.18 |
| Leptin (ng/mL) | 68.0 ± 53.9 | 54.9 ± 41.8 | p = 0.164 | −32.26, 6.18 |
| Adiponectin (ng/mL) | 10.2 ± 3.2 | 9.8 ± 3.1 | p = 0.697 | −2.55, 1.76 |
| Resistin (ng/mL) | 12.0 ± 3.5 | 11.0 ± 3.3 | p = 0.046 | −2.04, −0.24 |
| Total peptide YY (pg/mL) | 171.2 ± 63.2 | 209.8 ± 78.9 | p = 0.049 | 0.19, 77.01 |
| Active ghrelin (fmol/mL) | 5.8 ± 3.1 | 5.4 ± 2.2 | p = 0.456 | −1.51, 0.72 |
CI = Confidence interval
Plasma hormone concentrations
Table 2 presents the circulating, fasting concentrations of the hormones targeted in this study. The two hormones that changed concentration significantly with long-term exercise training were resistin (8% decrease) and total PYY (23% increase). Leptin, adiponectin, and active ghrelin concentrations did not change with long-term exercise training.
Correlation between changes in triglyceride concentration and changes in resistin concentration
Both triglyceride and resistin concentrations decreased with long-term exercise training. The changes from pre- to post-training in triglyceride concentrations correlated (r = 0.620, p ≤ 0.05) with the change in resistin concentrations. Figure 1 graphically depicts this positive relationship.
Figure 1.
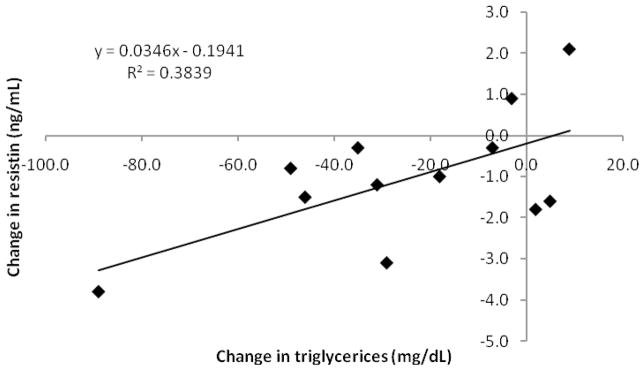
Relationship of changes in fasting triglyceride concentrations and changes in fasting resistin concentrations with long-term exercise training (p ≤ 0.05, 95% confidence interval (0.004, 0.065)).
Correlation between percent body fat and leptin concentrations
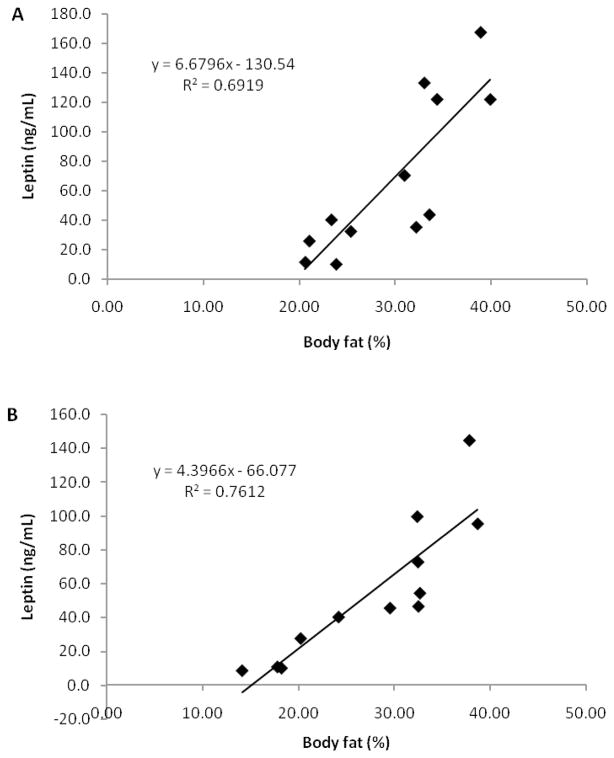
Leptin concentrations and percent body fat were correlated at pre-training (r = 0.832, p ≤ 0.001) and post-training (r = 0.872, p ≤ 0.0001). These positive relationships are graphically depicted in Figure 2A and 2B.
Figure 2.
Relationships between percent body fat and leptin concentrations (A) pre-training (p ≤ 0.001, 95% confidence interval (3.539, 9.820)) and (B) post-training (p ≤ 0.001, 95% confidence interval (2.661, 6.132)).
