Abstract
Objectives
The objectives of this study were to investigate: (1) the antibacterial activity of two antibacterial monomers, dimethylaminododecyl methacrylate (DMADDM) and dimethylammoniumethyl dimethacrylate (DMAEDM), against eight different species of oral pathogens for the first time; (2) the cytotoxicity of DMAEDM and DMADDM.
Methods
DMAEDM and DMADDM were synthesized by reacting a tertiary amine group with an organo-halide. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against eight species of bacteria were tested. Time-kill determinations were performed to examine the bactericidal kinetics. Cytotoxicity of monomers on human gingival fibroblasts (HGF) was assessed using a methyl thiazolyltetrazolium assay and live/dead viability assay.
Results
DMADDM showed strong bactericidal activity against all bacteria, with MIC of 1.2 to 9.8μg/mL. DMAEDM had MIC of 20 to 80mg/mL. Time-kill determinations indicated that DMADDM and DMAEDM had rapid killing effects against eight species of bacteria, and eliminated all bacteria in 30min at the concentration of 4-fold MBC. Median lethal concentration for DMADDM and DMAEDM was between 20 to 40μg/mL, which was 20-fold higher than 1 to 2μg/mL for BisGMA control.
Conclusions
DMAEDM and DMADDM were tested in time-kill assay against eight species of oral bacteria for the first time. Both were effective in bacteria-inhibition, but DMADDM had a higher potency than DMAEDM. Different killing efficacy was found against different bacteria species. DMAEDM and DMADDM had much lower cytotoxicity than BisGMA. Therefore, DMADDM and DMAEDM are promising for use in bonding agents and other restorative/preventive materials to combat a variety of oral pathogens.
Keywords: Antibacterial activity, cytotoxicity, oral bacteria, quaternary ammonium methacrylate, time-kill, human fibroblasts
1. Introduction
Composites are popular dental filling materials because of their esthetics and improved handling and load-bearing properties.1-3 After being bonded to dental tissue with adhesives,4 it is desirable for the restorations to function in the oral cavities durably. However, nearly half of all dental restorations fail within 10 years, and replacing them accounts for 50-70% of all restorations performed.5,6 One main problem is that resin composites tend to accumulate more biofilms and plaques than other restorative materials in vivo.7,8 In addition, microgap formation can be observed between the adhesive resin and the primed dentin, or between the adhesive resin and the hybrid layer.9,10 Biofilms at the restoration margins could penetrate into the bonded interface to produce acids and cause secondary caries, which was considered as one of the primary reasons for restoration failure. 11,12
Therefore, efforts have been made to develop antibacterial dental composites and adhesive systems.13-18 Novel polymers containing quaternary ammonium methacrylates (QAMs) were developed.14-21 Monomers such as 12-methacryloyloxydodecylpyridinium bromide (MDPB) could copolymerize with other dental monomers to form antibacterial polymer matrices that can effectively reduce bacteria growth.14,19 Previous studies showed that adhesives containing MDPB substantially reduced the growth of Streptococcus mutans (S. mutans).19,22 An adhesive with methacryloxylethylcetyl dimethyl ammonium chloride (DMAE-CB) also reduced biofilm growth.15 These polymerizable cationic monomers were covalently bonded within the polymer matrix and could kill bacteria upon contact without releasing compounds that might be toxic to mammalian cells. This was supported by the fact that the antibacterial capability of the resins was long-lasting.14-16,19,23
Recently, two QAMs were synthesized: dimethylaminododecyl methacrylate (DMADDM), and bis(2-methacryloyloxyethyl) dimethylammonium bromide (a quaternary ammonium dimethacrylate termed “QADM”).24-28 QADM is dimethylammoniumethyl dimethacrylate, which is referred to as DMAEDM in this article, to follow the same abbreviation pattern as DMADDM based on the chemical structure name of the compound. Primers and adhesives containing DMAEDM inhibited a dental plaque microcosm biofilm growth and lactic acid production.24,25 DMAEDM-containing resins suppressed the glucosyltransferases (gtf) gene expressions of S. mutans, which were important for the synthesis of extracellular glucans and for bacterial cell adhesion and biofilm formation.26 DMADDM exhibited a stronger antibacterial efficacy than DMAEDM.27 A bonding agent containing DMADDM showed no decrease in antibacterial activity after 6 months of water-aging, while the dentin bond strength after 6 months was higher for DMADDM-containing bonding agent than a commercial control.28 However, the antibacterial activity of DMAEDM and DMADDM against different species of oral bacteria and the cytotoxicity of DMAEDM and DMADDM remain to be investigated.
Accordingly, the objectives of this study were to investigate: (1) the antibacterial activity of DMAEDM and DMADDM against eight different species of oral pathogens; (2) cytotoxicity of DMAEDM and DMADDM. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were measured. Time-kill behavior was determined to examine the kinetics of DMADDM and DMAEDM against eight species of bacteria. Cytotoxicity was assessed using human gingival fibroblasts. It was hypothesized that: (1) DMADDM and DMAEDM have potent antibacterial functions against all eight species of bacteria; (2) There are significant differences in the monomers’ antibacterial efficacy against the different bacterial species; (3) Both DMADDM and DMAEDM have minimal cytotoxicity toward human gingival fibroblasts.
2. Materials and methods
2.1. Synthesis of antibacterial quaternary ammonium methacrylates
The synthesis of DMAEDM and DMADDM were recently described.20,24,27,28 Briefly, a modified Menschutkin reaction was employed, where a tertiary amine group was reacted with an organo-halide. To synthesize DMAEDM, 10 mmol of 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA, Sigma-Aldrich, St. Louis, MO) and 10 mmol of 2-bromoethyl methacrylate (BEMA, Monomer-Polymer and Dajec Labs, Trevose, PA) were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 60 °C for 24 h to complete the reaction. Then the solvent was removed by evaporation, yielding DMAEDM as a clear, colorless, and viscous liquid.20,24 To synthesize DMADDM, 10 mmol of 1-(dimethylamino)docecane (DMAD) (Tokyo Chemical Industry, Tokyo, Japan) and 10 mmol of BEMA were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 70 °C for 24 h. The solvent was then removed, yielding DMADDM as a clear, colorless, and viscous liquid.27,28 The structures of DMAEDM and DMADDM are shown in Fig. 1.
Figure 1.
