Abstract
Objective
Evidence suggests that SHBG affects glycemic control, predicts both T2D and metabolic syndrome, and is low in obese subjects. We sought to determine if resistance exercise training (RT) can increase sex hormone-binding globulin (SHBG) and ameliorate levels of related steroid hormones in overweight/obese, sedentary young men.
Materials/Methods
36 participants (BMI 31.4 kg/m2, age 22 years) were randomized into an RT (12 weeks of training, 3/week) or control group (C, 12 weeks no training), and assessed for changes in SHBG, cortisol, testosterone, free testosterone (FT) and free androgen index (FAI). In addition, body composition and oral glucose tolerance testing was performed.
Results
12 weeks of RT increased SHBG (P=0.01) and decreased FAI (P<0.05) and cortisol (P<0.05) compared to C. FT decreased in RT (P=0.01). Total testosterone did not change in either group. These changes were noted without weight loss, and in concert with increases in lean body mass (P=0.0002 vs C) and decreases in glucose area under the curve (AUC) (P= 0.004), insulin AUC (P=0.03), and total (P=0.002) and trunk (P=0.003) fat mass in RT.
Conclusion
In overweight/obese young men, RT increases SHBG and lowers FAI in obese young adult men.
Keywords: Steroid hormone, Cortisol, Testosterone, Insulin sensitivity, Strength training, Exercise
1. Introduction
Type 2 diabetes (T2D) has progressed into a major cause of preventable death over the past half century, increasing from just over one million diagnosed in 1958 to nearly 21 million in 2010 [1]. An additional ~7 million are undiagnosed with T2D and approximately 80 million exhibit prediabetes [2]. Thus, prevention of future T2D in present day young adults is a major public health challenge. Resistance training (RT) may represent a preventive strategy for T2D [3], as it improves insulin sensitivity and glucose tolerance, independent of weight loss [4–7]).
Independent of traditional risk factors, biochemical markers have been identified that may be associated with increased risk of T2D, such as sex-steroid hormones and sex hormone-binding globulin (SHBG). The function of SHBG has classically been ascribed to the binding of steroid hormones in circulation to regulate their bioavailability. Because SHBG is decreased with obesity, it was thought that SHBG may be a marker for obesity in relation to T2D risk. However, evidence suggests that SHBG independently affects glycemic control [8,9] and predicts both T2D [10–12] and metabolic syndrome [13]. In addition, it is known that insulin [14,15] and glucose [16] also have reciprocal action on SHBG to regulate SHBG production in the liver.
To date the studies that have investigated the effects of RT on SHBG [17–20] have generally noted that RT does not affect SHBG. However, these studies were performed in healthy young [17,20] and middle-aged men [18] or older men and women [19]. The effect of RT on SHBG in obese young subjects is unknown.
We investigated if an RT intervention (12 weeks, 3 sessions/week) can ameliorate low levels of SHBG as well as levels of related steroid hormones in sedentary, overweight/ obese young men. Our primary hypothesis was that RT would increase SHBG in concert with improved glucose tolerance and body composition, independent of weight loss.
2. Methods
2.1. Study participants
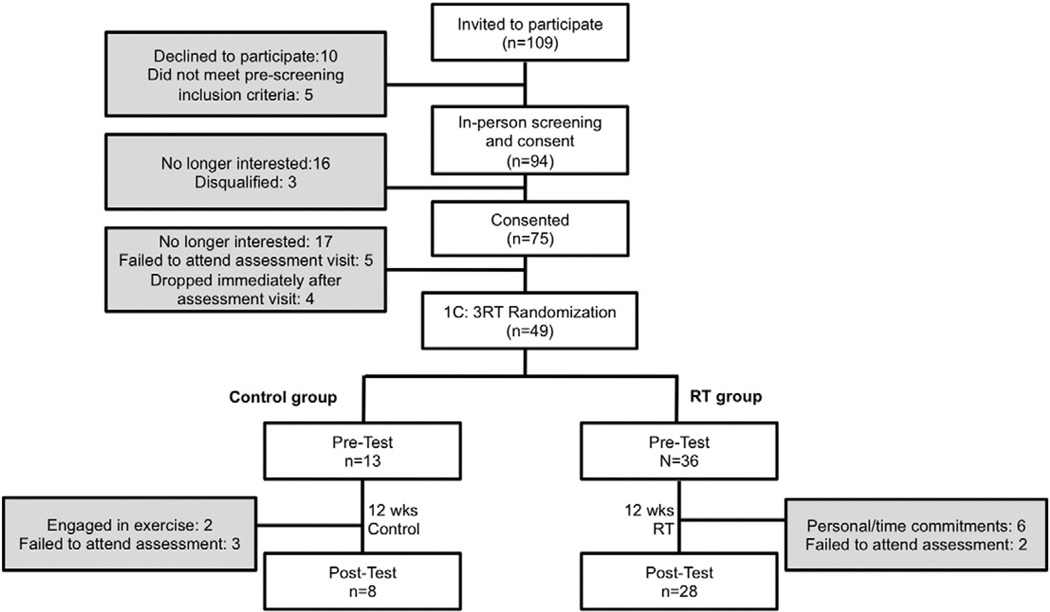
Of the 94 participants who attended the first screening visit, 75 were consented and qualified for the study, 49 attended their pre-test visit and were subsequently randomized to either RT or C. 36 young adult (ages 18–35) males who were overweight/ obese (BMI≥27 kg/m2) completed the study (Fig. 1).At baseline, participants were sedentary (participated in light-intensity physical activities ≤2 times/wk) and did not exhibit any other overt chronic disease symptoms, as indicated by a screening comprehensive history and physical examination. Potential participants who had: 1) documented CVD, cardiac surgery, or any heart arrhythmia found on an electrocardiogram (ECG) reading, 2) participated in a structured exercise, nutrition, or weight loss program within the previous 6 months, or 3) used tobacco products or medications that influence cardiovascular function, body composition or insulin indices in the prior 6 months, were excluded from the study. Participants were instructed to maintain their normal ad-libitum diet and normal activities of daily living. Pre- and post-intervention assessments were made at weeks 0 and 13, respectively. All of the study protocols were approved by the University of California, Los Angeles Institutional Review Board and were performed according to the Declaration of Helsinki.