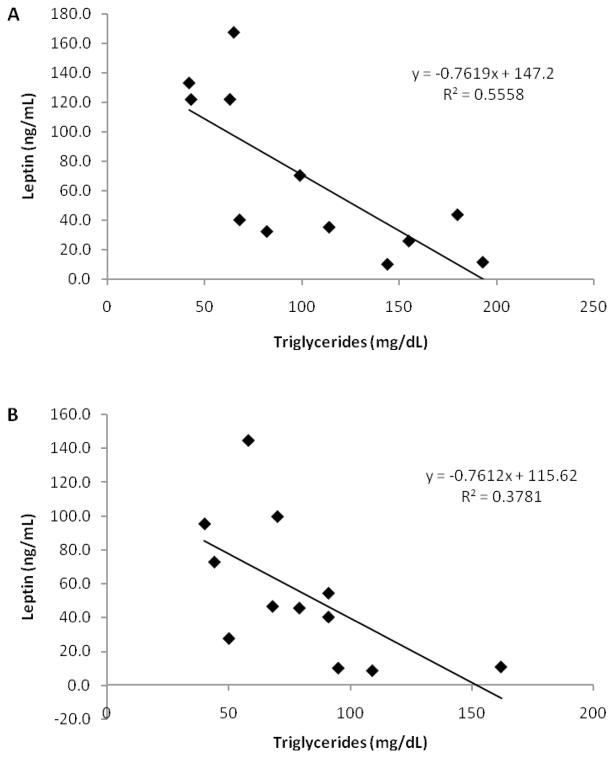
Correlation between triglyceride concentrations and leptin concentrations
Leptin concentrations and triglyceride concentrations were correlated at pre-training (r = −0.746, p ≤ 0.01) and post-training (r = −0.615, p ≤ 0.05). These positive relationships are graphically depicted in Figure 3A and 3B.
Figure 3.
Relationships between triglyceride concentrations and leptin concentrations (A) pre-training (p ≤ 0.01, 95% confidence interval (−1.242, −0.282)) and (B) post-training (p ≤ 0.05, 95% confidence interval (−1.449, −0.073)).
Discussion
We are interested in the role of long-term exercise training on hormones related to appetite (leptin, ghrelin, and PYY) and insulin sensitivity (adiponectin and resistin) in overweight adolescents, particularly with respect to concomitant changes in these hormones that might occur with exercise training. Participants in this study participated in eight months of supervised exercise sessions. We did not have a control group of non-exercising, overweight adolescents for pre- and post-comparison which is a limitation of our study. We do not advocate overweight teens be inactive for an extended period of time and therefore did not employ a non-exercise control group for ethical reasons. We found that our participants had decreased percent body fat, decreased concentrations of triglycerices and resistin and an increased concentration of PYY in response to long-term exercise training. Pre- and post-training leptin concentrations correlated with pre- and post-training percent body fat and pre- and post-training triglyceride concentrations. The change in triglycerides correlated with the change in resistin.
Long-term exercise training and cardiovascular disease risk factors
The adolescents in this study were greater than the 85th percentile of BMI for age and sex. Their circulating total and LDL cholesterol concentrations pre-training were in the acceptable range for 2–19 year olds (TC < 170 mg/dL and LDL cholesterol < 110mg/dL HDL) (24). Although there were not significant changes in post-training cholesterol concentrations, the means of TC and LDL cholesterol were lower. Mean total cholesterol decreased 9% and mean LDL cholesterol decreased 4%. The HDL: total cholesterol ratio was 3.7:1 in pre-training blood samples and was 3.5:1 in the post-training samples. 3.5:1 is the optimum ratio recommended by the American Heart Association. Circulating trigycerides were in the normal range in pre-training samples but did decrease significantly (23%) with training. Pre- and post-training lipid profiles in adolescents that participate in exercise training studies do not always show much change or improvements (25, 26).