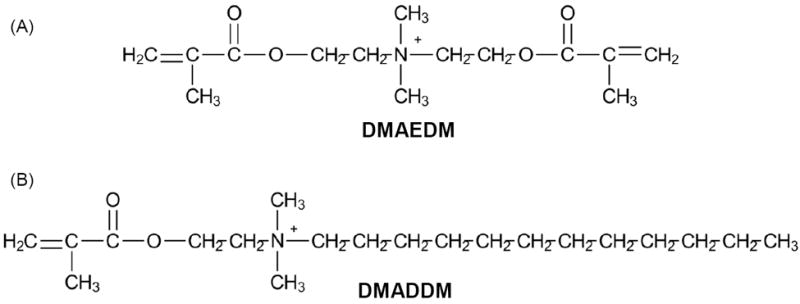
Chemical structures of the synthesized QAMs. (A) DMAEDM contains two methacrylate groups. It has a short alkyl chain length of 2. (B) DMADDM contains a single methacrylate group and a long alkyl chain with a chain length of 12.
2.2. Culture of eight different species of oral bacteria
The eight species of oral and perioral bacteria are listed in Table 1. Streptococcus mutans UA159, Actinomyces viscosus ATCC15987, Streptococcus sanguinis ATCC6715 and Enterococcus faecalis ATCC29212 (American Type Culture, Manassas, VA) were cultured in Brain Heart Infusion broth (BHI, Becton Dickinson, Sparks, MD). Lactobacillus acidophilus ATCC393 (American Type Culture) were cultured in Lactobacillus MRS broth (Research Product, Mount Prospect, IL). Staphylococcus aureus ATCC29213 (American Type Culture) were cultured in Tryptic Soy Broth (TSB) (Sigma-Aldrich). Porphyromonas gingivalis ATCC33277 were cultured in supplemented Tryptic Soy Broth (30 g/L tryptic soy broth, 0.5 g/L yeast extract, 0.5 g/L L-cysteine hydrochloride, 5 mg/L Hemin stock and 1 mg/L Vitamin K1). Prevotella melaninogenica ATCC25261 were cultured in BHI supplemented with 1% fetal bovine serum.29 Streptococci (Streptococcus mutans and Streptococcus sanguinis), Lactobacillus and Actinomyces are involved in the initiation and progression of caries.30,31 Porphyromonas and Prevotella predominate in periodontitis32 and produce virulence factors in periodontal pockets. Staphylococcus aureus are believed to be associated with peri-implantitis,33 and therapy-resistant (refractory) cases of periodontitis.34 Enterococcus faecalis are a commonly-isolated species from persistent apical periodontitis.35 Therefore, these eight species are important oral bacteria and hence were selected for the present study. The eight species of bacteria were grown at 37 °C under anaerobic conditions consisting of 80% N2, 10% CO2 and 10% H2 in MiniMACS anaerobic chamber (Microbiology International) until reaching the stationary phase which was the plateau of the growth curve after log growth, during which cell number remained constant. The culture of each type of bacteria thus obtained was adjusted with culture medium to 2×106 colony-forming units (CFU)/mL.
Table 1.
MIC and MBC of monomers against eight bacterial species
| Bacterial species | MIC/MBC (mg/mL)
|
||
|---|---|---|---|
| DMAEDM | DMADDM | Chlorhexidine | |
| Streptococcus mutans UA159 | 20/40 | 0.0049/0.0098 | 0.0012/0.0024 |
| Actinomyces viscosus ATCC15987 | 20/40 | 0.0049/0.0098 | 0.0024/0.0049 |
| Lactobacillus acidophilus ATCC393 | 40/80 | 0.0098/0.0195 | 0.0024/0.0049 |
| Staphylococcus aureus ATCC29213 | 40/80 | 0.0049/0.0098 | 0.0012/0.0024 |
| Streptococcus sanguinis ATCC6715 | 20/40 | 0.0012/0.0024 | 0.0006/0.0012 |
| Porphyromonas gingivalis ATCC33277 | 20/40 | 0.0024/0.0049 | 0.0012/0.0024 |
| Prevotella melaninogenica ATCC25261 | 20/40 | 0.0024/0.0049 | 0.0012/0.0024 |
| Enterococcus faecalis ATCC29212 | 80/160 | 0.0098/0.0195 | 0.0049/0.0098 |
2.3. MIC and MBC measurements
DMADDM was dissolved in the culture medium for each bacteria species at a starting concentration of 10 mg DMADDM per 1 mL of medium. DMAEDM was dissolved in medium to have a starting concentration of 640 mg/mL. Different concentrations were used because the antibacterial activity of DMAEDM was much lower than that of DMADDM, hence the MIC could only be tested at higher concentrations for DMAEDM than for DMADDM. Each monomer solution was diluted by twofold serially. Chlorhexidine acetate (CHX) served as control with a starting concentration at 10 mg/mL. A microtiter plate assay was used to determine the MIC and MBC for the eight bacterial species.36,37 MIC was measured as the lowest concentration of an antimicrobial agent where the bacterial growth was completely inhibited, with optical density (OD) increase of less than 0.05 which indicates no change in optical density and no bacterial growth. MBC was determined as the lowest concentration of the antimicrobial agent that killed 99.9% of the initial inoculum by a plate count of viable cells.36,37
An aliquot of 10 μL inoculum was added to a well of 96-well plates (Costar, Corning, Corning, NY) which contained 190 μL of a series of antibacterial monomer dilution broths. The OD value of each well was measured immediately using spectrophotometer (SpectraMax M5, Molecular Devices, Sunnyvale, CA) at 600 nm as an original OD value.36 After the 96-well plates were incubated at 37 °C for 24 h under anaerobic conditions, MIC was determined by reading the absorbance of each well.36 For MBC, 10 μL of aliquots from wells, where bacterial growth was inhibited, was spread on to bacterial culture agar and incubated under anaerobic conditions for 48 h at 37 °C. After calculating the colony-forming units (CFU), the MBC value was determined.36,37 The MIC and MBC determinations were repeated three times on different days.36
2.4. Time-kill assay
The antibacterial properties of DMAEDM and DMADDM against eight bacterial species were analyzed by a time-kill assay as previously described.29,38 Briefly, a bacterial suspension was adjusted to 1×107 CFU/mL.29 DMAEDM or DMADDM monomer was added to the suspension at concentrations of 1-, 2- and 4-fold of the MBC.29,38 The suspension (0.5 mL) was incubated at 37 °C with gentle agitation in a shaking water bath. After 1, 2, 5, 10, 30, 60, 120, 240 and 360 min of incubation,29,38 10 μL of the suspension was serially-diluted and inoculated on agar plates. After 48 h of anaerobic incubation at 37 °C, the number of viable bacteria colonies was counted. The time-kill assays were performed in triplicates.
