Abstract
Objectives
Antibacterial bonding agents are promising to combat bacteria and caries at tooth-restoration margins. The objectives of this study were to incorporate new quaternary ammonium methacrylates (QAMs) to bonding agent and determine the effects of alkyl chain length (CL) and quaternary amine charge density on dental plaque microcosm bacteria response for the first time.
Methods
Six QAMs were synthesized with CL = 3, 6, 9, 12, 16, 18. Each QAM was incorporated into Scotchbond Multi-purpose (SBMP). To determine the charge density effect, dimethylaminododecyl methacrylate (DMAHDM, CL = 16) was mixed into SBMP at mass fraction = 0%, 2.5%, 5%, 7.5%, 10%. Charge density was measured using a fluorescein dye method. Dental plaque microcosm using saliva from ten donors was tested. Bacteria were inoculated on resins. Early-attachment was tested at 4 hours. Biofilm colony-forming units (CFU) were measured at 2 days.
Results
Incorporating QAMs into SBMP reduced bacteria early-attachment. Microcosm biofilm CFU for CL = 16 was 4 log lower than SBMP control. Charge density of bonding agent increased with DMAHDM content. Bacteria early-attachment decreased with increasing charge density. Biofilm CFU at 10% DMAHDM was reduced by 4 log. The killing effect was similarly-strong against total microorganisms, total streptococci, and mutans streptococci.
Conclusions
Increasing alkyl chain length and charge density of bonding agent was shown for the first time to decrease microcosm bacteria attachment and reduce biofilm CFU by 4 orders of magnitude. Novel antibacterial resins with tailored chain length and charge density are promising for wide applications in bonding, cements, sealants and composites to inhibit biofilms and caries.
Keywords: Antibacterial bonding agent, Alkyl chain length, Quaternary amine charge density, Dental plaque microcosm biofilm, Caries inhibition
1. Introduction
Approximately 200 million dental restorations are performed in the United States each year.1 Composites are increasingly popular due to their excellent esthetics and direct-filling ability.2–6 Extensive studies have resulted in substantial improvements in composite fillers, polymer compositions, and handling and polymerization properties.2–6 Dental caries is a dietary carbohydrate-modified bacterial infectious disease, which is one of the most common bacterial infections in humans.7 Tooth demineralization is caused by acid generated by bacterial biofilms (dental plaque) in the presence of fermentable carbohydrates.8 Previous studies showed that composites in vivo had more biofilms and plaques than other restorative materials.9,10 These plaques could lead to secondary caries at the tooth-restoration margins, which has been suggested as a primary reason for restoration failure.6,11 The replacement of failed restorations accounts for more than half of all the restorations performed.12
Efforts were made to synthesize quaternary ammonium methacrylates (QAMs) for use in antibacterial dental resins.13–17 Through co-polymerization, 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) was covalently bonded in the resin matrix, thus becoming immobilized, achieving durable contact-killing capability against oral bacteria.18,19 Other antibacterial formulations were also prepared, including a methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB)-containing adhesive,20 antibacterial glass ionomer cements,21 and antibacterial nanocomposites and bonding agents using a quaternary ammonium dimethacrylate (QADM).22–24 Bonding agents are important in adhering the restorations to tooth structures.25,26 Bonding methods and procedures have been improved and the tooth-restoration bond strength has been enhanced.27–31 Antibacterial bonding agents are considered to be beneficial to reduce caries at the tooth-restoration margins.18–20 They can kill the residual bacteria in the prepared tooth cavity, and inhibit new bacteria at the tooth-restoration interface due to marginal leakage.13,18,19 For these reasons, efforts have been devoted to developing antibacterial primers and adhesive containing QAMs.13,18–20,23,24,32,33
Quaternary ammonium salts (QAS) can cause bacteria lysis by binding to cell membrane to cause cytoplasmic leakage.34,35 When the negatively charged bacteria contact the positive quaternary amine charge (N+), the electric balance is disturbed and the bacterium could explode under its own osmotic pressure.34,35 Long cationic polymers can penetrate bacterial cells to disrupt membranes, like a needle bursting a balloon.36,37 Indeed, previous studies revealed that the antibacterial potency of quaternary ammonium compounds increased with an increase in the alkyl chain length (CL) for the ammonium groups.21,38–40 One study showed that the antibacterial activity of quaternary ammonium monomers which were synthesized from dimethylaminoethyl methacrylate (DMAEMA) against Escherichia coli and Staphylococcus aureus increased with the number of carbon atoms in the alkyl chain.38 Another study reported that increasing the CL increased the biocidal activity of poly(quaternary ammonium salt) (PQAS)-containing glass-ionomer cement against Streptococcus mutans.21 Another investigation found that the antibacterial potency of quaternary ammonium thiol derivatives increased with CL, reach a maximal efficacy and then decreased with further increasing the CL.39 This is consistent with a separate study showing that the antibacterial activity of methacrylate monomers containing quaternary ammonium salt increased when CL was increased from 5 to 16, and then decreased when CL was further increased to 18.40 Therefore, CL and quaternary amine charge density of QAMs are two important factors. However, to date, there has been no report on the effects of CL and charge density of bonding agents on dental plaque microcosm bacterial behavior.