Fig. 1.
Participant Flow Diagram.
2.2. Randomization
Following their pre-intervention assessment, participants were randomized into one of two groups at a 1:3 control (C, n=8) to resistance training (RT, n=28) ratio. Both groups were reminded to maintain their normal ad-libitum diet and normal activities of daily living. Participants randomized to the C group completed a 12-week control period without RT. Pre-and post-intervention assessments were made at weeks 0 and 13, respectively.
2.3. Resistance training intervention and muscular strength testing
All training occurred at the John Wooden Recreation Center at UCLA. Participants in the RT group completed 12 weeks of RT at three supervised sessions/week, with each session lasting approximately one hour. The training overload was modified using a linear periodization model with 3 phases. During phase 1 (weeks 1–2), participants completed two sets of 12–15 repetitions for each exercise at 100% of their approximated 12–15 repetition maximum (RM). In phase 2 (weeks 3–7) participants completed three sets of 8–12 repetitions at 100% of their 8–12 RM, and in phase 3 (weeks 8–12) participants completed 6–8 repetitions at 100% of their 6–8 RM. As participants adapted to the training overload, the weight was increased to maintain the prescribed training intensity. All participants trained on 3 non-consecutive days/week, rotating between two daily workout regimens. Workout I consisted of dumbbell (DB) squat, cable row, DB front lunge, DB row, barbell (BB) deadlift, DB triceps extension, and DB curl. Workout II was DB step-up, BB chest press, machine squat, DB overhead press, DB incline chest press, DB side raise, DB reverse fly, and abdominal crunches. A certified personal trainer led all training sessions with a maximum 3:1 participant to trainer ratio.
Maximal strength testing consisted of 1-RM lifts for the barbell bench press, 45° incline leg press (Hammer Strength Linear Leg Press), and machine-seated row (Life Fitness Pro2 series; all Life Fitness products, Schiller Park, IL, USA). Participants first warmed up each muscle group by performing 8–10 repetitions with weight equivalent to 40%–60% of their estimated 1-RM. The weight was progressively increased while decreasing the repetitions until participants could safely attempt an estimated 1-RM for each exercise. A successful 1-RM occurred on the penultimate set, having failed their last set. Participants were allowed 3–4 min of rest between all sets. All participants performed a total of 2 maximal strength tests: the RT group participants performed one immediately preceding the first training session and the second immediately preceding their penultimate training session, while the C group participants performed the tests at weeks 0 and 13 after their outpatient visits to prevent any acute exercise effects. Relative strength measures were calculated by dividing each measure by participant body weight.
2.4. Outpatient visit procedures
Measurements were taken from participants at baseline (pre-test) and on week 13 (post-test). While selecting the precise time from the last bout of training to evaluate primary outcome variables is debatable, to assess predominately chronic adaptations of the training program, the outpatient visit occurred approximately 72 h after the last training session. Before each visit, participants were reminded to: 1) avoid all moderate to vigorous physical activity 24 hours prior to testing and 2) to abstain from all food and drink (except water) for approximately 12 h prior to each visit. Verbal confirmation of adherence to the aforementioned criteria was obtained immediately prior to all testing.
The outpatient procedures at the Clinical and Translational Research Center (CTRC) began at 7:30 am and typically lasted 3.5 h.A 12-lead ECG was administered as a safety measure and checked by a physician before allowing any participation in exercise testing/intervention. Height, weight and waist circumference were measured in duplicate in all participants. Fasting blood samples were collected and serum was separated and stored at −80 °C until assayed. Subsequently, a 2-h oral glucose tolerance test (OGTT) was performed.
2.5. Body composition
Body composition was determined by dual energy x-ray absorptiometry (DXA) scan (Hologic QDR4500 Fan Beam X-ray Densitometer, Hologic, Waltham, MA).
2.6. Steroid hormone assays
Plasma levels of SHBG, cortisol and testosterone were measured by an electrochemiluminescent immunoassay (ECLIA) on an Elecsys 2010 autoanalyzer (Roche Diagnostics, Indianapolis, IN) at the UCLA Clinical and Translational Research Laboratory (CTRL). The coefficients of variation for SHBG, cortisol and testosterone test results from blinded quality-control samples were 4.3%, 3.8%, and 6.2% respectively. Free testosterone (FT) was calculated via the Sodergard method [21]. FAI was calculated by 100*(Total Testosterone/SHBG).
2.7. Oral glucose tolerance test
The participants completed a 2-h OGTT using 75 g of anhydrous glucose dissolved in water. Venous blood samples were obtained at baseline and every 30 min (−30, 0, 30, 60, 90 and 120, relative to glucose ingestion), and were assayed for glucose and insulin. UCLA CTRL analyzed serum glucose via in vitro hexokinase method (Olympus AU400 Chemistry Analyzer, Beckman Coulter, North America Commercial Operations, Irving, TX 75063, USA). Serum insulin was measured by solid-phase, enzyme-labeled chemiluminescent immunometric assay (Immulite® 2000, Diagnostic Products, Los Angeles, CA) by the UCLA Clinical Laboratories.