2.5. Cytotoxicity testing
Human gingival fibroblasts (HGF, ScienCell, San Diego, CA) were cultured in a fibroblast medium (FM, ScienCell) supplemented with 2% fetal bovine serum, 100 IU/mL penicillin and 100 IU/mL streptomycin. DMAEDM, DMADDM and BisGMA monomers were each dissolved in FM, at concentrations of: 100, 60, 40, 20, 10, 5, 2, 1, and 0.5 μg/mL.29 HGF were seeded into the wells of 96-well micro plates at a density of 5,000 cells/well. 29 First, the HGF were incubated for 24 h in medium without monomer. Then, the culture medium in the 96-well microplates was discarded, and replaced with 100 μL of a medium containing a monomer. After 48 h, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) solution at a concentration of 5 mg/mL was added to each well.39 After incubating in a dark-room for 4 h, 150 μL/well of dimethylsulfoxide (DMSO, Sigma) was added.29,39 Absorbance of solutions was measured with a microplatereader (SpectraMax M5) at 492 nm.29 HGF culture without monomer served as control. Cell viability = absorbance of culture with monomer/absorbance of culture without monomer.29 The median lethal concentrations (LC50) of monomers were calculated from the dose-response results.
2.6. Live/dead viability assay
A live/dead viability assay (Molecular Probes, Eugene, OR) was used following the manufacturer’s instructions. Live and dead HGF were simultaneously determined with two probes, calcein AM and EthD-1. Live cells were distinguished by the enzymatic conversion of the nonfluorescent cell-permeant calcein AM to the intensely fluorescent calcein, producing a green fluorescence in live cells. Dead cells were distinguished by the entrance of EthD-1 into cells with damaged membranes, which generates a bright red fluorescence in dead cells upon binding to nucleic acids. HGF were seeded in wells of 96-well plates at 5,000 cells/well and cultured for 24 h in medium without monomer. Then the medium was replaced with 100 μL of a medium containing a monomer. After incubating for 24 h, each well was treated with 100 μL of the live/dead staining for 30 min. After fluorescence labeling, cells were examined with an epifluorescence microscope (TE2000-S, Nikon, Melville, NY).
2.7. Statistical analysis
One-way and two-way analyses-of-variance (ANOVA) were performed to detect the significant effects of variables. Tukey’s multiple comparison was used at p of 0.05.40
3. Results
The MIC and MBC values of DMAEDM and DMADDM against eight bacterial species are listed in Table 1. For each species and each monomer, the three replicates yielded the same MIC/MBC without a standard deviation. For DMAEDM, the MIC ranged from 20 to 80 mg/mL. DMADDM had much lower MIC ranging from 1.2 to 9.8 μg/mL, which approached the 0.6 to 4.9 μg/mL for CHX control. Among the eight bacterial species, Enterococcus faecalis was the most difficult to kill, with MIC of 80 mg/mL for DMAEDM, 9.8 μg/mL for DMADDM, and 4.9 μg/mL for CHX. Streptococcus sanguinis (S. sanguinis) was the easiest to kill, with MIC of 20 mg/mL for DMAEDM, 1.2 μg/mL for DMADDM, and 0.6 μg/mL for CHX.
The time-killing results of DMAEDM are plotted in Fig. 2. For all eight species of bacteria, the killing kinetics of DMAEDM was time-dependent. Higher DMAEDM concentrations led to a more rapid decrease in bacterial number. For Streptococcus mutans (S. mutans), at 1-fold MBC (40 mg/mL), bacteria were not eliminated by 360 min. At 2-fold MBC (80 mg/mL), S. mutans were all killed in 240 min. At 4-fold MBC (160 mg/mL), all S. mutans were killed in 30 min. A similar trend was observed for the other seven species. However, the killing efficacy of DMAEDM was different against different species, due to the different MBC. Three species (Lactobacillus acidophilus, Staphylococcus aureus, and Enterococcus faecalis) were more difficult to kill and required a DMAEDM concentration of 320 mg/mL to be killed in 30 min. The other species were killed in 30 min at 160 mg/mL of DMAEDM.
Figure 2.
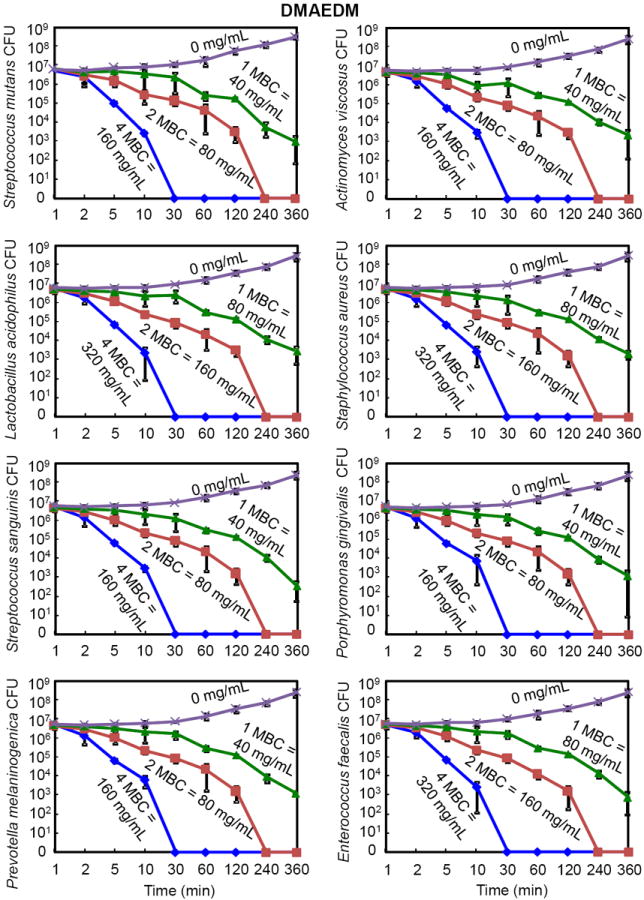
Time-kill curves of antibacterial monomer DMAEDM against the eight species of oral bacteria. The bacteria were cultured in medium containing DMAEDM at concentrations of 1-, 2-, and 4-fold of the MBC, as indicated for each curve in each plot. The bacteria species name is indicated in the y-axis label in each of the eight plots. Note the log scale for the y axis for colony-forming units (CFU). The surviving bacteria were plated at various time points as shown on the x axis. All data points represent mean ± sd of three independent experiments (n = 3).
