Abstract
BACKGROUND
Mammary analogue secretory carcinoma (MASC) is a recently described salivary gland neoplasm that is defined by ETV6-NTRK3 gene fusion. To the best of the authors’ knowledge, only rare case reports of the cytopathologic features of MASC have been published to date.
METHODS
A wide variety of archival salivary gland tumors were tested for ETV6 translocation by break-apart fluorescent in situ hybridization. Positive cases with preoperative fine-needle aspiration (FNA) specimens or intraoperative touch preparations were retrieved from the archives of The Johns Hopkins Hospital. All smears were reviewed and the cytologic characteristics were described.
RESULTS
Five cases of MASC with cytopathologic material (4 FNA specimens and 1 touch preparation) were identified. The cases occurred in 3 men and 2 women ranging in age from 21 years to 78 years (mean, 52 years). On the cytologic smears, the MASCs were variably cellular and exhibited 2 different architectural patterns: 1) intact tissue fragments with isomorphic cells arranged in a sheet-like or papillary configuration; and 2) dispersed and dissociated cells with a mostly “histiocyte-like” appearance with large cells containing abundant vacuolated cytoplasm. No matrix tissue or stromal spindled cells were present. The cells did not display acinic differentiation in the form of cytoplasmic zymogen granules. In each case, the preoperative FNA correctly identified a neoplasm, and the most frequent diagnostic considerations were acinic cell carcinoma, mucoepidermoid carcinoma, and pleomorphic adenoma.
CONCLUSIONS
MASC is a newly described salivary gland tumor that should be considered in the differential diagnosis of low-grade salivary gland neoplasms. Its cytologic features overlap considerably with those of other tumors, especially acinic cell carcinoma and mucoepidermoid carcinoma.
Keywords: mammary analogue secretory carcinoma, acinic cell carcinoma, cytopathology, salivary glands, fine-needle aspiration
INTRODUCTION
The interpretation of salivary gland tumor cytopathology is extremely challenging due to 1) marked morphologic diversity that includes 37 distinct epithelial neoplasms recognized by the World Health Organization1; 2) heterogeneous features even within individual tumors; and 3) a general absence of helpful ancillary studies such as immunohistochemistry.2 Despite these challenges, preoperative fine-needle aspiration (FNA) is an extremely useful tool that is widely used as the initial diagnostic technique for triaging patients to appropriate management.3–6 The increasingly widespread use of preoperative FNA for salivary gland lesions necessitates the recognition of even rare neoplasms.
Mammary analogue secretory carcinoma (MASC) is a recently described salivary gland neoplasm.7 MASC is defined by the t(12;15)(q13;q25) translocation that results in ETV6-NTRK3 gene fusion identical to that found in secretory breast carcinoma,8 infantile fibrosarcoma,9 and congenital mesoblastic nephroma.10 MASC is histologically characterized by a proliferation of uniform eosinophilic cells with vacuolated cytoplasm growing within a microcystic, macrocystic, and papillary architecture. These histologic features overlap considerably with those of other salivary gland neoplasms, especially acinic cell carcinoma (ACC), but MASC does not exhibit overt serous acinar differentiation in the form of cytoplasmic basophilic zymogen granules.
To the best of our knowledge, there are currently only rare case reports in the cytopathology literature that describe the cytopathologic features of MASC.11–14 The current study summarizes our experience with a series of 5 cases of molecularly proven MASC with cytopathologic material, and emphasizes the differential diagnosis of this newly recognized neoplasm.
MATERIALS AND METHODS
Cases of MASC from the archives of The Johns Hopkins Hospital were identified in 2 different ways. First, tissue microarrays containing a wide range of primary salivary gland neoplasms were tested for the ETV6 translocation. Second, paraffin-embedded tissue blocks of previously described cases of papillary cystic ACC from The Johns Hopkins Hospital15 were also tested for the ETV6 translocation. These cases were targeted because of the morphologic overlap of MASC with the papillary cystic variant of ACC.