Total area under the glucose and insulin curves (AUC) was calculated by trapezoidal rule. AUC from 0 to 120 min was calculated for glucose (GAUC(0–120)) and insulin (IAUC(0–120)). Glucose and insulin at time points 0 (fasting) and 120 min (2-h), and AUC measures were used as indicators of glucose tolerance and insulin resistance. Glycated hemoglobin (HbA1c) was measured via DCA Vantage® Analyzer (Siemens Medical Solutions Diagnostics, New York, USA).
2.8. Statistical analyses
Non-parametric analyses were chosen for statistical inference due to the presence of non-normally distributed data, unequal samples sizes, and heteroscedasticity. The overall effects of the intervention were tested for evaluable participants. Significance was calculated using Wilcoxon signed-rank test. Confidence intervals were derived from bias-corrected bootstrap methods (100,000 permutations). Data are reported as median (interquartile range) unless stated otherwise. Statistical analyses were performed with the use of Stata 11.2 statistical software (StataCorp LP., College Station, TX). A p-value of <0.05 was considered statistically significant.
3. Results
A total of 36 participants, 28 in the RT group and 8 in the C group, finished the pre- and post-test visits (Fig. 1). At baseline, there were no significant differences between groups. Additionally, comparing baseline anthropometrics, there were no differences between the 13 participants who did not continue following their pre-test visit and the 36 participants who completed the entire study (all P>0.2).
3.1. Body composition, strength and OGTT
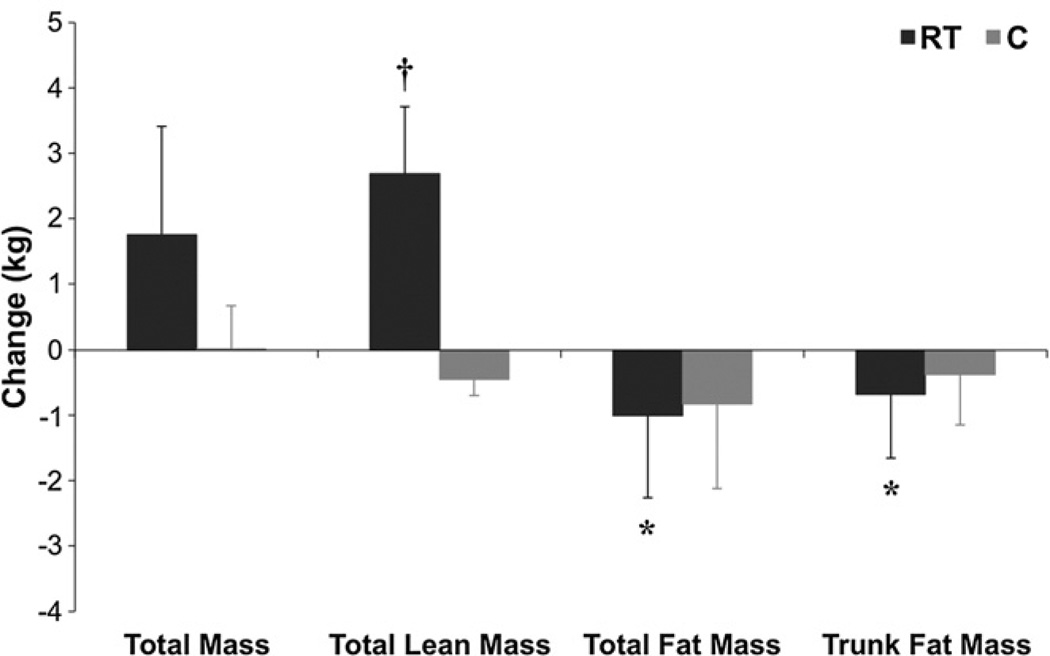
In total, participants in the RT group attended 99.7% of their training sessions. Table 1 illustrates changes in anthropometric data for between- and within-group effects. LBM (P=0.0002, Fig. 2) and 1RM strength in chest, leg, and row significantly increased in the RT compared to C (all P<0.05). Total (P<0.002) and trunk (P<0.003) fat mass decreased significantly in the RT group (Fig. 2). There was no change in BMI, waist circumference (WC), or body weight between groups, although body weight (P=0.07) and BMI (P=0.06) trended to increase in RT vs. C and BMI increased within the RT group (P=0.03). There was no change in HbA1c within- or between-groups. Both fasting insulin and glucose trended to increase in RT vs. C (P=0.054, P=0.05, respectively). However, glucose AUC and 2-h glucose trended to decrease in RT vs. C (P=0.07, P=0.05, respectively). Glucose AUC (P=0.004), insulin AUC (P= 0.03), 2-h glucose (P=0.007), and 2-h insulin (P=0.002) all decreased significantly in RT.
Table 1.
Effects of RT on Body Composition, Strength, and OGTT.