The time-killing kinetics of DMADDM is plotted in Fig. 3. At 1-fold MBC (9.8 μg/mL), S. mutans were killed in 360 min. At 2-fold MBC (19.5 μg /mL), S. mutans were killed in 120 min. At 4-fold MBC (39 μg/mL), S. mutans were killed in 10 min. The trend of the killing kinetics against S. mutans was similar to the other seven species. However, there were differences among the different species. At 4-fold of MBC, S. mutans had a shorter killing time of 10 min, while the other seven species all took 30 min to kill. At 2-fold of MBC, Actinomyces viscosus had the longest killing time of 240 min, while all other seven species were killed in 120 min. At 1-fold of MBC, Actinomyces viscosus and Lactobacillus acidophilus were not completely eliminated by 360 min, while complete killing was achieved for the other 6 species. Furthermore, there were differences in MBC for the different species. Streptococcus sanguinis were the easiest to kill, and were eradicated in 30 min at the lowest DMADDM concentration (9.8 μg/mL) among the eight species. In contrast, Lactobacillus acidophilus, Staphylococcus aureus, and Enterococcus faecalis were the most difficult to eradicate and were killed in 30 min at the highest DMADDM concentration of 78 μg/mL.
Figure 3.
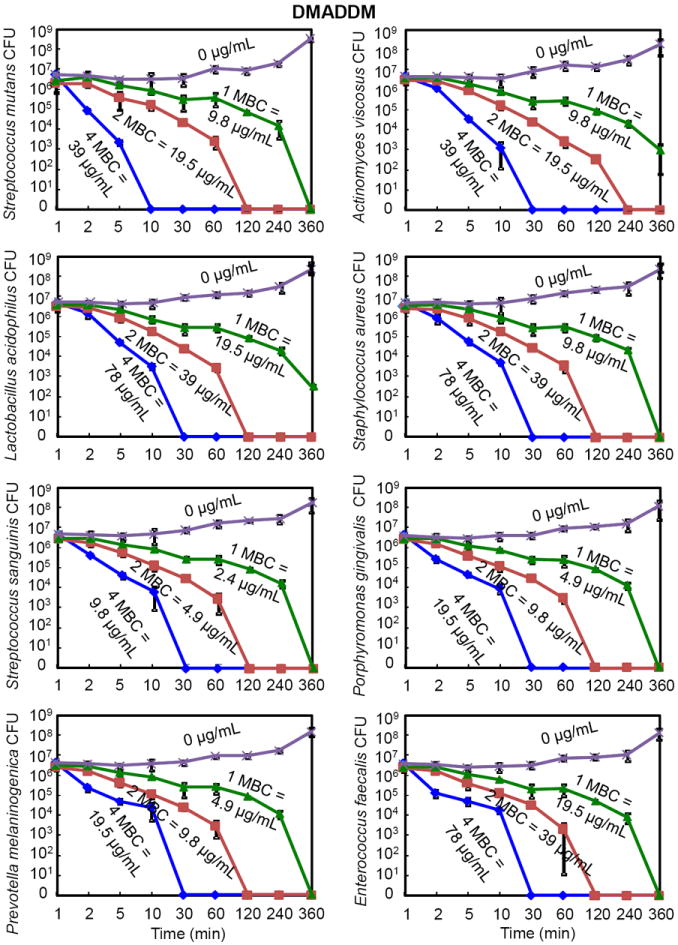
Time-kill curves of antibacterial monomer DMADDM against the eight species of oral bacteria (mean ± sd; n = 3). The bacteria were cultured in medium containing DMADDM at concentrations of 1-, 2-, and 4-fold of MBC, as indicated for each curve in each plot. The bacteria species name is indicated in the y-axis label in each of the eight plots. Note the log scale for the y axis for colony-forming units (CFU). The surviving bacteria were plated at various time points as shown on the x axis.
Comparing DMAEDM in Fig. 2 with DMADDM in Fig. 3, the killing kinetics was similar when their respective MBC was used as the unit. However, their MBC values differed greatly as listed in each plot. DMADDM at a concentration of 39 μg/mL killed all S. mutans in 10 minutes. In contrast, DMAEDM killed all S. mutans in 30 minutes, via a concentration of 160 mg/mL, which was nearly 4,000 times the DMADDM concentration.
Fig. 4 plots the HGF viability vs. concentration of monomer in culture medium. BisGMA exhibited the strongest cytotoxicity, yielding a sharp drop in cell viability at a concentration of 1 μg/mL (p < 0.05). For DMAEDM and DMADDM, with the increase of monomer concentration, cell viability showed a milder decrease than BisGMA. At 20 μg/mL, both DMAEDM and DMADDM had HGF viability of approximately 70-80%, while that of BisGMA was only 10% (p < 0.05). The median lethal concentration LC50 for DMAEDM and DMADDM was 20 to 40 μg/mL, much higher than the 1 to 2 μg/mL for BisGMA.
Figure 4.
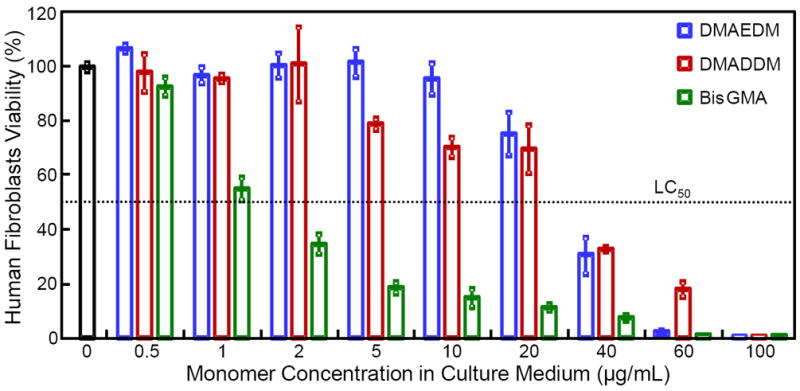
Cytotoxicity of monomers against human gingival fibroblasts (means ± sd, n = 4). Different concentrations of DMAEDM, DMADDM and BisGMA monomers were added to the culture medium for human gingival fibroblasts. The results are expressed as percentage of the control group containing no monomers. BisGMA exhibited the strongest cytotoxicty. DMAEDM and DMADDM showed a milder cytotoxicty.