Break-apart fluorescent in situ hybridization for the ETV6 translocation was performed using a commercially available ETV6 dual-color break-apart probe (TEL; Abbott Molecular Inc, Des Plaines, Ill). Before hybridization, the slides were deparaffinized using a VP 2000 processor (Abbott Molecular Inc) in which pretreatment with protease I was used (Abbott Molecular Inc). After deparaffinization, the slides and the ETV6 probe were codenatured at 75°C for 7 minutes and allowed to hybridize for 22 hours at 37°C in humidified atmosphere. At the end of the incubation, the slides were washed in 2 × standard saline citrate/0.3% NP-40 for 2 minutes at 72°C and for 2 minutes at room temperature with agitation. Traces of detergent were removed by washing the slides in 2 × standard saline citrate at room temperature with agitation. The slides were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) and a coverslip was applied using Vectashield mounting medium (Vector Laboratories Inc, Burlingame, Calif). A fluorescence microscope was used to evaluate the probe pattern in 50 nuclei. Although cells with 2 fusion signals of 1 orange and 1 green fluorochrome were scored as normal, cells with rearrangements of the ETV6 gene had 1 normal fusion signal and 1 orange and 1 green signal at a distance from each other. Tumors were interpreted as being positive for rearrangement when > 10% of cells had a split signal.
The cases that were found to have ETV6 rearrangements for which archival cytopathologic material was available were pulled for review, and the cytopathologic features were described. FNA was performed with on-site evaluation by a cytopathologist with or without ultrasound guidance. The smears were air-dried and fixed in 95% ethanol and stained with Diff-Quik and Papanicolaou stains, respectively. Cell block preparations were stained with hematoxylin and eosin. In addition, the surgical pathology material from the corresponding resection specimen was also reviewed.
RESULTS
Patient Demographics and Clinical Data
Five cases of MASC with available cytopathology material were identified over a 14-year span from 1992 to 2005. Three of the cases were identified from the salivary gland tissue microarrays, and 2 were found in the group of neoplasms previously reported to be papillary cystic ACCs. The clinical features are summarized in Table 1. The MASCs were diagnosed in 3 men and 2 women ranging in age from 21 years to 78 years (mean, 52 years). Each MASC presented as a painless mass. In 4 of 5 cases (80%), the MASC arose in the parotid gland; the remaining case had a submandibular location. Three cases of MASC were on the left side and 2 were right-sided.
TABLE 1.
Patient Demographics and Clinical Data
| Case No. |
Age, Years |
Sex | Anatomic Location |
Tumor Size, cm |
Original Cytopathologic Diagnosis |
Original Histologic Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 21 | Man | Right parotid gland | 4.0 | Carcinoma, favor low- to intermediate-grade mucoepidermoid carcinomaa | Acinic cell carcinoma |
| 2 | 53 | Man | Left parotid gland | 2.0 | Salivary gland neoplasm, pleomorphic adenoma vs mucoepidermoid carcinoma | Acinic cell carcinoma |
| 3 | 76 | Man | Left parotid gland | 0.8 | Salivary gland neoplasm, acinic cell carcinoma vs mucoepidermoid carcinoma vs sebaceous gland neoplasm | Acinic cell carcinoma |
| 4 | 78 | Woman | Right parotid gland | 1.8 | Favor acinic cell carcinoma | Acinic cell carcinoma |
| 5 | 34 | Woman | Left submandibular gland | 0.9 | Epithelial neoplasm, with features suggestive of cellular pleomorphic adenoma | Acinic cell carcinoma |
Diagnosis was made in conjunction with a concurrent frozen section.
Cytopathologic Features
Because this neoplasm was not described until 2010, none of the tumors were initially interpreted as MASC. In each case, a diagnosis of a neoplasm was correctly made, but the diagnoses rendered were quite variable (Table 1). A definitive diagnosis was not given in any case, but in 3 cases a diagnosis was favored (mucoepidermoid carcinoma, ACC, and pleomorphic adenoma, respectively). In the remaining 2 cases, a differential diagnosis was given, and the entities considered included mucoepidermoid carcinoma (2 cases), ACC (1 case), pleomorphic adenoma (1 case), and a sebaceous gland neoplasm (1 case).