| Outcomes | Median (25th–75th percentile) |
Median (95% CI) |
P value |
||
|---|---|---|---|---|---|
| Pre-test | Post-test | Within-Group Changes |
Between-Group Changes |
||
| Age (years) | − | −b | |||
| Control | 22.0 (20.8–22.8) | − | −w | ||
| RT | 21.5 (20.0–23.0) | − | −w | ||
| Height (m) | − | − | |||
| Control | 1.74 (1.70–1.77) | − | − | ||
| RT | 1.77 (1.73–1.81) | − | − | ||
| Weight (kg) | 1.7 (−0.56 to 3.7) | 0.07 | |||
| Control | 98.5 (91.6–106.2) | 98.0 (90.9–105.9) | 0.02 (−1.4 to 0.57) | 0.58 | |
| RT | 96.6 (90.0–103.5) | 97.1 (91.2–105.4) | 1.8 (0.00 to 3.0) | 0.06 | |
| BMI (kg/m2) | 0.58 (−0.22 to 1.4) | 0.06 | |||
| Control | 33.6 (31.2–34.7) | 33.2 (31.3–34.6) | −0.19 (−0.57 to 0.19) | 0.16 | |
| RT | 30.9 (29.7–32.7) | 31.2 (30.3–32.7) | 0.39 (−0.18 to 0.96) | 0.03 | |
| WC (cm) | −0.27 (−3.4 to 2.9) | 0.77 | |||
| Control | 106.5 (96.1–110.8) | 106.7 (93.6–112.0) | −0.28 (−2.0 to 1.9) | 0.83 | |
| RT | 103.3 (99.4–111.3) | 101.4 (96.8–108.7) | −0.55 (−2.4 to 0.85) | 0.24 | |
| Total Fat Mass (kg) | −0.18 (−2.7 to 1.1) | 0.25 | |||
| Control | 25.0 (22.3–32.3) | 26.1 (22.1–31.0) | −0.82 (−1.9 to 1.3) | 0.58 | |
| RT | 27.9 (23.7–32.6) | 26.2 (20.5–30.1) | −1.0 (−2.9 to −0.44) | 0.002 | |
| Trunk Fat Mass (kg) | −0.30 (−1.5 to 0.78) | 0.36 | |||
| Control | 12.7 (11.5–17.2) | 12.7 (11.2–16.4) | −0.38 (−1.3 to 0.90) | 0.48 | |
| RT | 13.6 (11.6–16.9) | 12.8 (10.4–15.8) | −0.68 (−1.5 to −0.33) | 0.003 | |
| Lean Mass (kg) | 3.1 (1.2 to 4.1) | 0.0002 | |||
| Control | 69.3 (68.0–71.9) | 70.2 (68.6–71.4) | −0.45 (−0.70 to 1.4) | 0.58 | |
| RT | 69.5 (64.7–72.8) | 70.9 (66.5–76.2) | 2.7 (2.0 to 3.4) | <0.0001 | |
| 1RM Chest (kg) | 15.9 (10.2 to 21.6) | <0.001 | |||
| Control | 70.3 (65.8–96.4) | 70.3 (65.8–99.8) | 0.00 (0.00 to 13.6) | 0.45 | |
| RT | 70.3 (56.7–80.5) | 86.2 (70.3–94.1) | 15.9 (13.6 to 19.3) | <0.0001 | |
| 1RM Leg (kg) | 59.0 (15.9 to 95.3) | <0.001 | |||
| Control | 272.2 (272.2–347.0) | 281.2 (260.8–349.3) | 9.1 (−9.1 to 54.4) | 0.45 | |
| RT | 251.7 (237.0–293.7) | 331.1 (311.9–365.1) | 68.0 (56.7 to 90.7) | <0.0001 | |
| 1RM Row (kg) | 18.1 (4.5 to 29.5) | 0.015 | |||
| Control | 81.7 (70.3–95.3) | 88.5 (82.8–89.6) | −2.3 (−4.5 to 17.0) | 0.67 | |
| RT | 79.4 (72.0–90.7) | 94.1 (90.2–104.9) | 15.9 (13.6 to 20.4) | <0.0001 | |
| Strength Score (kg) | 77.1 (28.4 to 124.7) | <0.001 | |||
| Control | 455.9 (399.2–511.4) | 437.7 (411.6–526.2) | 22.7 (−11.3 to 77.1) | 0.40 | |
| RT | 409.4 (373.7–470.6) | 517.1 (479.1–560.8) | 99.8 (88.5 to 129.3) | <0.0001 | |
| Fasting Glucose (mg/dL) | 8.5 (0.75 to 17.0) | 0.05 | |||
| Control | 95.3 (92.0–98.5) | 87.5 (85.6–89.4) | −7.8 (−13.5 to 2.0) | 0.05 | |
| RT | 88.0 (85.5–93.9) | 90.0 (84.9–94.8) | 0.75 (−2.5 to 3.5) | 0.89 | |
| Fasting Insulin (µIU/mL) | 2.8 (−0.50 to 7.5) | 0.054 | |||
| Control | 6.5 (5.8–8.0) | 4.3 (3.6–4.9) | −3.3 (−6.5 to 1.0) | 0.05 | |
| RT | 9.0 (5.5–12.0) | 9.0 (6.0–11.5) | −0.50 (−2.0 to 2.0) | 0.88 | |
| HbA1c (%) | 0.00 (−0.20 to 0.70) | 0.79 | |||
| Control | 5.6 (5.2–5.6) | 5.5 (5.3–5.5) | 0.00 (0.00 to 0.40) | 1.00 | |
| RT | 5.3 (5.2–5.6) | 5.3 (5.2–5.4) | 0.00 (−0.05 to 0.20) | 0.99 | |
| Glucose AUC (mg/dL)*min | −2137.5 (−3420.0 to 315.0) | 0.07 | |||
| Control | 15,461.3 (13,680.0–15,656.3) | 14,118.8 (13,621.9–15,673.1) | 780.0 (−1357.5 to 1762.5) | 0.83 | |
| RT | 14,812.5 (13,665.0–16,155.0) | 13,357.5 (12,195.0–1475.0) | −1357.5 (−2062.5 to −352.5) | 0.004 | |
| Insulin AUC (µIU/mL)*min | −258.8 (−2408.8 to 2197.5) | 0.80 | |||
| Control | 4200.0 (3791.3–5486.3) | 3903.8 (3671.3–4524.4) | −788.8 (−2418.8 to 1260.0) | 0.25 | |
| RT | 7008.8 (5381.3–9543.7) | 6180.0 (4657.5–9255.0) | −1042.5 (−1252.5 to −438.8) | 0.03 | |
| 2-h Glucose (mg/dL) | −22.5 (−37.0 to −1.0) | 0.05 | |||
| Control | 110.5 (104.8–114.8) | 112.5 (104.5–123.5) | 9.0 (9.0 to 23.0) | 0.46 | |
| RT | 111.0 (100.3–121.8) | 97.0 (87.8–113.0) | −13.5 (−19.0 to −3.0) | 0.007 | |
| 2-h Insulin (µIU/mL) | −15.0 (−27.5 to 21.0) | 0.27 | |||
| Control | 29.0 (16.8–55.5) | 24.5 (19.3–29.0) | −6 (−24.5 to 14.0) | 0.25 | |
| RT | 54.0 (36.0–82.0) | 35.0 (25.0–48.0) | −21.0 (−22.0 to −1.0) | 0.002 | |
Abbreviations: BMI: body mass index; WC: waist circumference; 1RM: 1 repetition maximum; Strength Score: sum of all 3 strength measures. Relative strength measures were calculated by dividing each measure by participant body weight. HbA1c: Glycated hemoglobin. AUC: area under the curve. RT (n=28) C (n=8).