In the present study, a series of new QAMs with CL varying from 3 to 18 were synthesized and incorporated into bonding agent, and the antibacterial properties were measured using a dental plaque microcosm model with human saliva as inoculum. The objectives of this study were to investigate for the first time: (1) the CL effect on microcosm early-attachment to bonding agent resin as well as biofilm colony-forming units (CFU); (2) the charge density effect on microcosm early-attachment and biofilm CFU, by varying QAM mass fraction in the bonding agent. It was hypothesized that: (1) Microcosm attachment and biofilm CFU will be inversely proportional to the CL of QAM in bonding agent; (2) bacteria attachment and biofilm CFU will be inversely proportional to bonding agent charge density; (3) dental plaque microcosm biofilm CFU can be reduced by several orders of magnitude via the new antibacterial bonding agent.
2. Materials and methods
2.1. Antibacterial bonding agents with QAMs of different CL
The synthesis of QAMs was recently described, which employed a Menschutkin reaction via the addition reaction of tertiary amines with organo-halides.15,22 The 2-(dimethy-lamino) ethyl methacrylate (DMAEMA, Sigma-Aldrich, St. Louis MO) was the methacrylate-containing tertiary amine. The following is an example to make dimethylaminododecyl methacrylate (DMADDM) with CL of 12. Ten mmol of DMAEMA, 10 mmol of 1-bromododecane (BDD) (TCI America, Portland, OR), and 3 g of ethanol as solvent were added to a vial. The vial was capped and stirred at 70 °C for 24 h for the reaction to proceed.41 After the reaction was completed, the ethanol solvent was removed via evaporation. This process yielded DMADDM as a clear viscous liquid, and the reaction product was verified in a pilot study via Fourier transform infrared spectroscopy.41 The same method was used to synthesize six QAMs with a series of CL. Namely, DMAEMA was reacted with 1-bromopropane (BP) to form dimethylami-nopropyl methacrylate (DMAPM, CL = 3). DMAEMA was reacted with 1-bromohexane (BH) to form dimethylamino-hexyl methacrylate (DMAHM, CL = 6). DMAEMA was reacted with 1-bromononane (BN) to form dimethylaminononyl methacrylate (DMANM, CL = 9). DMAEMA was reacted with 1-bromododecane (BDD) to form dimethylaminododecyl methacrylate (DMADDM, CL = 12). DMAEMA was reacted with 1-bromohexadecane (BHD) to form dimethylaminohexadecyl methacrylate (DMAHDM, CL = 16). DMAEMA was reacted with 1-bromooctadecane (BOD) to form dimethylaminooctadecyl methacrylate (DMAODM, CL = 18). These six QAMs are listed in Table 1.
Table 1.
Reaction of 2-(dimethylamino) ethyl methacrylate (DMAEMA) with various organo-halides to synthesize new QAMs with a series of alkyl chain length (CL).
| Tertiary Amine | Alkyl Organo-Halide | Product | CL |
|---|---|---|---|
| 2-(dimethylamino) ethyl methacrylate (DMAEMA) | 1-bromopropane (BP) | DMAPM | 3 |
| 1-bromohexane (BH) | DMAHM | 6 | |
| 1-bromononane (BN) | DMANM | 9 | |
| 1-bromododecane (BDD) | DMADDM | 12 | |
| 1-bromohexadecane (BHD) | DMAHDM | 16 | |
| 1-bromooctadecane (BOD) | DMAODM | 18 |
To formulate antibacterial bonding agents, Scotchbond Multi-Purpose (SBMP, 3M, St. Paul, MN) was used as the parent system. According to the manufacturer, SBMP adhesive contained 60–70% of bisphenol A diglycidyl methacrylate (BisGMA) and 30–40% of 2-hydroxyethyl methacrylate (HEMA), tertiary amines and photo-initiator. SBMP primer contained 35–45% of HEMA, 10–20% of a copolymer of acrylic and itaconic acids, and 40–50% water. Each QAM from Table 1 was mixed at a QAM/(SBMP primer + QAM) mass fraction of 10%, following previous studies.23,24 The SBMP adhesive was also incorporated with 10% QAM.24 This yielded six antibacterial bonding agents, corresponding to the six QAMs in Table 1. The unmodified SBMP served as control.
2.2. Antibacterial bonding agents with different QAM mass fractions and charge densities
Because the CL results showed that DMAHDM (CL = 16) had the strongest antibacterial activity, it was selected for testing the effect of charge density. Charge density was varied by varying DMAHDM/(SBMP primer + DMAHDM) mass fraction of 0%, 2.5%, 5%, 7.5%, and 10%. Similarly, DMAHDM was mixed with SBMP adhesive at the same mass fractions. To make resin disks, the cover of a 96-well plate was used as molds following a previous study.20 Ten μL of a primer was placed in the bottom of each dent. After drying with a stream of air, 20 μL of adhesive was applied to the dent and photo-polymerized for 10 s (Optilux VCL 401, Demetron Kerr, Danbury, CT) using a Mylar strip covering to obtain a disk of approximately 8 mm in diameter and 0.5 mm in thickness. The cured disks were immersed in 200 mL of distilled water and magnetically-stirred with a bar at a speed of 100 rpm (Bellco Glass, Vineland, NJ) for 1 h to remove any uncured monomers, following a previous study.13
The density of quaternary ammonium groups present on the polymer disk surfaces was quantified using a fluorescein dye method as described previously.15,36 Resin disks were placed in a 48-well plate. Fluorescein sodium salt (200 μL of 10 mg/mL) in deionized (DI) water was added into each well, and specimens were left for 10 min at room temperature in the dark. After removing the fluorescein solution and rinsing extensively with DI water, each sample was placed in a new well, and 200 μL of 0.1% (by mass) of cetyltrimethylammonium chloride (CTMAC) in DI water was added. Samples were shaken for 20 min at room temperature in the dark to desorb the bound dye. The CTMAC solution was supplemented with 10% of 100 mM phosphate buffer at pH 8. This was prepared with 0.94 mg/mL monosodium phosphate-monohydrate and 13.2 mg/mL disodium phosphate-anhydrous in DI water. Sample absorbance was read at 501 nm using a plate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA).15,36 The fluorescein concentration was calculated using Beers Law and an extinction coefficient of 77 mM−1 cm−1.15,36 Using a ratio of 1:1 for fluorescein molecules to the accessible quaternary ammonium groups, the surface charge density was calculated as the total molecules of charge per exposed surface area (the summation of top, bottom and side areas, measured independently for each polymer disk due to slight variations in disk sizes).15 Six replicates were tested for each group.