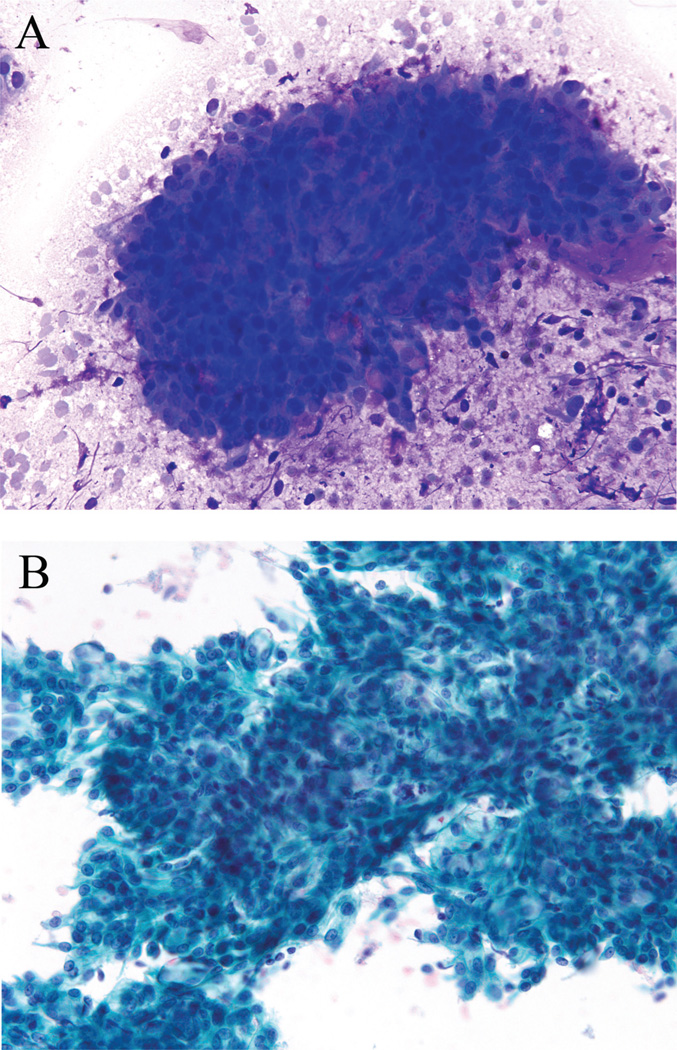
Cytomorphologic characteristics included hypercellular smears comprised of cellular fragments of various sizes and singly dispersed neoplastic cells. The predominant architectural pattern was a round to oval “sheet-like” or “papillary” pattern, often observed in a granular or cystic smear background (Figs. 1A and 1B). These tumor fragments displayed irregular and jagged outer borders with cells demonstrating round to oval uniform nuclei and small nucleoli. No matrix material or spindled stromal tissue were evident. Occasional cells demonstrated cytoplasmic vacuoles with pale green secretory material. No recognizable mucin was present.
FIGURE 1.
Fine-needle aspiration of a mammary analogue secretory carcinoma is shown. The smears were hypercellular and contained predominantly round to oval “sheet-like” or “papillary” cellular fragments that were often observed in a granular or cystic smear background (A, Diff-Quik stain; B, Papanicolaou stain, × 200).