Bold data denotes P<0.05.
between group significance was calculated using Wilcoxon rank-sum test on post-test -- pre-test change scores.
within group significance was calculated using Wilcoxon signed-rank test.
Fig. 2.
Effects of RT on Body Composition. Bar graphs present median and median absolute deviation (MAD) for pre- and post-test. *P<0.01 within RT; †P=0.0002 between RT (n=28) and C (n=8).
3.2. Steroid hormones
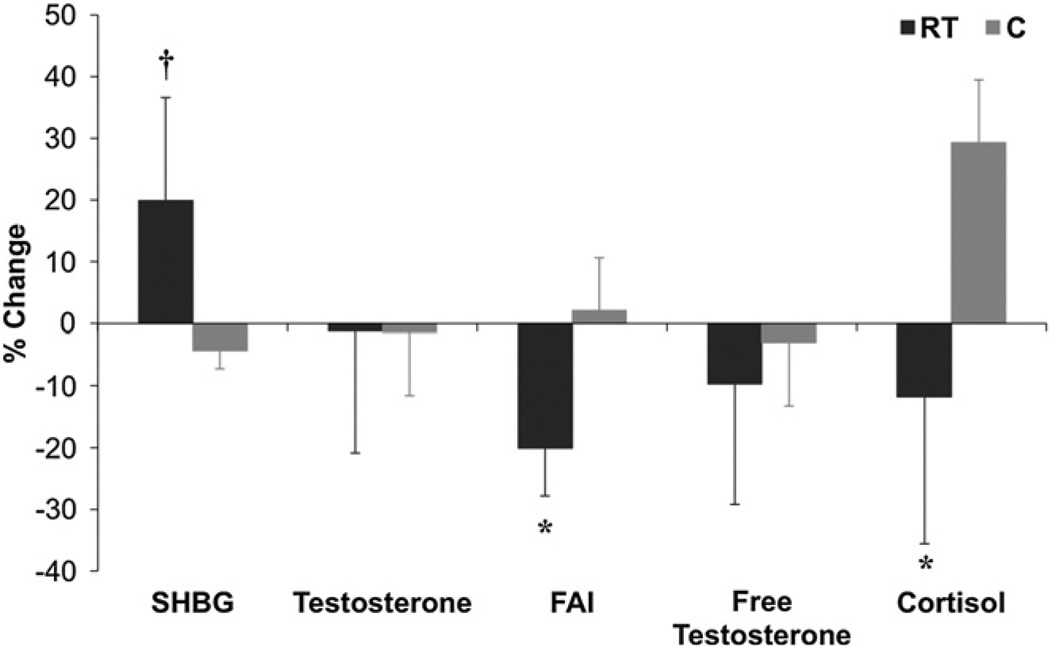
Table 2 indicates that compared to C, SHBG increased (P= 0.005) and FAI (P<0.05) and cortisol (P<0.05) decreased in RT. FT decreased in RT (P=0.01) and total testosterone did not change in either group. Percent changes in steroid hormone indices are depicted in Fig. 3.
Table 2.
Effects of RT on Steroid Hormone Indices.
| Outcomes | Median (25th–75th percentile) |
Median (95% CI) |
P value |
||
|---|---|---|---|---|---|
| Pre-test | Post-test | Within-Group Changes |
Between-Group Changes |
||
| Cortisol (µg/dL) | −4.6 (−7.2 to −0.78) | 0.04b | |||
| Control | 13.5 (13.4–14.6) | 17.5 (17.2–18.0) | 2.9 (1.2 to 4.6) | 0.03w | |
| RT | 14.8 (12.5–19.1) | 12.7 (11.2–14.9) | −1.7 (−3.5 to 1.1) | 0.17w | |
| SHBG (nmol/L) | 4.0 (1.7 to 7.7) | 0.005 | |||
| Control | 15.2 (11.8–17.9) | 16.7 (11.7–17.8) | −0.73 (−1.2 to 2.6) | 0.35 | |
| RT | 14.3 (11.4–19.7) | 19.4 (13.7–24.3) | 3.3 (1.6 to 7.0) | 0.0001 | |
| Testosterone (ng/mL) | 0.06 (−0.76 to 0.86) | 1.0 | |||
| Control | 4.1 (4.1–5.3) | 4.2 (3.5–5.7) | −0.10 (−0.60 to 0.68) | 0.75 | |
| RT | 4.6 (4.1–5.6) | 4.5 (3.8–5.4) | −0.05 (−0.69 to 0.29) | 0.64 | |
| Free Testosterone (ng/dL) | −0.57 (−3.1 to 1.2) | 0.40 | |||
| Control | 11.9 (9.6–12.5) | 10.3 (10.2–13.8) | −0.49 (−1.8 to 2.0) | 0.60 | |
| RT | 11.6 (9.8–14.0) | 10.4 (8.6–12.3) | −1.1 (−2.6 to −0.10) | 0.01 | |
| FAI | −23.3 (−47.4 to −4.1) | 0.04 | |||
| Control | 99.4 (79.9–135) | 99.8 (88.8–115) | 0.61 (−9.5 to 21) | 0.75 | |
| RT | 113.0 (77.3–134) | 80.2 (64.9–105) | −22.7 (−41.6 to −9.0) | 0.0005 | |
between group significance was calculated using Wilcoxon rank-sum test on post-test - pre-test change scores.