2.3. Bacteria early-attachment on resin and confocal laser scanning microscopy (CLSM)
The dental plaque microcosm model was approved by the University of Maryland. Human saliva from ten healthy donors was collected following previous studies.42,43 The ten donors had natural dentitions without active caries or periopathology, and without using antibiotics within the last 3 months.23,42,43 They did not brush teeth for 24 h and abstained from food/drink intake for 2 h prior to donating saliva.23,24 Stimulated saliva was collected during parafilm chewing and kept on ice. An equal volume of saliva from each of the ten donors was combined to form the saliva sample, which was then diluted to 70% saliva with glycerol.23,42,43 Aliquots of 1 mL were stored at −80 °C for subsequent experiments.
The saliva-glycerol stock was added, with 1:50 final dilution, into brain heart infusion (BHI) broth supplied with 0.2% sucrose as inoculums. An inoculum of 1.5 mL was added to each well of 24-well plates containing a resin disk and incubated with 5% CO2 at 37 °C. To examine bacteria early-attachment, the incubation lasted for 4 h.15,44 After 4 h, samples were washed with phosphate buffered saline (PBS) to remove loose bacteria, fixed with 37 mg/mL formaldehyde, and stained for 1 h with 1 μmol/L SYTOX green.15,44 Samples were examined using a confocal laser scanning microscope (CLSM) (LSM510, Carl Zeiss, Thornwood, NY). A software (bioImageL, Malmö University, Malmö, Sweden) was used to measure the surface area covered by bacteria in each image.45 The surface area coverage was plotted as a percentage of the unmodified SBMP control. Six specimens were tested for each test group (n = 6).
2.4. Microcosm biofilm response in 2-day culture and colony-forming units (CFU)
The saliva-glycerol stock was added, with 1:50 final dilution, into BHI with 0.2% sucrose as inoculums. An inoculum of 1.5 mL was added to each well of 24-well plates containing a resin disk and incubated with 5% CO2 at 37 °C. After incubating in 5% CO2 at 37 °C for 8 h, the disks were transferred to new 24-well plates with fresh medium of BHI plus 0.2% sucrose. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for another 24 h. The total culture time was 48 h, previously demonstrated to successfully form biofilms on resins.23,24,43 To measure the biofilm CFU, disc with 48 h biofilms were rinsed with PBS to remove loose bacteria. Disks were transferred into tubes with 2 mL PBS, and the biofilms were harvested by sonication (3510R-MTH, Branson, Danbury, CT) for 5 min, followed by vortexing at 2400 rpm for 30 s using a vortex mixer (Fisher Scientific, Pittsburgh, PA). Three types of agar plates were prepared. Tryptic soy blood agar culture plates were used to determine total microorganisms.23,24 Mitis salivarius agar (MSA) culture plates with 15% sucrose were used to determine total streptococci.23,24,46 This is because MSA contains selective agents crystal violet, potassium tellurite and trypan blue, which inhibit most gram-negative bacilli and most gram-positive bacteria except streptococci, thus enabling streptococci to grow.46 In addition, cariogenic mutans streptococci are resistant to bacitracin, hence this property can be used to isolate mutans streptococci from the highly heterogeneous oral microflora. Therefore, the third type of agar plates was MSA agar plates plus 0.2 units/mL of bacitracin, to determine mutans streptococci.23,24,47 The bacterial suspensions were serially diluted and spread onto agar plates for CFU analysis.23,24 Six specimens were tested for each test group (n = 6).
2.5. Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL). One way analyses of variance (ANOVA) were used to detect the significant effects of the variables. Tukey’s multiple comparison was performed using a p value of 0.05.