Fig. 5 shows live/dead staining photos of HGF cultured in mediums containing monomers. BisGMA showed the most rapid decrease in green cells with a high proportion of red cells even at a monomer concentration of 2 μg/mL. DMAEDM and DMADDM started to exhibit an increasing proportion of red cells at a monomer concentration of 40 μg/mL. There was no noticeable difference between the DMAEDM and DMADDM groups.
Figure 5.
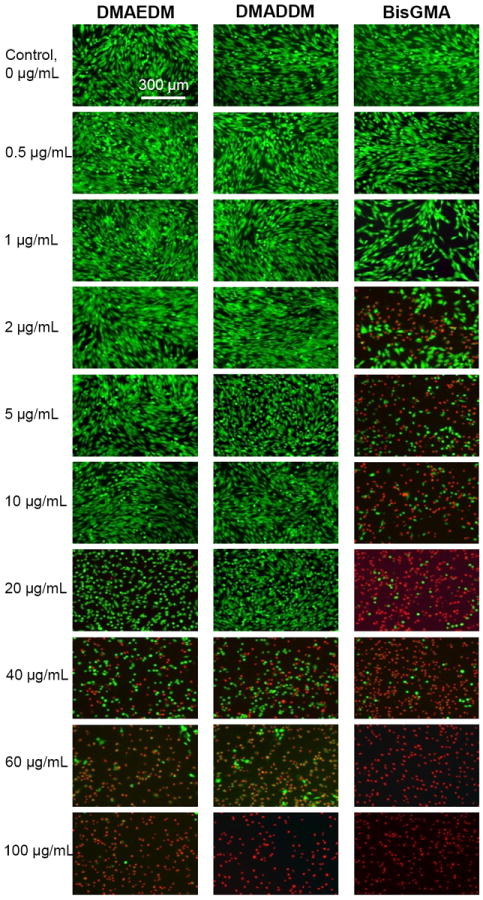
Representative live/dead staining images of human gingival fibroblasts (HGF) cultured in medium containing monomers. The top titles indicate the monomer names. The left side labels indicate the monomer concentrations in the culture medium. Live cells were stained green, and dead cells were stained red. BisGMA showed the most rapid decrease in green cells with a higher proportion of red cells when the monomer concentration was increased.
4. Discussion
This study investigated the antibacterial activities of two experimental quaternary ammonium methacrylates (DMAEDM and DMADDM) against eight species of oral pathogens for the first time. This study showed that: (1) both antibacterial monomers were effective in bacterial inhibition; (2) different monomers had different antibacterial potency; (3) different killing efficacy was found against different species of bacteria; (4) both monomers had a low cytotoxicity. Oral cavity is highly heterogeneous and supports a diverse microbial consortium comprising at least several hundred species of bacteria.41 To test the antibacterial activity of the new monomers, eight representative species of oral bacteria were chosen. The MIC/MBC results for DMADDM suggest that the degree of difficulty to inhibit the bacteria was: Lactobacillus acidophilus = Enterococcus faecalis > Staphylococcus aureus = Actinomyces viscosus = Streptococcus mutans > Porphyromonas gingivalis = Prevotella melaninogenica > Streptococcus sanguinis. The time-kill results for DMADDM (Fig. 3) indicate that the degree of difficulty to eradicate the bacteria was: Lactobacillus acidophilus = Enterococcus faecalis = Staphylococcus aureus > Actinomyces viscosus > Streptococcus mutans > Porphyromonas gingivalis = Prevotella melaninogenica > Streptococcus sanguinis. The two different tests showed slight differences in the ranking of bacteria resistance to antibacterial monomer. However, the general trend was consistent: Lactobacillus acidophilus, Staphylococcus aureus and Enterococcus faecalis were the most difficult to kill; Streptococcus sanguinis were the easiest to kill; the rest were in between, under the conditions of the present study.
Among these eight species, streptococci (S. mutans and S. sanguinis), Lactobacillus, and Actinomyces are known to be involved in the initiation and progression of dental caries.30,31 They can rapidly digest carbohydrates such as sucrose, fructose and glucose into acids, primarily lactic acid.42,43 Change of environmental pH could activate a shift in the proportions of the resident microbiota which could then promote the occurrence of caries.42,43 Porphyromonas and Prevotella predominate in periodontitis and primary endodontic infections, and produce virulence factors in periodontal pockets,32 thereby causing progressive loss of the alveolar bone and periapical bone. This can then lead to the loosening and subsequent loss of teeth or acute endodontic infections. Staphylococcus aureus are believed to be associated with peri-implantitis,33 and therapy-resistant (refractory) cases of periodontitis.34 Enterococcus faecalis is a commonly-isolated species from persistent apical periodontitis.35 Some possible factors facilitating its long-term survival in the root canal system are its ability to adhere to dentin and invade dentinal tubules44 and to form communities organized in biofilms, which may contribute to bacterial resistance and persistence after intracanal antimicrobial procedures.45 The mechanism for Enterococcus faecalis’ less susceptibility to QAMs is still unclear. Previous studies indicated that Enterococci exhibited intrinsic resistance to certain antibiotics such as cephalosporins, clindamycin and aminoglycosides, and had acquired genetic determinants that confer resistance to many classes of antimicrobials, including tetracycline, erythromycin, chloramphenicol, and vancomycin.46-48 This is consistent with the observation that Enterococcus faecalis was the most difficult to kill in the present study.