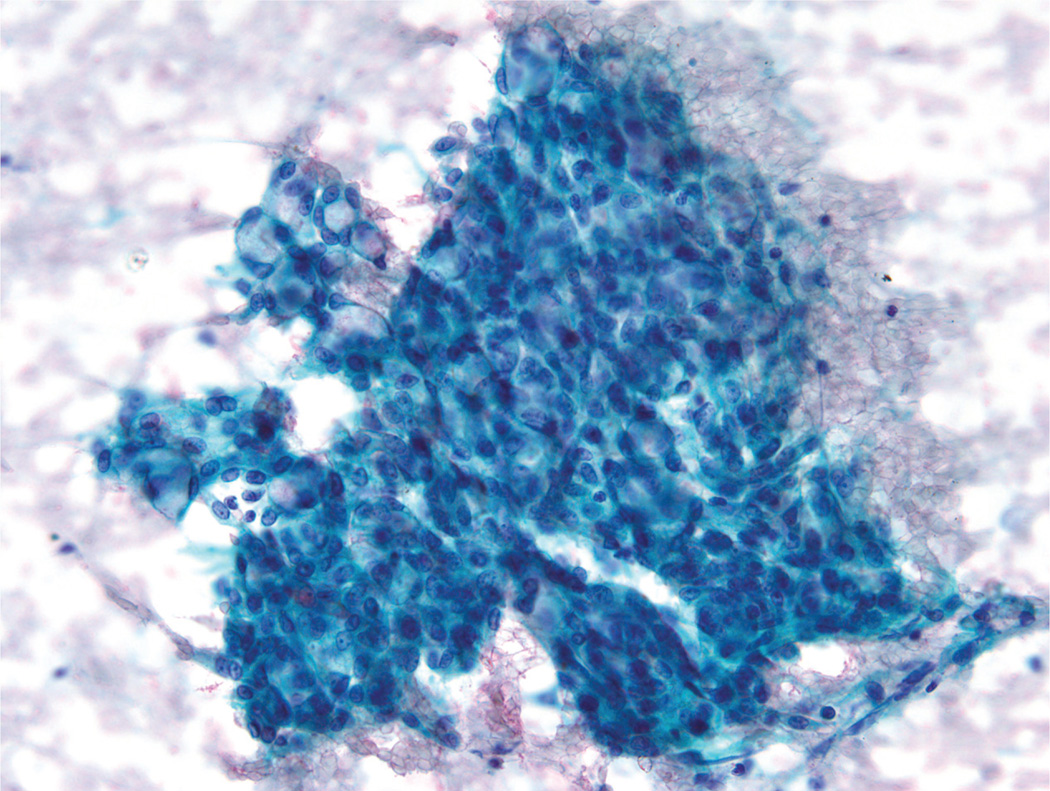
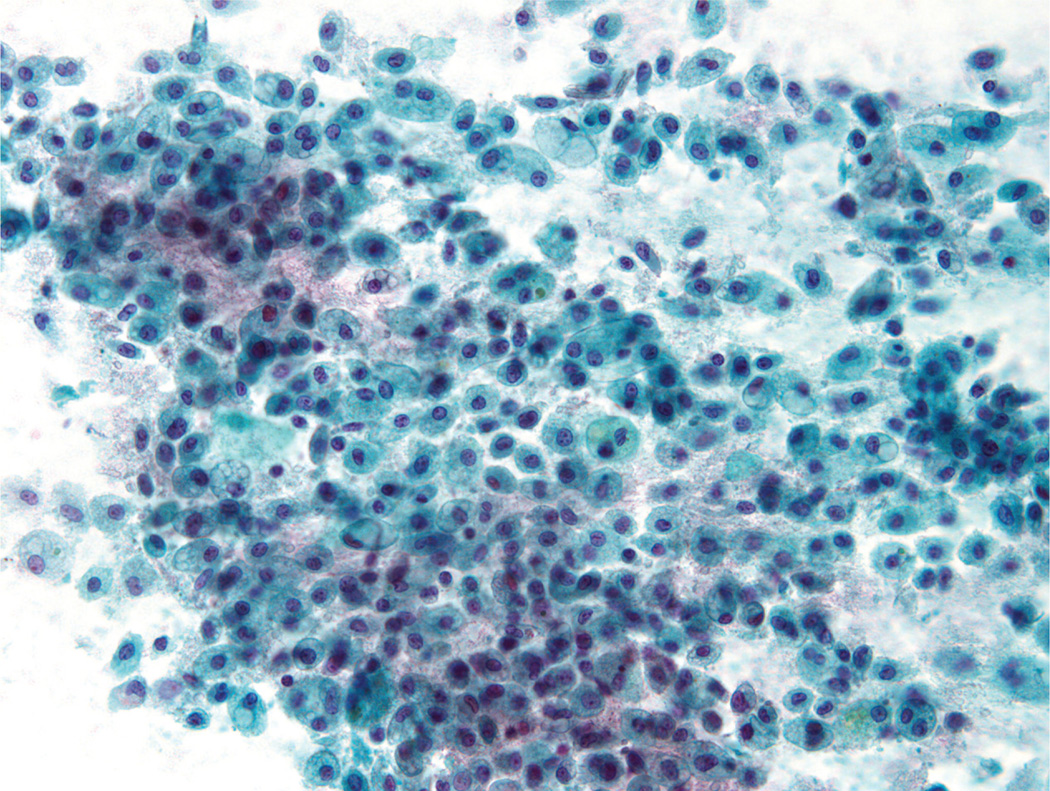
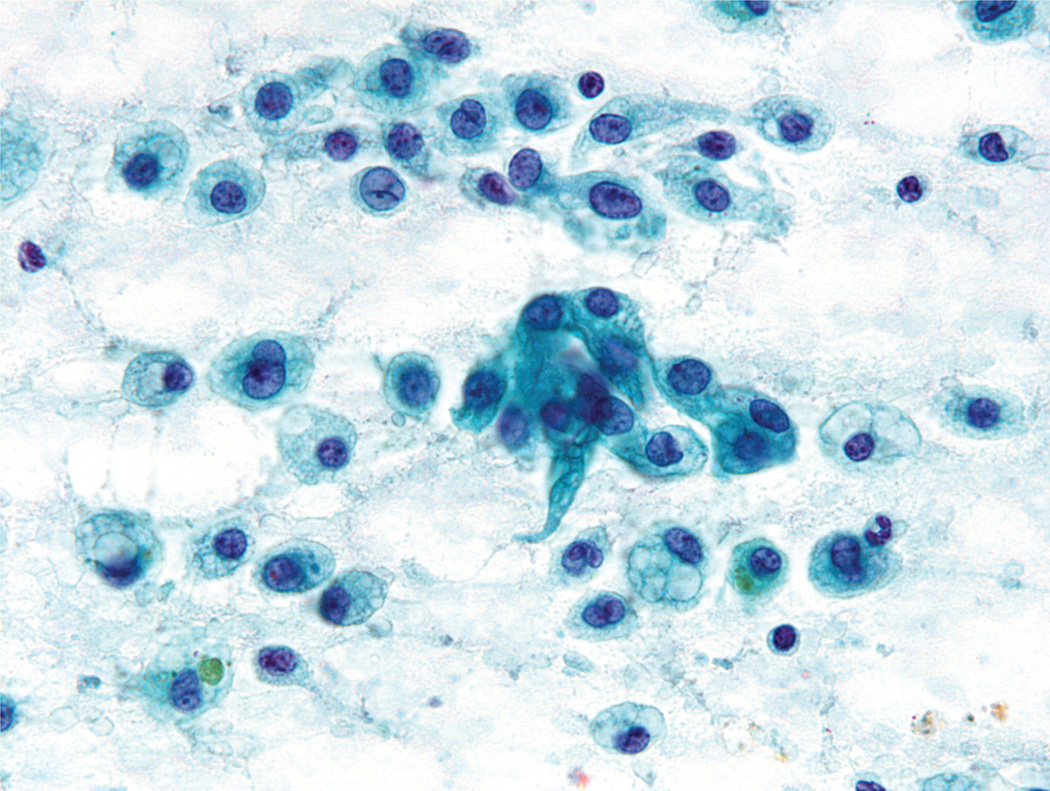
A second cytomorphologic pattern was highlighted by the predominance of single cells admixed with histiocytes and granular debris (Fig. 2). Neoplastic cells were large and round to polygonal, with well-defined cytoplasmic borders and a moderate amount of vacuolated cytoplasm (either a solitary large vacuole or multiple small “soap bubble-type” vacuoles) (Fig. 3). The cytoplasm was devoid of any noticeable granularity or other characteristics of acinic differentiation. These malignant cells closely resembled the histiocytes present in the smear background. The nuclei displayed open chromatin, irregular envelopes, mild anisonucleosis, and inconspicuous nucleoli (Fig. 4). Naked/stripped-off nuclei were often noted within the smears.