3. Results
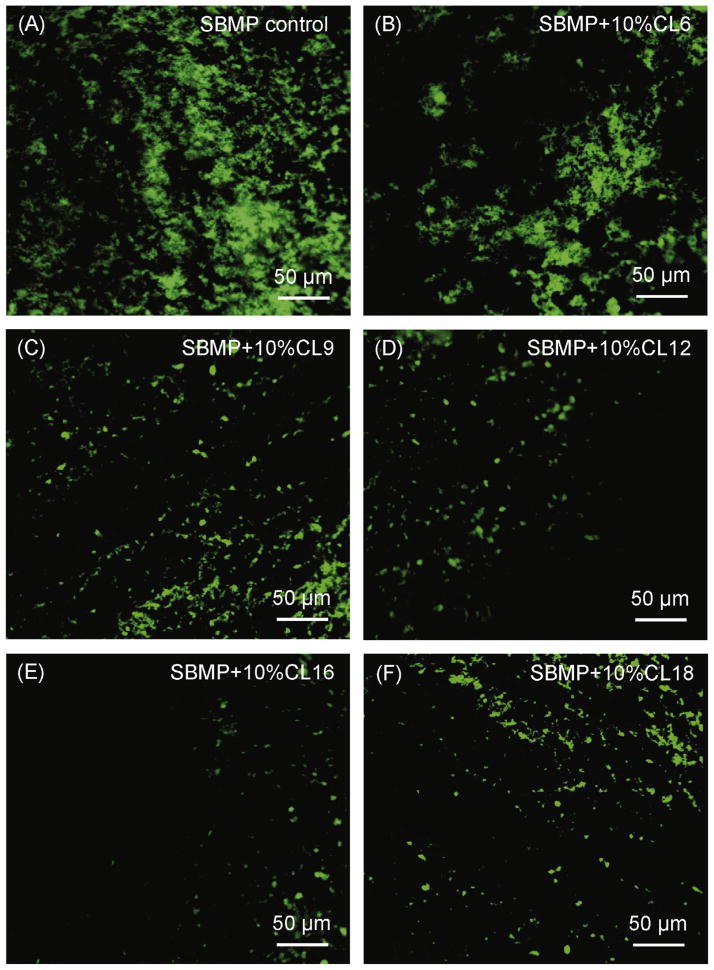
Fig. 1 shows representative SYTOX green-stained CLSM images of microcosm bacteria early-attachment at 4 h vs. QAM chain length. The resin disks were SBMP containing 10% QAM with different CL. SBMP control without QAM had the most bacteria attachment. Increasing the CL gradually reduced bacteria early-attachment, with the least bacteria colonization on resin containing DMAHDM with CL of 16. However, with further increasing the CL to 18, the bacteria attachment increased.
Fig. 1.
Representative confocal laser scanning microscopy (CLSM) images of SYTOX green-stained microcosm bacteria on resins: Effect of chain length CL. Human saliva microcosm bacteria were inoculated on resin disks and cultured for 4 h to examine early-attachment. The resin disks were SBMP bonding agent containing 10% of QAM with different chain length CL. SBMP control had no QAM.
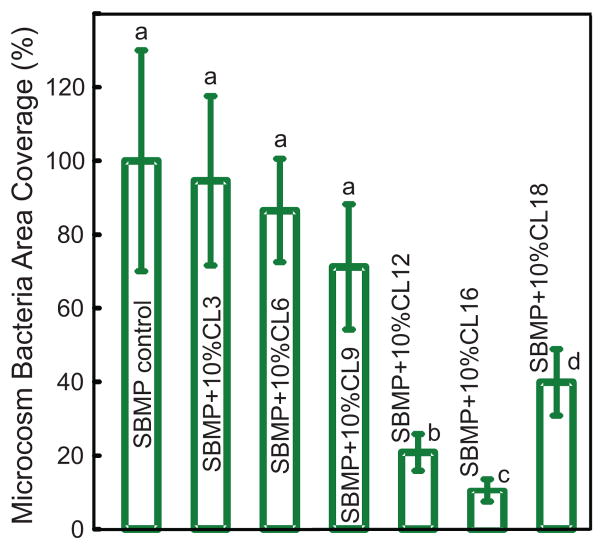
The quantitative coverage of resin area by microcosm bacteria at 4 h vs. CL is plotted in Fig. 2. Starting from CL of 3, the bacteria coverage decreased with increasing CL, reaching a minimum at CL of 16 ( p < 0.05). Then the bacteria coverage increased with a further increase in CL to 18. Therefore, CL of 16 showed the strongest antibacterial potency.
Fig. 2.
Resin surface area coverage by microcosm bacteria early-attachment: Effect of chain length CL. Saliva microcosm bacteria were inoculated on resin disks and cultured for 4 h. Each value is mean ± sd (n = 6). Values with dissimilar letters are significantly different from each other ( p < 0.05).
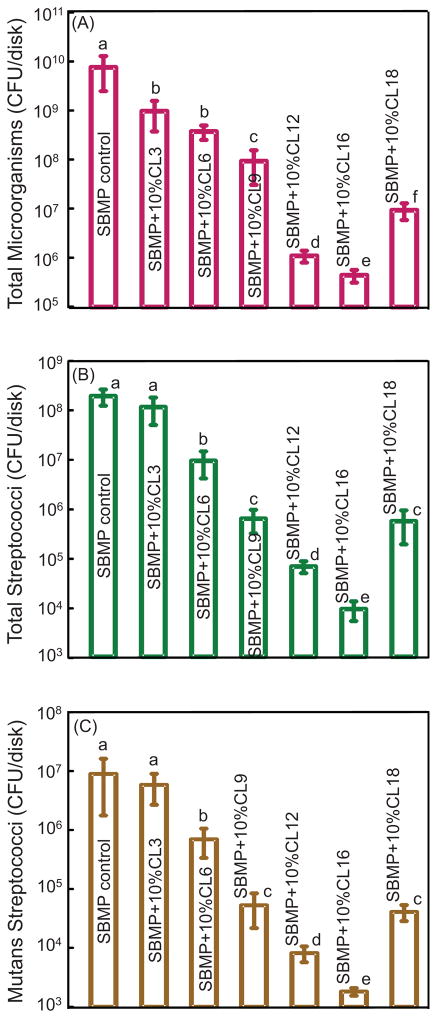
Fig. 3 plots the CFU of dental plaque microcosm biofilms on resins cultured for 2 d vs. CL: (A) Total microorganisms; (B) total streptococci; and (C) mutans streptococci. The biofilm CFU (mean ± sd; n = 6) decreased with increasing CL ( p < 0.05). The CFU at CL of 16 was 4 log lower than that of SBMP control. However, the biofilm CFU at CL of 18 was higher than that at CL of 16 ( p < 0.05).