On the other hand, S. sanguinis was the easiest to kill according to the results of the present study. This was consistent with a previous report on the antibacterial effect of fluoride-releasing materials against oral bacteria.49 A literature search revealed no explanation on the high efficacy of QAMs in killing S. sanguinis. It may be related to the unique membrane component of S. sanguinis such as membrane-associated adenosine triphosphate ATPases,50 which may be sensitive to the charge change of QAMs. S. sanguinis is a member of the human indigenous oral microbiota, and is known to be a pioneering colonizer to serve as a scaffold in the formation of dental plaque.51 S. sanguinis is also one of the most common agents of infective endocarditis (IE) among the viridans streptococci.52 IE is a serious endovascular infection that carries a high risk of morbidity and mortality, and is the fourth leading cause of life-threatening infectious disease syndromes.53 Therefore, it is highly beneficial that S. sanguinis was the easiest to kill via QAMs in the present study. The high efficacy of the new QAMs against S. sanguinis could help inhibit the formation of dental biofilm, and could benefit the prevention of IE. One concern is that, S. sanguinis is antagonistic against S. mutans via hydrogen peroxide production,54 hence it could be argued that the high activity of QAMs on S. sanguinis could increase the relative proportion of S. mutans in the dental plaque. However, the QAMs of the present study also effectively killed S. mutans. Especially for DMADDM, the 4-fold MBC could eradiate the S. mutans within 10 min. Therefore, the rapid antibacterial effect could lower the quantity of S. mutans in dental biofilm, thus help inhibiting caries.
In addition, the present study showed that both DMAEDM and DMADDM could effectively kill all the eight bacterial species. At 4-fold of their respective MBC, both monomers killed all bacteria in 30 min. However, DMAEDM had a much higher MBC than DMADDM. According to the MIC and MBC results, the antibacterial activity of DMADDM was four orders of magnitude stronger than that of DMAEDM. The difference in antibacterial performance between these two monomers may be due to their difference in molecular structures. Previous studies indicated that the antimicrobial effects of quaternary ammonium salts were parabolically related to the length of the alkyl chains (the hydrophobic moiety).55,56 For Gram-positive bacteria, such activity maximized at chain lengths of C12 to C14, while for Gram-negative bacteria, optimal activity was achieved for compounds with chain lengths of C14 to C16. Compared with the ethyl group attached on the positive nitrogen ions in DMAEDM (C2), DMADDM had an alkyl chain of 12 carbons (C12) which boosted its antibacterial efficacy. This indicates that DMADDM, due to its potent antibacterial activity, would render a dental resin with a strong antimicrobial potency even at a low concentration.
Unlike previous antibacterial monomers such as MDPB and DMAE-CB which were monomethacrylates, DMAEDM is a dimethacrylate, with reactive groups on both ends of the molecule. Dimethacrylates, such as BisGMA and TEGDMA, are used as the cross-linking monomers in dental resins to form the matrix after polymerization and provide mechanical strength.57 Therefore, DMAEDM had the potential to serve both as a cross-linking monomer and as an antibacterial functional monomer. In a previous study, DMAEDM was incorporated into dental resin at relatively high concentrations without compromising the mechanical property.58 Together with the fact that DMAEDM was readily miscible with other dental methacrylates, this double-role monomer has the potential to be polymerized into dental resins at high concentrations to obtain desirable antibacterial effects.
DMADDM with a chain length of 12 was far more potent in antibacterial efficacy than DMAEDM.27 The MIC and MBC of DMADDM were orders of magnitude lower than DMAEDM. In a previous study,27 DMADDM was incorporated into a primer and an adhesive. The uncured primer with DMADDM had much larger inhibition zones than DMAEDM; the cured primer/adhesive resin with DMADDM could inhibit microcosm biofilm growth, metabolic activity, and lactic acid production. These results showed that DMADDM was promising to kill the residual bacteria in the prepared tooth cavity and the invading bacteria at the tooth-restoration margins to inhibit caries. Furthermore, the DMADDM-containing bonding agent exhibited a long-term antibacterial performance, with no significant decrease from 1 d to 6 months of water-aging.27 In addition, the incorporation of DMAEDM and DMADDM into primer and adhesive did not adversely affect the dentin bond strength, compared to commercial control.25,27 After 6 months of water-aging, the DMADDM-containing bonding agent had no loss in dentin bond strength, while a commercial control lost a third of the dentin bond strength.28 Regarding the synthesis of DMAEDM and DMADDM, it is anticipated that both monomers had a relatively high purity. During the synthesis of these monomers, the primary contaminants would most likely be unreacted starting compounds. For example, for DMADDM, the primary contaminants would be unreacted BEMA, DMAH and DMAD. Additionally, there might be trace amounts of ethanol solvent remaining. However, care was taken to remove all of these potential impurities prior to use via evaporation at room temperature under vacuum. A preliminary experiment on the antimicrobial activity of the starting materials showed that they were not antibacterial, hence the antibacterial activity of the present study was due to DMADDM, not the chemicals used to make it. A previous study used Fourier transform infrared spectroscopy (FTIR) and found that the starting materials BEMA, DMAH and DMAD were not present in the final product.27 In the FTIR spectrum of the final product, the C-Br peak and the tertiary amine peak of the DMAH and DMAD were not detected, indicating a high purity of the product.27 Further study is needed to quantify the monomer purity using a gas chromatography-mass spectrometry method.
Time-kill determines how fast an antimicrobial material can kill the bacteria. Both DMADDM and DMAEDM had rapid bactericidal actions against all eight species of bacteria. While DMAEDM exhibited a killing speed as fast as DMADDM at 4-fold of MBC, the absolute value of concentration was much high for DMAEDM (4 MBC = 160 mg/mL for S. mutans) than DMADDM (4 MBC = 39 μg/mL for S. mutans). In addition, the smaller amount of culture medium for DMAEDM when tested for 4 MBC further impacted the bacterial growth. The total medium amount was fixed at 1 mL for both DMAEDM and DMADDM. In the case of DMAEDM, the 1 mL of medium (which was about 1000 mg) contained 160 mg of DMAEDM plus 840 mg of the normal culture medium. Hence, only about 84% of the total culture medium could support the bacterial growth. In contrast, in the case of DMADDM, there was only 39 μg of DMADDM in 1 mL culture medium, hence the normal culture medium was still nearly 100%. Therefore, in the case of DMADDM, the bacteria had more access to more medium with more nutrients than in the case of DMAEDM. Nonetheless, DMADDM could still kill the eight species of bacteria faster than DMAEDM. Therefore, DMADDM could kill bacteria much faster at a much low concentration than DMAEDM. This characteristic of DMADDM could be useful for incorporation into adhesive systems, for example, at a concentration of 5% (which is about 50 mg/mL).27 This concentration is about 4 orders of magnitude higher than the MBC of the present study. This suggests that the bonding agent containing 5% of DMADDM could readily kill the residue bacteria in the tooth cavity within a small time window when the antibacterial adhesive was applied in the unpolymerized form. It usually ranges from tens of seconds to several minutes from the time the primer is applied to the time the bonding agent is photo-cured. Hence the ability to kill residual bacteria in the dentinal tubules at a fast speed would be clinically important, especially for minimally invasive management of dental caries when cavity preparation does not remove all carious tissues.59 In addition, the bonding agent containing DMADDM after being polymerized could further kill the invading bacteria along the tooth-restoration margins to inhibit recurrent caries.