FIGURE 2.
Fine-needle aspiration of a mammary analogue secretory carcinoma is shown. A secondary cytomorphologic pattern was comprised of single cells admixed with histiocytes and granular debris (Papanicolaou stain, × 200).
FIGURE 3.
Fine-needle aspiration of a mammary analogue secretory carcinoma is shown. The tumor cells were large, round to polygonal, and contained well-defined cytoplasmic borders with vacuolated cytoplasm. These neoplastic cells closely resembled histiocytes present in the smear background (Papanicolaou stain, × 200).
FIGURE 4.
Fine-needle aspiration of a mammary analogue secretory carcinoma is shown. At high power, the tumor nuclei were found to possess open chromatin, irregular envelopes, mild anisonucleosis, and inconspicuous nucleoli (Papanicolaou stain, × 400).
Macroscopic and Histopathologic Features
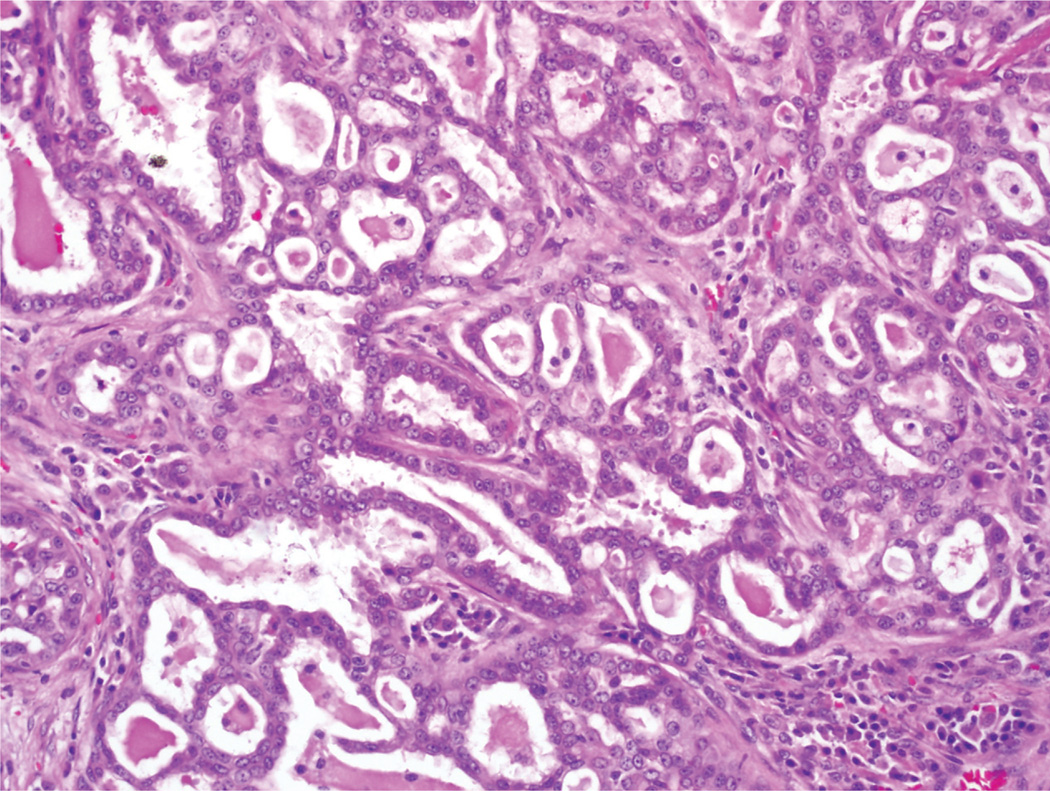
All the MASCs were surgically resected. Macroscopically, the tumors were all solitary, well-circumscribed masses, ranging in size from 0.8 cm to 4 cm (mean, 1.9 cm). Three MASCs (60%) were noted to be cystic with intracystic fluid described as green, dark red, and brown, respectively. Histopathologic correlation was available in all cases. Each MASC was originally diagnosed as an ACC. All MASCs had a similar histologic appearance, exhibiting a mixture of microcystic, papillary, macrocystic, and follicular architectural patterns. The tumor cells demonstrated eosinophilic to focally clear cytoplasm that often had a vacuolated quality, and every case had lightly eosinophilic intraluminal secretions (Fig. 5). None of the MASCs exhibited overt serous acinar differentiation in the form of basophilic cytoplasmic granules.