Fig. 3.
Effect of chain length CL on dental plaque microcosm biofilm colony-forming units (CFU): (A) Total microorganisms, (B) total streptococci, and (C) mutans streptococci. Two-day biofilms on resins were used for CFU measurements (mean ± sd; n = 6). Note the log scale for the y-axis. The CFU at CL of 16 was 4 log lower than that of SBMP control. In each plot, values with dissimilar letters are significantly different from each other ( p < 0.05).
Quaternary amine surface charge density results are listed in Table 2. Control samples with 0% DMAHDM had slight nonspecific interaction with and absorption of the fluorescein salt. With increasing the DMAHDM mass fraction, the fluorescein binding to the cationic quaternary groups revealed statistically significant increases in the quaternary ammonium sites present on the surfaces of the cured bonding agent disks (p < 0.05).
Table 2.
Quaternary amine surface charge density of bonding agents (mean ± sd; n = 6).*
| DMAHDM mass% in bonding agent | 0% | 2.5% | 5% | 7.5% | 10% |
| Surface charge density (1015 N+/cm2) | 1.1 ± 0.1 | 1.6 ± 0.1 | 2.2 ± 0.3 | 4.2 ± 0.9 | 6.9 ± 0.8 |
DMAHDM was incorporated into SBMP bonding agent at different mass fractions. The group with 0% indicates SBMP control. All the values are significantly different from each other (p < 0.05).
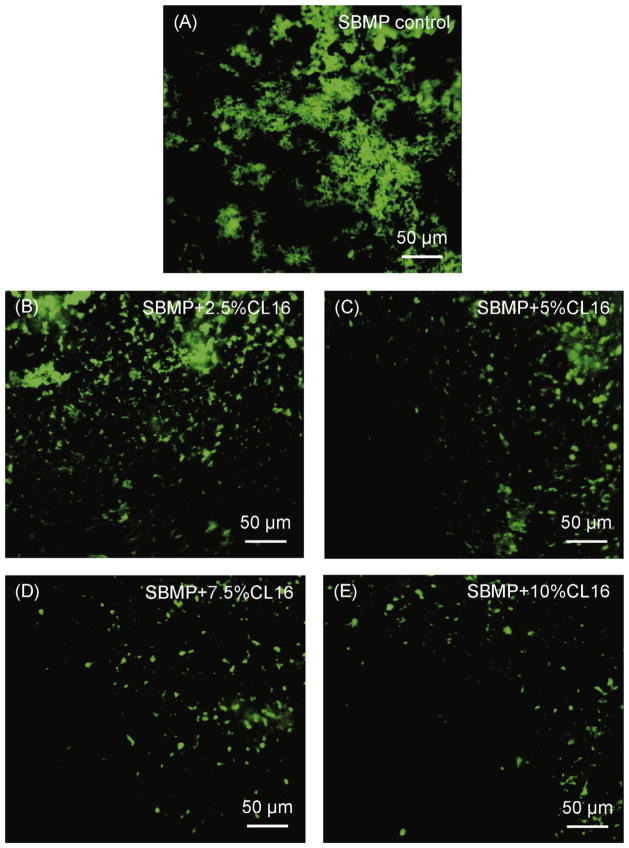
Fig. 4 shows representative CLSM images of microcosm bacteria early-attachment at 4 h vs. QAM mass fraction in the bonding agent. The bonding agent contained DMAHDM at mass fractions from 0% to 10%. SBMP control with 0% QAM had the most bacteria attachment. Increasing the DMAHDM mass fraction in SBMP decreased the microcosm bacteria colonization. The microcosm early-attachment was greatly reduced at 10% DMAHDM, compared to SBMP control.
Fig. 4.
Representative CLSM images of SYTOX green-stained microcosm bacteria on resins: Effect of quaternary amine surface charge density. The bonding agent contained DMAHDM at mass fractions ranging from 0% to 10%.