within group significance was calculated using Wilcoxon signed-rank test. RT (n=28) C (n=8).
Fig. 3.
Effects of RT on Steroid Hormone Changes. Bar graphs present median and median absolute deviation (MAD) for pre- and post-test. *P<0.05 between RT and C; †P=0.005 between RT (n=27) and C (n=7).
3.3. Individual responses
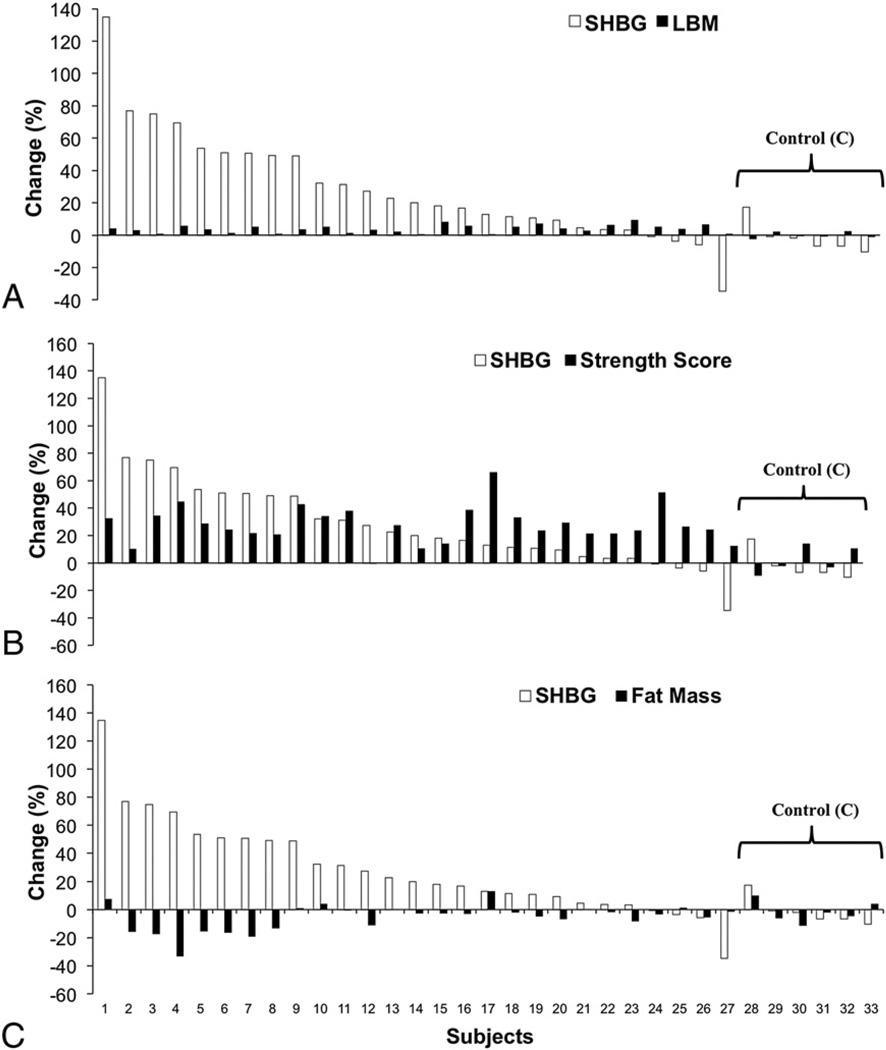
Fig. 4 represents the percent change from pre-test to post-test in each individual for SHBG sorted by LBM (4A), strength score (4B) and total fat mass (4C). Notably, although nearly all subjects in RT exhibited an increase in SHBG, the effect was highly variable. The individualized effect of RT on SHBG was generally unrelated to the effects on these outcomes.
Fig. 4.
Individual Responses. Individual responsiveness to 12-week RT intervention presented as a percent change from pre-test values and sorted from greatest to least training response for SHBG sorted by LBM (A), strength score (B) and total fat mass (C). RT (n=27) C (n=6).
4. Discussion
Recently, there is evidence to suggest that steroid hormone biology plays a role in metabolic disease. For example, although the function of SHBG has classically been ascribed to the binding of steroid hormones in circulation to regulate their bioavailability, SHBG has been demonstrated to affect glycemic control [8,9] and to predict both T2D [10–12] and metabolic syndrome [13].
We investigated the effects of an RT intervention on SHBG, cortisol, testosterone and indices of free androgens in sedentary, overweight/obese young men. We noted that: 1) RT increased SHBG, and decreased cortisol and FAI, compared to C; 2) FT decreased in RT; 3) these changes occurred in conjunction with improvements in glucose tolerance, strength, LBM, and decreases in total and trunk fat mass, but in the absence of weight loss; and 4) the effects of RT on SHBG exhibited significant individual variability. These results supported our primary hypothesis, that RT would increase SHBG, independent of weight loss.