As cationic agents, quaternary ammonium compounds are suggested to exert biocide activity by reacting with the negatively charged bacterial surface, causing membrane damage and irreversible loss of cytoplasmic constituents.60,61 A higher concentration of quaternary ammonium compound would increase the charge density, which could disrupt the membranes of many more bacteria quickly, compared to a lower concentration (such as 1-fold of MBC). The cationic agents exhibit antibacterial activity by the absorption of positively-charged monomer onto the negatively-charged cell surfaces of the bacteria. However, the high affinity of the cationic monomer to cell membranes is not limited to bacteria. Cells of surrounding tissues may also be affected, raising the concern about the toxicity of cationic monomers to human cells. The monomer-containing material used in an adhesive or composite would be polymerized and immobilized in the resin matrix, and would exhibit contact-inhibition without significant leachout.19,22,62 However, the photo-polymerization of resins is not complete, hence the cytotoxicity of antibacterial monomers is a concern. The present study showed that the LC50 for DMAEDM and DMADDM was between 20 and 40 μg/mL for human gingival fibroblasts, while the LC50 for BisGMA was between 1 and 2 μg/mL. Previous studies ranked the cytotoxicity of monomers in the order of BisGMA > UDMA > TEGDMA > HEMA.63 Since BisGMA is widely used in dental resins, DMAEDM and DMADDM with twenty times less toxicity than BisGMA should also be acceptable for clinical usage. It should be noted that the tested monomer concentrations (Fig. 4) were quite high and should be considered as the worst-case scenario. For the purpose of illustration, use an example in which DMADDM was mixed into a bonding agent at a DMADDM/(DMADDM + bonding agent) mass fraction of 10%. Assume that a tooth cavity would use 50 μg of a bonding agent to bond a composite to the tooth structure. In the unrealistic worst-case scenario, assume all 50 μg of the bonding agent were completely leached out in 1 day. The normal human saliva flow rate is about 1000-1500 mL daily.64 This would yield a DMADDM concentration of 0.05 to 0.033 μg/mL. Therefore, even the lowest monomer concentration of 0.5 μg/mL in Fig. 4 was 10 times more concentrated than the worst-case scenario, and the fibroblast viability was still excellent for all three monomers tested. Further studies should investigate the application of DMAEDM and DMADDM in dental resins and their antibacterial and biocompatibility properties in vivo.
5. Conclusions
Experimental antibacterial monomers DMAEDM and DMADDM were tested in time-kill assay against eight species of oral bacteria for the first time. This study showed that: (1) both monomers were effective in bacterial inhibition; (2) different monomers had different antibacterial potency; (4) different killing efficacy was found against different species of bacteria; and (4) both DMAEDM and DMADDM had a low cytotoxicity. The time-kill results for DMADDM showed that the degree of difficulty to eradicate the bacteria was: Lactobacillus acidophilus = Staphylococcus aureus = Enterococcus faecalis > Actinomyces viscosus > Streptococcus mutans > Porphyromonas gingivalis = Prevotella melaninogenica > Streptococcus sanguinis. DMADDM had strong bactericidal activity and could rapidly kill all the eight species of oral bacteria. DMADDM showed a stronger antibacterial activity than DMAEDM. The cytotoxicity of both monomers against human fibroblasts was twenty times less than that of BisGMA. Therefore, DMADDM and DMAEDM are promising for incorporation into bonding agents and other restorative/preventive materials to combat a variety of oral pathogens.
Acknowledgments
We thank Drs. Joseph M. Antonucci, Nancy J. Lin and Sheng Lin-Gibson of the National Institute of Standards and Technology for discussions. This study was supported by NIH R01 DE17974 (HX), National Natural Science Foundation of China 81100772 (FL), and a bridge funding from the University of Maryland School of Dentistry (HX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Jouranl of Dental Research. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NHF. State-of-the-art techniques in Operative Dentistry: contemporary teaching of posterior composites in UK and Irish dental schools. British Dental Journal. 2010;209:129–136. doi: 10.1038/sj.bdj.2010.674. [DOI] [PubMed] [Google Scholar]
- 3.Ferracane JL. Resin composite--state of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Godoy F, Kramer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. in vivo. Dental Materials. 2010;26:1113–8. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 6.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Primary Dental Care. 2002;9:31–6. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 7.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. Journal of Esthetic Dentistry. 1998;10:187–90. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 8.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Loguercio AD, Reis A, Bortoli G, Patzlaft R, Kenshima S, Rodrigues Filho LE, et al. Influence of adhesive systems on interfacial dentin gap formation in vitro. Operative Dentistry. 2006;31:431–41. doi: 10.2341/05-53. [DOI] [PubMed] [Google Scholar]
- 10.Awliya WY, El-Sahn AM. Leakage pathway of Class V cavities restored with different flowable resin composite restorations. Operative Dentistry. 2008;33:31–6. doi: 10.2341/07-22. [DOI] [PubMed] [Google Scholar]
- 11.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commission Project 2-95. International Dental Journal. 2001;51:117–58. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Summary of discussion from the Portland Composites Symposium (POCOS) June 17-19, 2004, Oregon Health and Science University, Portland, Oregon. Dental Materials. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Sarasam AR, Brown P, Khajotia SS, Dmytryk JJ, Madihally SV. Antibacterial activity of chitosan-based matrices on oral pathogens. Journal of Materials Science-materials in Medicine. 2008;19:1083–90. doi: 10.1007/s10856-007-3072-z. [DOI] [PubMed] [Google Scholar]
- 14.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, et al. Anti-biofilm effect of dental adhesive with cationic monomer. Jouranl of Dental Research. 2009;88:372–6. doi: 10.1177/0022034509334499. [DOI] [PubMed] [Google Scholar]
- 16.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dental Materials. 2011;27:487–96. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1151–62. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng Y, Howard L, Guo X, Chong VJ, Gregory RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. Journal of Materials Science-materials in Medicine. 2012;23:1553–61. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dental Materials. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 20.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dental Materials. 2012;28:561–72. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 23.Cheng L, Weir MD, Zhang K, Xu SM, Chen Q, Zhou X, et al. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. Jouranl of Dental Research. 2012;91:460–6. doi: 10.1177/0022034512440579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Jouranl of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dental Materials. 2013;29:450–61. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HHK. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into calcium phosphate dental nanocomposite to inhibit caries. Dental Materials. 2013 doi: 10.1016/j.dental.2013.05.005. accepted for publication, http://dx.doi.org/10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed]