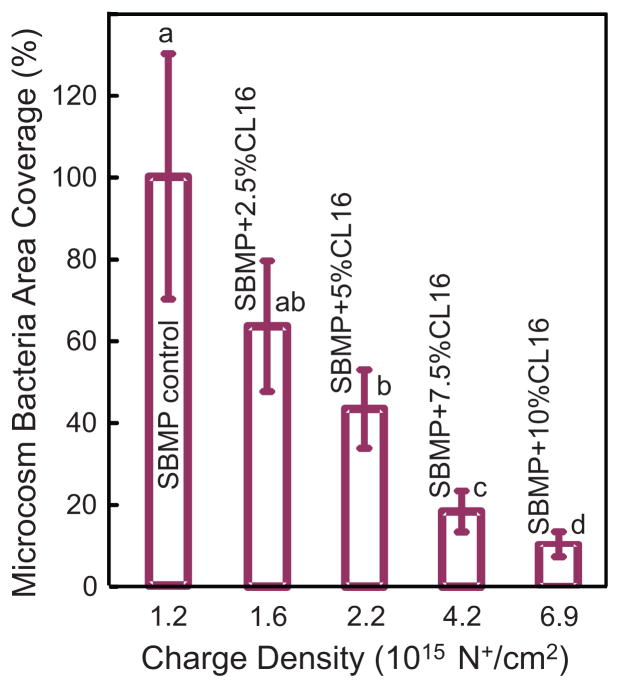
Fig. 5 plots the quantitative resin area covered by microcosm bacteria vs. charge density (mean ± sd; n = 6). The DMAHDM mass fraction is listed inside the plot. The corresponding quaternary ammine charge density is shown on the x-axis. Human saliva microcosm bacteria were cultured on the resin disks for 4 h to examine early-attachment and contact-killing effects. The bacteria coverage area on the resin disks decreased with increasing the DMAHDM mass fraction in the bonding agent ( p < 0.05).
Fig. 5.
Resin surface area coverage by microcosm bacteria early-attachment: Effect of quaternary amine surface charge density. Saliva microcosm bacteria were inoculated on resin disks and cultured for 4 h. The DMAHDM mass fraction is listed inside the plot. The corresponding quaternary ammine charge density of bonding agent disks is shown on the x-axis. Each value is mean ± sd (n = 6). Values with dissimilar letters are significantly different from each other ( p < 0.05).
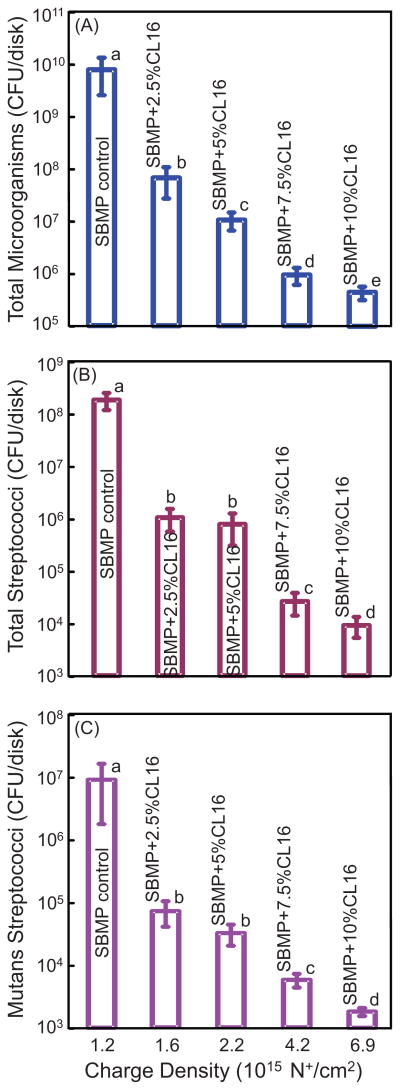
The antibacterial effect against microcosm biofilms at 2 d is plotted in Fig. 6 vs. charge density (mean ± sd; n = 6): (A) Total microorganisms; (B) total streptococci; and (C) mutans streptococci. SBMP control had the highest CFUs. The microcosm biofilm CFU decreased with increasing DMAHDM concentration ( p < 0.05). The microcosm biofilm CFU at 10% DMAHDM was reduced by 4 orders of magnitude compared to SBMP control. The extent of CFU reduction was similar for total microorganisms, total streptococci, and mutans streptococci.
Fig. 6.
Effect of quaternary amine surface charge density on dental plaque microcosm biofilm colony-forming units (CFU): (A) Total microorganisms, (B) total streptococci, and (C) mutans streptococci. Two-day biofilms on resins were used for CFU measurements (mean ± sd; n = 6). The DMAHDM mass fraction in the bonding agent is listed inside the plot. The corresponding quaternary ammine charge density is shown on the x-axis. Note the log scale for the y-axis. The CFU of biofilm adherent on bonding agent resin disks with a charge density of 6.9 × 1015 N+/ cm2 was 4 log lower than that of SBMP control. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
4. Discussion
This study investigated the effects of alkyl chain length and quaternary amine charge density of bonding agents against human saliva microcosm bacteria for the first time. QAMs with a series of CL from 3 to 18 were synthesized and incorporated into a bonding agent. Previous studies showed the prevalence of dental caries worldwide,48,49 and the basic mechanism of caries is demineralization via acids from bacterial biofilms.8 In particular, recurrent caries at the tooth-restoration margins is a primary reason for restoration failure.12 Antibacterial primers and adhesives in the uncured state could flow into dentinal tubules and kill residual bacteria in the tooth cavity.14,18,32 Antibacterial primers and adhesives in the cured state could inhibit bacteria invasion along the margins, which could be especially beneficial with the inevitable formation of marginal microgaps over time. The present study achieved substantial reduction in bacteria early-attachment to bonding agents, with biofilm CFU decreasing by 4 log. These results showed that the new antibacterial bonding agents with tailored chain length and charge density could be highly promising for the inhibition of biofilms and dental caries.