FIGURE 5.
Histologic section of a mammary analogue secretory carcinoma is shown. In the resection specimens, the tumors often grew in a microcystic pattern, with eosinophilic secretions noted in the luminal spaces. The tumor cells were eosinophilic with uniform nuclei (H & E, × 200).
DISCUSSION
MASC, first described in 2010 by Skalova et al,7 is a salivary carcinoma defined by a specific genetic alteration: t(12; 15) (p13; q25). Because it has only recently been recognized, much remains to be elucidated about this neoplasm. For example, although MASC has a low-grade appearance, to our knowledge its biologic behavior is not yet known. One study comparing the clinical behavior of MASC and ACC found a trend toward increased lymph node metastases in MASC, suggesting that it may be a more aggressive tumor.16 In addition, in the initial description, MASC was noted predominantly in the parotid gland, but subsequent studies have reported that approximately one-third of MASCs involve the submandibular gland and minor salivary glands of the oral cavity.7,12,16–19 Finally, the cytopathologic findings of MASC have yet to be clarified because to date there have only been rare case reports that included the cytologic features of MASC.12–14 To that end, herein we described the cytopathology of 5 cases of MASC.
The cytopathologic differential diagnosis of MASC may include many low-grade epithelial neoplasms. Pleomorphic adenoma (PA) may be considered because PA is the most common salivary gland tumor and both PA and MASC are comprised of bland cells. Indeed, in 2 of the cases in the current study, PA was a diagnostic consideration. However, unlike in PA, we did not observe extracellular matrix, mesenchymal stroma, or spindled myoepithelial cells in these cases of MASC. The isomorphic cleared and vacuolated cells of MASC may result in its being confused with both low-grade mucoepidermoid carcinoma (MEC) and ACC. In fact, the diagnosis of MEC was favored in 1 of the current study cases and considered in the differential diagnosis of 2 others, and ACC was a consideration in 2 cases. However, MASC does not have the intimate mixture of epidermoid cells, intermediate cells, and mucinous cells that characterize MEC. Although MASC has cells with vacuolated cytoplasm and focal mucin has been reported,14 the abundant intracellular and extracellular mucin characteristic of low-grade MEC are not observed in MASC. Unlike MASC, ACC should at least focally exhibit basophilic cytoplasmic granules. Other helpful features of ACC are nuclei with macronucleoli and abundant arborizing vessels. Finally, metastatic renal cell carcinoma (RCC) could be a consideration, given the low-grade appearance with common clear cells and histiocyte-like cells we observed in MASC. However, RCC is characterized by perivascular nesting, macronucleoli, and often cytoplasmic metachromatic stromal material. Certainly, a history of a renal tumor would also be helpful in this differential diagnosis. If doubt remains, performing immunohistochemistry for renal markers (eg, RCC, CD10, and especially paired box gene 8 [PAX8]) on cell block material would resolve the diagnosis.
Previous studies have shown that MASC is usually positive for mammaglobin and S-100 protein by immunohistochemistry.7,16,17 Although these studies may be helpful, S-100 protein expression is observed in many salivary tumors and to our knowledge the specificity of mammaglobin for the diagnosis of MASC is not yet known. Ultimately, a definitive diagnosis of MASC currently rests on documenting ETV6 rearrangement, which to the best of our knowledge has not been noted in any other salivary gland tumor.