Our findings are in contrast to studies that have demonstrated SHBG does not change in young men with RT [17,20,22], and these findings were also noted in middle-aged [18] or older men and women [19]. However, Daly et al. [23] noted that in older overweight adults with T2D, despite no change in SHBG with weight loss (low calorie diet) compared with weight loss+RT, there was a within group increase in SHBG after 6 months in the group performing RT. To our knowledge, ours is the first study to determine the effects of RT alone on SHBG in young obese men. We noted an increase in SHBG of ~25% relative to the change in the control group (Fig. 3), and it is likely that the main difference in the results of the aforementioned studies and our study was the baseline phenotypes of the subjects. The implications of an increase in SHBG is unknown, however it has been suggested that low SHBG is associated with higher rates of obesity [24] and T2D [10–12]. Interestingly, the increase in SHBG that we noted occurred in the absence of weight loss. In fact, due to greater increases in LBM than decreases in fat mass, body weight of these overweight/obese young men increased with RT. It is apparent that the relationships between metabolic disease risk factors (body weight, fat mass, SHBG, etc) is complex and not simply a function of weight loss improving metabolic risk profile and weight gain resulting in a more deleterious profile. The increase in SHBG was accompanied by improvements in other metabolic risk factors associated with risk of T2D, metabolic syndrome or mortality including LBM, strength, fat mass and glucose tolerance. The increase in SHBG may be related to the change in glucose tolerance as it is known that both insulin [14,15] and glucose [16] regulate SHBG production in the liver. Selva et al. [16] elegantly demonstrated that elevated glucose (and fructose), rather than insulin might be the primary stimulus to lower SHBG. In this study, transgenic mice expressing different SHBG transgenes exposed to diets with elevated monosaccharides led to large decreases in SHBG.
Levels of testosterone have also been found to be associated with insulin resistance [25,26], visceral adiposity [27], diabetes [28,29] and metabolic syndrome [30]. However, we did not note a change in testosterone after RT, although FT did exhibit a small but statistically significant decrease. The implications of this finding are unknown, but the improvements in body composition (increased LBM, decreased total and trunk fat mass), strength, and insulin dynamics occurred without increases in total or FT. The decrease in FAI, indicative of biologically active testosterone, is likely due to the increase in SHBG since total testosterone did not change. This suggests that increased levels of bioavailable androgens are not required for the aforementioned improvements in metabolic risk variables with RT intervention. Similarly, the improvement in strength and increase in LBM noted are likely independent of change in androgen levels [31].
Historically, it has been demonstrated that higher cortisol levels are noted in obesity, and thus may be noted in obese patients with T2D and/or metabolic syndrome. Indeed, several studies have reported increased cortisol in subjects with T2D and metabolic syndrome [32,33]. However, in a recent study by DeSantis et al. [34], salivary cortisol was not related to metabolic syndrome. We noted that RT decreased cortisol compared to C. This would be consistent with the expected change in cortisol associated with an improved metabolic profile. Given that the effects of cortisol on metabolic syndrome and T2D are controversial, the implications of this finding remain unclear.
What also has been underappreciated is the individual responsiveness to different forms of exercise training. It has previously been demonstrated that aerobic exercise training responses are highly variable (see Fig. G.2.4 in Refs. [3] and [35]). Determination of individual responses with RT is important as we move toward individualizing exercise training programs. We noted strength increases of 2%–60% and LBM of <1% to 9%, with decreases in fat mass of −10% (a 10% gain to 30% decrease). This occurred in conjunction with highly variable responsiveness for SHBG (35% decrease to 130% increase). Thus, the improvements in SHBG and other phenotypic outcomes – across subject LBM, strength and fat mass improvements – were highly variable, and suggest the existence of a complex relationship, whereby genetic variation, training motivation and other factors likely account for the spectrum of responses noted.
In summary, in overweight/obese young men, RT increases SHBG and decreases both cortisol and FAI.The primary strength of this study is the novel investigation of the effects of RT on SHBG and steroid hormones in an early risk population of young obese men. Limitations of this study include the small sample size and gender-specific population. The implications of the effects of RT on SHBG and its relationship with T2D and metabolic syndrome warrant further study.
Acknowledgments
We would like to thank Brian Le, Mary M. Lee, Shannon L. Krell, Christopher S. Oh, Michael Katiraie, and the entire Exercise and Metabolic Disease Research team for their commitment to this study. We thank the dedicated nurses and staff of the UCLA CTRC, Gonda (Goldschmied) Diabetes Center, and Elisa Terry and colleagues at the John Wooden Recreation Center. Furthermore, we thank all participants for their time and effort.
Funding
This work was supported by the American Heart Association (BGIA # 0765139Y to C.K.R.), the National Heart, Lung and Blood Institute (P50 HL105188 to C.K.R.), the National Institute of Diabetes and Digestive and Kidney Diseases (DK090406 to C.K.R.) and the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124.