This study showed a strong CL effect of bonding agent on microcosm bacteria. QAMs possess bacteriolysis effects, because their positively-charged quaternary amine N+ can attract the negatively-charged cell membrane of bacteria, which can disrupt membranes and cause cytoplasmic leakage.34,35 The positively-charged ammonium group could interact with negatively-charged bacterial membrane to disrupt membrane functions, alter the balance of essential ions (i.e., K+, Na+, Ca2+, and Mg2+), interrupt protein activity, and damage bacterial DNA.50 Furthermore, long-chained quaternary ammonium compounds impart antimicrobial activity by inserting into bacterial membrane, resulting in physical disruption.50 These mechanisms suggest that chain length and charge density are key factors that determine the antibacterial efficacy. A previous study on glass ionomers showed that quaternary ammonium with chain length 16 had the highest antibacterial activity, whereas that with chain length 2 had the lowest.21 However, little has been reported on the CL effect of dental bonding agents. The present study incorporated QAMs into bonding agent and demonstrated that the antibacterial potency greatly increased with increasing CL from 3 to 16.
Furthermore, the present study showed a strong effect of charge density of bonding agent on microcosm bacteria response. A previous study on polymeric brushes showed that high density cationic surfaces could effectively killed bacteria.37 The charge density of a resin was increased with higher quaternary ammonium concentration.15 To date there has been no report on the effects of resin charge density on dental microcosm bacteria response. The present study demonstrated that the antibacterial potency of the resin was directly proportional to charge density. For early attachment at 4 h, the microcosm bacteria coverage was greatly reduced with higher charge density. For 2-day microcosm biofilms, a reduction of 4 log in CFU was achieved.
Since SBMP control did not contain QAM, they should have zero charge density. However, when using the fluorescein binding assay, baseline fluorescein absorption was exhibited in SBMP control, indicating unspecific background staining. Similar results were found in previous studies.15,36 For example, a charge density of 1.4 × 1013 N+/cm2 was reported in the control material prepared with 1:1 (by mass) of BisGMA:TEGDMA (triethylene glycol dimethacrylate) resin without any QAM,15 which indicated fluorescein background staining by the resin. In the present study, a charge density of 1.1 × 1015 N+/cm2 in the background staining was measured for SBMP control. The difference in the extent of fluorescein background staining was likely due to the difference in resin composition. Compared to the BisGMA-TEGDMA resin in the previous study,15 SBMP was the control in the present study. SBMP primer had 35–45% HEMA. SBMP adhesive had 30–40% HEMA. HEMA is hydrophilic and can cause significant water-uptake after the resin is cured.51 In contrast, BisGMA and TEGDMA of the previous study15 are hydrophobic with much less water-uptake.51 Therefore, the control samples in the present study were more hydrophilic than those in the previous study15 with greater water-uptake and easier adsorption of florescent staining. This likely caused more background staining of fluorescein in the SBMP control samples. Assuming that the SBMP resin disks with various DMAHDM mass fractions had approximately the same background fluorescein staining, the control background staining value could be deducted from all the samples. This yielded net charge density (1015 N+/cm2) of 0 for SBMP control, 0.5 for 2.5% DMAHDM, 1.1 for 5% DMAHDM, 3.1 for 7.5% DMAHDM, and 5.8 for 10% DMAHDM.
Saliva is ideal for growing biofilms to maintain much of the complexity and heterogeneity in vivo.52 In the present study, to represent the diverse bacterial populations, saliva from ten donors was collected and mixed together for use in the experiments. Dental biofilm models can be divided into three groups: Single species, defined consortium, and microcosm.52 Several studies investigated antibacterial resins using single species bacteria.15,20,32,34 In the present study, a dental plaque microcosm biofilm model was used to evaluate bonding agents with various chain length and charge density for the first time. In saliva, streptococci play an important role in caries formation. The oral streptococci include several groups, such as the mutans streptococci group, which is considered to be the main pathogens of dental caries.7 Members of the mutans streptococci group include Streptococcus mutans and Streptococcus sorbrinus, which are major players in causing caries. The present study showed that the killing efficacy of bonding agent with different chain length and charge density was similar in the three types of CFU measurements: total microorganisms, total streptococci, and mutans streptococci. This indicates that the antibacterial bonding agent had a similar killing efficacy against different types of bacterial species. Further study is needed to test the antibacterial efficacy of the new bonding agents using a human in vivo model, as well as the application of the new QAMs to other dental resins, sealants, cements and composites.
5. Conclusions
The effects of alkyl chain length and quaternary amine charge density of bonding agent on dental plaque microcosm bacteria response were investigated for the first time. The antibacterial potency of bonding agent containing a series of new QAMs increased with increasing CL from 3 to 16, but then decreased at CL of 18. The microcosm early-attachment was decreased on antibacterial bonding agent surfaces, and the biofilm CFU was reduced by 4 log, compared to commercial control. Bonding agent containing DMAHDM with different mass fractions enabled the variation of charge density on resin. Increasing the charge density reduced bacteria attachment, and decreased biofilm CFU by 4 log. The killing effect vs. chain length or charge density of bonding agent showed a similar potency against different species, manifested by similar reductions in total microorganisms, total streptococci, and mutans streptococci CFU. Novel resins with tailored chain length and charge density are promising for use in dental bonding agents, cements, sealants and composites to inhibit biofilms and caries.
Acknowledgments
We thank Drs. Joseph M. Antonucci, Nancy J. Lin and Sheng Lin-Gibson of the National Institute of Standards and Technology for discussions. This study was supported by NIH R01 DE17974 (HX), West China School of Stomatology (HZ), and a bridge funding from the Department of Endodontics, Prosthodontics and Operative Dentistry of University of Maryland School of Dentistry (HX).