Long-term exercise training effects on appetite-related hormones
PYY is the intestine-derived hormone that is produced in response to caloric load and inhibits appetite and food intake (13, 14). It has been demonstrated that PYY concentrations increase within minutes of ingesting a meal, peak at approximately 60 min, and remain elevated for up to 6 h (13). An increase in fasting PYY would suggest a reduction in appetite. A long-term exercise training study measuring PYY has not been previously published. The largest change in appetite-related hormones in the present study occurred in circulating total PYY concentrations (+23%). An acute exercise study that measured PYY in adult subjects found that PYY release was increased during exercise and similar to non-exercisers after exercise (27). Our results suggest that long-term exercise training, as opposed to acute exercise, increases fasting total PYY in overweight adolescents. PYY circulates as PYY1–36 and PYY3–36. PYY3–36 administered to humans reduces food intake suggesting it is the relevant form for appetite control (15). In the fed state, PYY3–36 is approximately 63% of circulating total PYY and in the fasted state is approximately 37% of circulating total PYY(28). A limitation to our study is that we did not determine the concentrations of PYY1–36 and PYY3–36 so we do not know if the increase was in the relevant form of PYY for appetite reduction. Additionally, we did not measure PYY after feeding which would have further defined the long-term exercise effect on this hormone that is sensitive to caloric load. Our subjects did lose fat mass, but we did not collect any information regarding food intake or diet; therefore, we do not know what role increased total PYY might have played in the loss of fat mass. Determining the concentrations of PYY3–36 with long-term exercise training in the fasted and fed state would further define the potential for exercise to regulate PYY concentrations in adolescents as well as adults.
In our participants, long-term exercise training did not alter mean active ghrelin concentrations in the fasted state. Ghrelin is produced in the stomach and concentrations increase before each meal and rapidly decline in the interval between meals suggesting ghrelin initiates food intake (12, 29). Exercise may not have a direct effect on ghrelin production because studies that test this typically report changes in ghrelin concentrations only if weight loss occurred as a result of the exercise program (30, 31). The cited studies were performed on either healthy weight (31) or overweight (30) participants. Ghrelin circulates in an active (octanoylated) form and inactive form (32). The present study is the first to determine active (octanoylated) ghrelin concentrations in response to long-term exercise training in overweight adolescents. Our participants did not lose weight and did not exhibit a change in active ghrelin concentration with long-term exercise training. Our results measuring active ghrelin parallel other studies reporting that exercise training does not affect total ghrelin concentrations if weight-loss does not occur with training. A limitation to our study is that we did not measure ghrelin in an interval between meals to determine if long-term exercise impacts the decline of ghrelin seen between meals.
Mean fasting leptin was reduced 19% by long-term exercise training in overweight adolescents in our study, but this difference was not significant (p = 0.165). Although an effect of elevated leptin is to suppress eating, leptin concentrations are elevated in overweight adolescents (33). Leptin release from fat cells is related to fat cell size and content (10). There are several reports of leptin concentrations being related to changing body composition (BMI, body fat) and waist circumference in children and adolescents (34, 35). We found that leptin concentrations were significantly correlated with pre- and post-training weights (r = 0.696, p ≤ 0.02 and r = 0.887 p ≤ 0.001, respectively) (data not shown) and with pre- and post-waist circumferences which did not change with long-term exercise training (r = 0.599, p ≤ 0.05 and r = 0.775, p ≤ 0.01, respectively) (data not shown). In this study percent body fat correlated with leptin concentration before long-term exercise training (r = 0.832, p ≤ 0.001) and remained correlated (r = 0.872, p ≤ 0.001) after a significant decrease in percent body fat (2.2%). It has been demonstrated that leptin concentrations remain correlated with fat mass after a small fat loss in children (mean age nine years) (36). It has also been demonstrated that changes in fat mass were related to changes in leptin in obese boys and girls after three weeks of low-calorie diet and physical activity (37). This has not been demonstrated in overweight adolescents and we did not see a relationship between the change in leptin concentrations and the change in percent body fat. Pre- and post-training triglyceride concentrations negatively correlated with the corresponding concentrations of leptin (r = −0.746, p ≤ 0.01 and r = −0.615, p ≤ 0.0.05, respectively). There was also a negative relationship in both pre- and post-training percent body fat and triglycerides (r = −0.572, p ≤ 0.10 and r = −0.663 p ≤ 0.0.02, respectively) (data not shown). Although percent body fat was positively correlated with leptin concentrations pre- and post-training, we cannot explain why an increase in circulating triglycerides would be inversely related with decrease in both percent body fat and leptin concentrations.
Long-term exercise training effects on insulin sensitivity hormones
Mean fasting resistin concentrations were significantly decreased (9%) by long-term exercise training in our participants. A reduction in resistin concentration would be interpreted as a positive change because resistin plays a role in insulin resistance. We are not aware of any published data on the effect of long-term exercise training on resistin concentrations in adolescents. In adult subjects that are diabetic or have impaired glucose tolerance, there are mixed results as to whether long-term exercise training increases or does not alter resistin concentrations, suggesting that training does not consistently produce favorable changes in resistin concentration (38, 39). Kadoglou et al. found a reduction in resistin levels of 31% in adults with type 2 diabetes with long-term exercise training (38). Our participants had a reduction of 9% in resistin concentration with long-term exercise training. The similarity between our two studies is the intensity and duration of each aerobic exercise session. It is difficult to interpret if the 9% decrease in resistin impacts insulin resistance since our subjects were not insulin resistant. Our study does suggest that long-term exercise alters the concentration of resistin and further study is warranted in determining the impact of exercise-regulation of resistin in those that are insulin resistant.