- 28.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013 doi: 10.1016/j.jdent.2013.03.011. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Xiao YH, Xing XD, Li F, Ma S, Qi LL, et al. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Archives of Oral Biology. 2011;56:367–73. doi: 10.1016/j.archoralbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiological Reviews. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyvad B. Microbial colonization of human tooth surfaces. APMIS Supplementum. 1993;32:1–45. [PubMed] [Google Scholar]
- 32.Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiology and Molecular Biology Reviews. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persson GR, Renvert S. Cluster of Bacteria Associated with Peri-Implantitis. Clinical Implant Dentistry and Related Research. 2013 doi: 10.1111/cid.12052. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Fine DH. Microbial identification and antibiotic sensitivity testing, an aid for patients refractory to periodontal therapy. A report of 3 cases. Journal of Clinical Periodontology. 1994;21:98–106. doi: 10.1111/j.1600-051x.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 35.Rocas IN, Siqueira JF, Jr, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. Journal of Endodontics. 2004;30:315–20. doi: 10.1097/00004770-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Tong Z, Dong L, Zhou L, Tao R, Ni L. Nisin inhibits dental caries-associated microorganism in vitro. Peptides. 2010;31:2003–8. doi: 10.1016/j.peptides.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Branen JK, Davidson PM. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. International Journal of Food Microbiology. 2004;90:63–74. doi: 10.1016/s0168-1605(03)00172-7. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Tao R, Tong Z, Ding Y, Kuang R, Zhai S, et al. Effect of a novel antimicrobial peptide chrysophsin-1 on oral pathogens and Streptococcus mutans biofilms. Peptides. 2012;33:212–9. doi: 10.1016/j.peptides.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 40.Hannigan A, Lynch CD. Statistical methodology in oral and dental research: Pitfalls and recommendations. Journal of Dentistry. 2013;41:385–92. doi: 10.1016/j.jdent.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. Journal of Bacteriology. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh PD. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proceedings of the Finnish Dental Society. 1991;87:515–25. [PubMed] [Google Scholar]
- 43.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Advances in Dental Research. 1994;8:263–71. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 44.Chivatxaranukul P, Dashper SG, Messer HH. Dentinal tubule invasion and adherence by Enterococcus faecalis. International Endodontic Journal. 2008;41:873–82. doi: 10.1111/j.1365-2591.2008.01445.x. [DOI] [PubMed] [Google Scholar]
- 45.Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. Journal of Endodontics. 2002;28:689–93. doi: 10.1097/00004770-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Mundy LM, Sahm DF, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clinical Microbiology Reviews. 2000;13:513–22. doi: 10.1128/cmr.13.4.513-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray BE. The life and times of the Enterococcus. Clinical Microbiology Reviews. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepard BD, Gilmore MS. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes and Infection. 2002;4:215–24. doi: 10.1016/s1286-4579(01)01530-1. [DOI] [PubMed] [Google Scholar]
- 49.Marczuk-Kolada G, Jakoniuk P, Mystkowska J, Łuczaj-Cepowicz E, Waszkiel D, Dabrowski JR, et al. Fluoride release and antibacterial activity of selected dental materials. Advances in Hygiene and Experimental Medicine. 2006;60:416–20. [PubMed] [Google Scholar]
- 50.Sutton SV, Marquis RE. Membrane-associated and solubilized ATPases of Streptococcus mutans and Streptococcus sanguis. Journal of Dental Research. 1987;66:1095–8. doi: 10.1177/00220345870660060201. [DOI] [PubMed] [Google Scholar]
- 51.Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. Interspecies interactions within oral microbial communities. Microbiology and Molecular Biology Reviews. 2007;71:653–70. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mylonakis E, Calderwood SB. Infective endocarditis in adults. New England Journal of Medicine. 2001;345:1318–30. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 53.Bayer AS, Bolger AF, Taubert KA, Wilson W, Steckelberg J, Karchmer AW, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–48. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 54.Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. Journal of Bacteriology. 2005;187:7193–203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daoud NN, Dickinson NA, Gilbert P. Antimicrobial activity and physico-chemical properties of some alkyldimethylbenzylammonium chlorides. Microbios. 1983;37:73–85. [PubMed] [Google Scholar]
- 56.Gilbert P, Al-Taae ANA. Antimicrobial activity of some alkyl-trimethyl ammonium bromides. Letters in Applied Microbiology. 1985;1:101–05. [Google Scholar]
- 57.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 58.Cheng L, Weir MD, Limkangwalmongkol P, Hack GD, Xu HH, Chen Q, et al. Tetracalcium phosphate composite containing quaternary ammonium dimethacrylate with antibacterial properties. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:726–34. doi: 10.1002/jbm.b.32505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. Journal of the American Dental Association. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 60.Yoshimatsu T, Hiyama K. Mechanism of the action of didecyldimethylammonium chloride (DDAC) against Escherichia coil and morphological changes of the cells. Biocontrol Science. 2007;12:93–9. doi: 10.4265/bio.12.93. [DOI] [PubMed] [Google Scholar]
- 61.Sumitomo T, Nagamune H, Maeda T, Kourai H. Correlation between the bacterioclastic action of a bis-quaternary ammonium compound and outer membrane proteins. Biocontrol Science. 2006;11:115–24. doi: 10.4265/bio.11.115. [DOI] [PubMed] [Google Scholar]
- 62.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. Jouranl of Dental Research. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 63.Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. Journal of Dental Research. 1995;74:1602–6. doi: 10.1177/00220345950740091601. [DOI] [PubMed] [Google Scholar]
- 64.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. Journal of Prosthetic Dentistry. 2001;85:162–9. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]