Conclusions
MASC is a recently described salivary gland tumor that is defined by ETV6-NTRK3 gene fusion. On FNA, MASC may closely mimic other salivary lesions and as a result should be considered in the differential diagnosis of lowgrade salivary gland neoplasms, especially those with clear cell changes and vacuolated cytoplasm. If cell block material is available, demonstration of the ETV6 rearrangement is diagnostic.
Footnotes
FUNDING SUPPORT
No specific funding was disclosed.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Barnes L, Eveson JW, Relchart P, Sidransky D, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. Tumours of the salivary glands; pp. 209–281. [Google Scholar]
- 2.Faquin WC, Powers CN. Introduction to FNA and salivary gland neoplasia. In: Syed ZA, Douglas PC, Yener SE, editors. Salivary Gland Cytopathology. New York: Springer; 2008. pp. 1–16. [Google Scholar]
- 3.Al-Khafaji BM, Nestok BR, Katz RL. Fine-needle aspiration of 154 parotid masses with histologic correlation: ten-year experience at the University of Texas M. D. Anderson Cancer Center. Cancer. 1998;84:153–159. [PubMed] [Google Scholar]
- 4.Cohen EG, Patel SG, Lin O, et al. Fine-needle aspiration biopsy of salivary gland lesions in a selected patient population. Arch Otolaryngol Head Neck Surg. 2004;130:773–778. doi: 10.1001/archotol.130.6.773. [DOI] [PubMed] [Google Scholar]
- 5.Seethala RR, LiVolsi VA, Baloch ZW. Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck. 2005;27:217–223. doi: 10.1002/hed.20142. [DOI] [PubMed] [Google Scholar]
- 6.Zbaren P, Schar C, Hotz MA, Loosli H. Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope. 2001;111(11 pt 1):1989–1992. doi: 10.1097/00005537-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 8.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 9.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 10.Rubin BP, Chen CJ, Morgan TW, et al. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am J Pathol. 1998;153:1451–1458. doi: 10.1016/S0002-9440(10)65732-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito S, Ishida E, Skalova A, Matsuura K, Kumamoto H, Sato I. Case report of mammary analog secretory carcinoma of the parotid gland. Pathol Int. 2012;62:149–152. doi: 10.1111/j.1440-1827.2011.02759.x. [DOI] [PubMed] [Google Scholar]
- 12.Petersson F, Lian D, Chau YP, Yan B. Mammary analogue secretory carcinoma: the first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol. 2012;6:135–139. doi: 10.1007/s12105-011-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisharodi L. Mammary analog secretory carcinoma of salivary gland: cytologic diagnosis and differential diagnosis of an unreported entity [published online ahead of print April 30, 2012] Diagn Cytopathol. doi: 10.1002/dc.21766. [DOI] [PubMed] [Google Scholar]
- 14.Levine P, Fried K, Krevitt LD, Wang B, Wenig BM. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: another diagnostic dilemma in matrix-containing tumors of the salivary glands [published online ahead of print July 16, 2012] Diagn Cytopathol. doi: 10.1002/dc.22886. [DOI] [PubMed] [Google Scholar]
- 15.Ali SZ. Acinic-cell carcinoma, papillary-cystic variant: a diagnostic dilemma in salivary gland aspiration. Diagn Cytopathol. 2002;27:244–250. doi: 10.1002/dc.10167. [DOI] [PubMed] [Google Scholar]
- 16.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61:387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 17.Fehr A, Loning T, Stenman G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol. 2011;35:1600–1602. doi: 10.1097/PAS.0b013e31822832c7. [DOI] [PubMed] [Google Scholar]
- 18.Rastatter JC, Jatana KR, Jennings LJ, Melin-Aldana H. Mammary analogue secretory carcinoma of the parotid gland in a pediatric patient. Otolaryngol Head Neck Surg. 2012;146:514–515. doi: 10.1177/0194599811419044. [DOI] [PubMed] [Google Scholar]
- 19.Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36:27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]