References
- 1.American Dental Association (ADA) The 1999 Survey of Dental Services Rendered. Chicago, IL: ADA Survey Center; 2002. [Google Scholar]
- 2.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. Journal of the American Dental Association. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 3.Ferracane JL. Resin composite—state of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Dental Materials. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NHF. State-of-the-art techniques in operative dentistry: contemporary teaching of posterior composites in UK and Irish dental schools. British Dental Journal. 2010;209:129–36. doi: 10.1038/sj.bdj.2010.674. [DOI] [PubMed] [Google Scholar]
- 6.Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: not only a matter of materials. Dental Materials. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 7.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 8.Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Research. 2007;41:467–73. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 9.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. Journal of Esthetic Dentistry. 1998;10:187–90. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 10.Beyth N, Domb AJ, Weiss E. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Mjor IA, Toffeneti F. Secondary caries: a literature review with caries reports. Quintessence International. 2000;31:165–79. [PubMed] [Google Scholar]
- 12.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 13.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 14.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 15.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng Y, Howard L, Guo X, Chong VJ, Gregory RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. Journal of Materials Science. 2012;23:1553–61. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2012;100:1151–62. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–6. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37:289–96. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dental Materials. 2011;27:487–96. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dental Materials. 2012;28:561–72. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Dental Materials. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer P, Ye Q, Park JG, Topp EM, Misra A, Marangos O, et al. Adhesive/dentin interface: the weak link in the composite restoration. Annals of Biomedical Engineering. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 28.Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dental Materials Journal. 2008;27:315–29. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 29.Ritter AV, Swift EJ, Jr, Heymann HO, Sturdevant JR, Wilder AD., Jr An eight-year clinical evaluation of filled and unfilled one-bottle dental adhesives. Journal of the American Dental Association. 2009;140:28–37. doi: 10.14219/jada.archive.2009.0015. [DOI] [PubMed] [Google Scholar]
- 30.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J. State of the art of self-etch adhesives. Dental Materials. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Blum IR, Hafiana K, Curtis A, Barbour ME, Attin T, Lynch CD, et al. The effect of surface conditioning on the bond strength of resin composite to amalgam. Journal of Dentistry. 2012;40:15–21. doi: 10.1016/j.jdent.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of clearfil protect bond. Dental Materials. 2006;22:527–32. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. Journal of Dentistry. 2010;38:496–502. doi: 10.1016/j.jdent.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, et al. Antibacterial effect of bactericide immobilized in resin matrix. Dental Materials. 2009;25:424–30. doi: 10.1016/j.dental.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Tiller J, Liao C, Lewis K, Klibanov A. Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5981–5. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surfaces -2: how high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–9. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Lu G, Wu D, Fu R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacryalte. Reactive and Functional Polymers. 2007;67:355–66. [Google Scholar]
- 39.Thebault P, Taffin de Glvenchy E, Levy R, Vandenberghe Y, Guittard F, Geribaldi S. Preparation and antimicrobial behavior of quaternary ammonium thiol derivatives able to be grafted on metal surfaces. European Journal of Medical Chemistry. 2009;44:717–81. doi: 10.1016/j.ejmech.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 40.He J, Söderling E, Österblad M, Vallittu PK, Lassila LVJ. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules. 2011;16:9755–63. doi: 10.3390/molecules16119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HHK. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013 doi: 10.1016/j.jdent.2013.01.004. http://dx.doi.org/10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed]
- 42.Pratten J, Wilson M, Spratt DA. Characterization of in vitro oral bacterial biofilms by traditional and molecular methods. Oral Microbiology and Immunology. 2003;18:45–9. doi: 10.1034/j.1399-302x.2003.180107.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K, Cheng L, Imazato S, Antonucci JM, Lin NJ, Lin-Gibson S, et al. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentin bond properties. Journal of Dentistry. 2013 doi: 10.1016/j.jdent.2013.02.001. http://dx.doi.org/10.1016/j.jdent.2013.02.001. [DOI] [PMC free article] [PubMed]
- 44.Zeiger DN, Stafford CM, Cheng Y, Leigh SD, Lin-Gibson S, Lin NJ. Effects of sample preparation on bacterial colonization of polymers. Langmuir The ACS Journal of Surfaces and Colloids. 2010;26:2659–64. doi: 10.1021/la902920n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chávez de Paz LE. Image analysis software based on color segmentation for characterization of viability and physiological activity of biofilms. Applied and Environment Microbiology. 2009;75:1734–9. doi: 10.1128/AEM.02000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shewmaker PL, Gertz RE, Jr, Kim CY, de Fijter S, DiOrio M, Moore MR, et al. Streptococcus salivarius meningitis case strain traced to oral flora of anesthesiologist. Journal of Clinical Microbiology. 2010;48:2589–91. doi: 10.1128/JCM.00426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre Dos Santos M, et al. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. European Journal of Oral Sciences. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 48.Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. American Journal of Dentistry. 2009;22:3–8. [PubMed] [Google Scholar]
- 49.Dye BA, Thornton-Evans G. Trends in oral health by poverty status as measured by healthy people 2010 objectives. Public Health Reports. 2010;125:817–30. doi: 10.1177/003335491012500609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simoncic B, Tomcis B. Structures of novel antimicrobial agents for textiles—a review. Textile Research Journal. 2010;80:1721–37. [Google Scholar]
- 51.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 52.McBain AJ. In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]