Abbreviations
- T2D
Type 2 diabetes
- RT
Resistance training
- C
Control
- SHBG
Sex hormone-binding globulin
- FT
Free testosterone
- FAI
Free androgen index
- AUC
Area under the curve
- CVD
Cardiovascular disease
- ECG
Electrocardiogram
- RM
Repetition maximum
- DB
Dumbbell
- BB
Barbell
- CTRC
Clinical and Translational Research Center
- OGTT
Oral glucose tolerance test
- DXA
Dual energy x-ray absorptiometry
- CTRL
Clinical and Translational Research Laboratory
- LBM
Lean body mass
- BMI
Body mass index
- WC
Waist circumference
- MAD
Median absolute deviation
Footnotes
Author contributions
C.K.R. conception and design of research; D.M.C. led training intervention; N.A. performed experiments; D.M.C. analyzed data. C.K.R., D.M.C. interpreted results of experiments; C.K.R. drafted manuscript; C.K.R., D.M.C., N.A., A.W.B, and C.C.L. edited manuscript; C.K.R., D.M.C., N.A., A.W.B, and C.C.L. approved final version of manuscript.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- 1. [Accessed August 31, 2012]; http://www.cdc.gov/diabetes/statistics In.
- 2. [Accessed October 10, 2011]; http://www.diabetes.org/diabetes-basics/diabetes-statistics/ In.
- 3.Physical activity guideline advisory committee report. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 4.Zachwieja JJ, Toffolo G, Cobelli C, et al. Resistance exercise and growth hormone administration in older men: effects on insulin sensitivity and secretion during a stable-label intravenous glucose tolerance test. Metabolism. 1996;45:254–260. doi: 10.1016/s0026-0495(96)90063-3. [DOI] [PubMed] [Google Scholar]
- 5.Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91:5107–5112. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 6.Ryan AS, Pratley RE, Goldberg AP, Elahi D. Resistive training increases insulin action in postmenopausal women. J Gerontol A Biol Sci Med Sci. 1996;51:M199–M205. doi: 10.1093/gerona/51a.5.m199. [DOI] [PubMed] [Google Scholar]
- 7.Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38:1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 8.Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metabol. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 10.Ding EL, Song Y, Malik VS, Liu S, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 11.Lindstedt G, Lundberg PA, Lapidus L, et al. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM. 12-yr follow-up of population study of women in Gothenburg, Sweden. Diabetes. 1991;40:123–128. doi: 10.2337/diab.40.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhasin S, Jasjua GK, Pencina M, et al. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men. Diabetes Care. 2011;34:2464–2470. doi: 10.2337/dc11-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plymate SR, Matej LA, Jone RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by Insulin and prolactin. J Clin Endocrinol Metabol. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 15.Pasquali R, Casimirri F, De Iasio R, et al. Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J Clin Endocrinol Metabol. 1995;80:654–658. doi: 10.1210/jcem.80.2.7852532. [DOI] [PubMed] [Google Scholar]
- 16.Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117:3979–3987. doi: 10.1172/JCI32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCall GE, Byrnes WC, Fleck SJ, et al. Acute and chronic hormonal responses to resistance training designed to promote muscle hypertrophy. Can J Appl Physiol. 1999;24:96–107. doi: 10.1139/h99-009. [DOI] [PubMed] [Google Scholar]
- 18.Cadore EL, Lhullier FL, Brentano MA, et al. Hormonal responses to resistance exercise in long-term trained and untrained middle-aged men. J Strength Cond Res. 2008;22:1617–1624. doi: 10.1519/JSC.0b013e31817bd45d. [DOI] [PubMed] [Google Scholar]
- 19.Hakkinen K, Kraemer WJ, Pakarinen A, et al. Effects of heavy resistance/power training on maximal strength, muscle morphology, and hormonal response patterns in 60-75-year-old men and women. Can J Appl Physiol. 2002;27:213–231. doi: 10.1139/h02-013. [DOI] [PubMed] [Google Scholar]
- 20.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 21.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer WJ, Staron RS, Hagerman FC, et al. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol Occup Physiol. 1998;78:69–76. doi: 10.1007/s004210050389. [DOI] [PubMed] [Google Scholar]
- 23.Daly R, Dunstan D, Owen N, et al. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int. 2005;16:1703–1712. doi: 10.1007/s00198-005-1906-4. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen TL, Hagen C, Wraae K, et al. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metabol. 2007;92:2696–2705. doi: 10.1210/jc.2006-1847. [DOI] [PubMed] [Google Scholar]
- 25.Endre T, Mattiasson I, Berglund G, Hulthen UL. Low testosterone and insulin resistance in hypertension-prone men. J Hum Hypertens. 1996;10:755–761. [PubMed] [Google Scholar]
- 26.Haffner SM, Karhapaa P, Mykkanen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes. 1994;43:212–219. doi: 10.2337/diab.43.2.212. [DOI] [PubMed] [Google Scholar]
- 27.Seidell JC, Bjorntorp P, Sjostrom L, et al. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 28.Haffner SM, Shaten J, Stern MP, et al. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143:889–897. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- 29.Defay R, Papoz L, Barny S, et al. Hormonal status and NIDDM in the European and Melanesian populations of New Caledonia: a case–control study. The CALedonia DIAbetes Mellitus (CALDIA) Study Group. Int J Obes Relat Metab Disord. 1998;22:927–934. doi: 10.1038/sj.ijo.0800697. [DOI] [PubMed] [Google Scholar]
- 30.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 31.West DWD, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010;42:1371–1375. doi: 10.1016/j.biocel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Chiodini I, Torlontano M, Scillitani A, et al. Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. Eur J Endocrinol. 2005;153:837–844. doi: 10.1530/eje.1.02045. [DOI] [PubMed] [Google Scholar]
- 33.Welles B. Glucocorticoids in type 2 diabetes mellitus and the metabolic syndrome. Drug Dev Res. 2006;67:570–573. [Google Scholar]
- 34.DeSantis AS, DiezRoux AV, Hajat A, et al. Associations of salivary cortisol levels with metabolic syndrome and its components: the Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metabol. 2011;96:3483–3492. doi: 10.1210/jc.2011-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2 max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;87:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]