Adiponectin is associated with increased insulin sensitivity and plays a role in reducing hyperglycemia (16). In our study, mean fasting adiponectin was not altered by long-term exercise training in overweight adolescents which had normal glucose and insulin concentrations. In a study looking at insulin sensitivity changes in overweight and obese adolescents with 12-weeks of aerobic exercise training, insulin sensitivity did increase, but adiponectin concentrations did not (40). Body weight and percentage fat did not change in this study. Kraemer and Castracane reviewed the literature on exercise and adiponectin and concluded that exercise of sufficient intensity for a period of two months or greater had a beneficial effect on adiponectin; however, it could not be determined whether long-term exercise training directly altered adiponectin or whether it was the weight reduction resulting from long-term exercise training that directly upregulated adipose tissue production of adiponection (41).
Relationship of ghrelin, resistin, and leptin pre- and post-training
We found a relationship between pre-training ghrelin concentration and pre-training resistin concentration (r = 0.648, p ≤ 0.05) (data not shown). Post-training concentration of these two hormones were not correlated (r = 0.217). We did not find a change in ghrelin after training and decreases in ghrelin do not appear to occur unless there is weight loss, which did not occur in our participants. Resistin is linked with obesity; percent body fat and resistin concentrations decreased in our participants. These results may account for the loss of the relationship of ghrelin and resistin post-training. Exercise results in better regulation of the balance between energy intake and energy expenditure which may result in the loss of the relationship of ghrelin and resistin.
Additionally, there was a relationship between post-training leptin concentration and post-training resistin concentration (r = 0.646, p ≤ 0.05) (data not shown). Pre-training concentrations of these hormones were not related (r = 0.528) at the criterion confidence level of p ≤ 0.05 but was at a level of p ≤ 0.10 (data not shown). These findings are indicative of leptin and resistin release being related to fat mass. In agreement with our findings in overweight adolescents is a study completed by Jung et al. in which obese adults who lost weight and percent body fat with a diet and exercise intervention also had decreases in leptin and resistin concentrations (42).
In summary, we found that long-term exercise training increased total PYY and decreased resistin in overweight adolescents. These are favorable changes in as PYY plays a role in decreasing appetite and resistin is related to increasing insulin resistance. Circulating leptin, ghrelin, and adiponectin concentrations were not altered with long-term exercise training. Leptin was correlated with weight, waist circumference, and percent body fat. Circulating triglyceride concentrations were inversely related to leptin concentrations, which might suggest a relationship between fat metabolism with exercise and the production of leptin. We conclude that long-term exercise training has beneficial effects for overweight adolescents with respect to PYY and resistin, two hormones related to appetite and insulin sensitivity.
Acknowledgments
We appreciate the technical assistance of Leslie Holmes, Alan Sirk, Katherine Stephenson, and Julia King. TEJ supported by East Carolina University Faculty Senate. MRM supported by the Pitt Memorial Hospital Foundation. RCH supported by NIH 1R01DK071081-01.
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, et al. Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the united states. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 3.I’allemand D, Wiegand S, Reinehr T, et al. Cardiovascular risk in 26,008 european overweight children as established by a multicenter database. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.259. [DOI] [PubMed] [Google Scholar]
- 4.Kelley GA, Kelley KS. Efficacy of aerobic exercise on coronary heart disease risk factors. Prev Cardiol. 2008;11:71–75. doi: 10.1111/j.1751-7141.2008.08037.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson MS, Figueroa-Colon R, Herd SL, et al. Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics. 2000;106:E50. doi: 10.1542/peds.106.4.e50. [DOI] [PubMed] [Google Scholar]
- 8.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 9.Muoio DM, Dohm GL, Fiedorek FT, Jr, Tapscott EB, Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997;46:1360–1363. doi: 10.2337/diab.46.8.1360. [DOI] [PubMed] [Google Scholar]
- 10.Maffei M, Fei H, Lee GH, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohrt WM, Landt M, Birge SJ., Jr Serum leptin levels are reduced in response to exercise training, but not hormone replacement therapy, in older women. J Clin Endocrinol Metab. 1996;81:3980–3985. doi: 10.1210/jcem.81.11.8923847. [DOI] [PubMed] [Google Scholar]
- 12.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 13.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 14.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 15.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 17.Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 18.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 19.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 20.Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and TNF{alpha} with insulin resistance. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson AS, Pollock ML. Practical assessment of body composition. Phys Sportsmed. 1985;13:76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 22.Siri WE. Body composition from fluid space and density: Analysis of methods. In: Brozek j, Henschel A., editors. Techniques for Measuring Body Composition. Washington, DC: National Academy of Sciences; 1961. pp. 223–244. [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Fletcher B, Berra K, Ades P, et al. Managing abnormal blood lipids: A collaborative approach. Circulation. 2005;112:3184–3209. doi: 10.1161/CIRCULATIONAHA.105.169180. [DOI] [PubMed] [Google Scholar]
- 25.Bell LM, Watts K, Siafarikas A, et al. Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab. 2007;92:4230–4235. doi: 10.1210/jc.2007-0779. [DOI] [PubMed] [Google Scholar]
- 26.Caranti DA, de Mello MT, Prado WL, et al. Short- and long-term beneficial effects of a multidisciplinary therapy for the control of metabolic syndrome in obese adolescents. Metabolism. 2007;56:1293–1300. doi: 10.1016/j.metabol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol. 2007;193:251–258. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- 28.Grandt D, Schimiczek M, Beglinger C, et al. Two molecular forms of peptide YY (PYY) are abundant in human blood: Characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 29.Avram AM, Jaffe CA, Symons KV, Barkan AL. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endocrinol Metab. 2005;90:2982–2987. doi: 10.1210/jc.2004-1785. [DOI] [PubMed] [Google Scholar]
- 30.Foster-Schubert KE, McTiernan A, Frayo RS, et al. Human plasma ghrelin levels increase during a one-year exercise program. J Clin Endocrinol Metab. 2005;90:820–825. doi: 10.1210/jc.2004-2081. [DOI] [PubMed] [Google Scholar]
- 31.Leidy HJ, Gardner JK, Frye BR, et al. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J Clin Endocrinol Metab. 2004;89:2659–2664. doi: 10.1210/jc.2003-031471. [DOI] [PubMed] [Google Scholar]
- 32.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 33.Falorni A, Bini V, Molinari D, et al. Leptin serum levels in normal weight and obese children and adolescents: Relationship with age, sex, pubertal development, body mass index and insulin. Int J Obes Relat Metab Disord. 1997;21:881–890. doi: 10.1038/sj.ijo.0800485. [DOI] [PubMed] [Google Scholar]
- 34.Pilcova R, Sulcova J, Hill M, Blaha P, Lisa L. Leptin levels in obese children: Effects of gender, weight reduction and androgens. Physiol Res. 2003;52:53–60. [PubMed] [Google Scholar]
- 35.Tsolakis C, Vagenas G, Dessypris A. Growth and anabolic hormones, leptin, and neuromuscular performance in moderately trained prepubescent athletes and untrained boys. J Strength Cond Res. 2003;17:40–46. doi: 10.1519/1533-4287(2003)017<0040:gaahla>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Gutin B, Ramsey L, Barbeau P, et al. Plasma leptin concentrations in obese children: Changes during 4-mo periods with and without physical training. Am J Clin Nutr. 1999;69:388–394. doi: 10.1093/ajcn/69.3.388. [DOI] [PubMed] [Google Scholar]
- 37.Sudi KM, Gallistl S, Borkenstein MH, et al. Effects of weight loss on leptin, sex hormones, and measures of adiposity in obese children. Endocrine. 2001;14:429–435. doi: 10.1385/ENDO:14:3:429. [DOI] [PubMed] [Google Scholar]
- 38.Kadoglou NP, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care. 2007;30:719–721. doi: 10.2337/dc06-1149. [DOI] [PubMed] [Google Scholar]
- 39.Corpeleijn E, Feskens EJ, Jansen EH, Mensink M, Saris WH, Blaak EE. Lifestyle intervention and adipokine levels in subjects at high risk for type 2 diabetes: The study on lifestyle intervention and impaired glucose tolerance maastricht (SLIM) Diabetes Care. 2007;30:3125–3127. doi: 10.2337/dc07-0457. [DOI] [PubMed] [Google Scholar]
- 40.Nassis GP, Papantakou K, Skenderi K, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Kraemer RR, Castracane VD. Exercise and humoral mediators of peripheral energy balance: Ghrelin and adiponectin. Exp Biol Med (Maywood) 2007;232:184–194. [PubMed] [Google Scholar]
- 42.Jung SH, Park HS, Kim KS, et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J Nutr Biochem. 2008;19:371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]