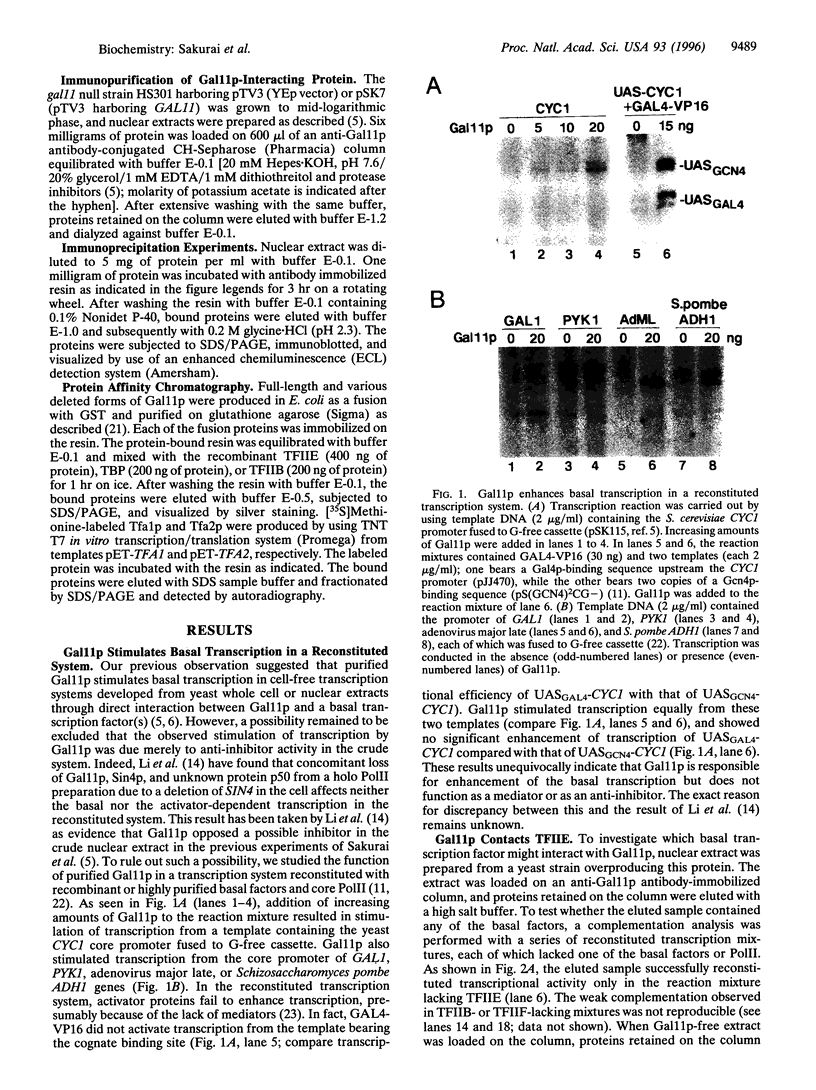
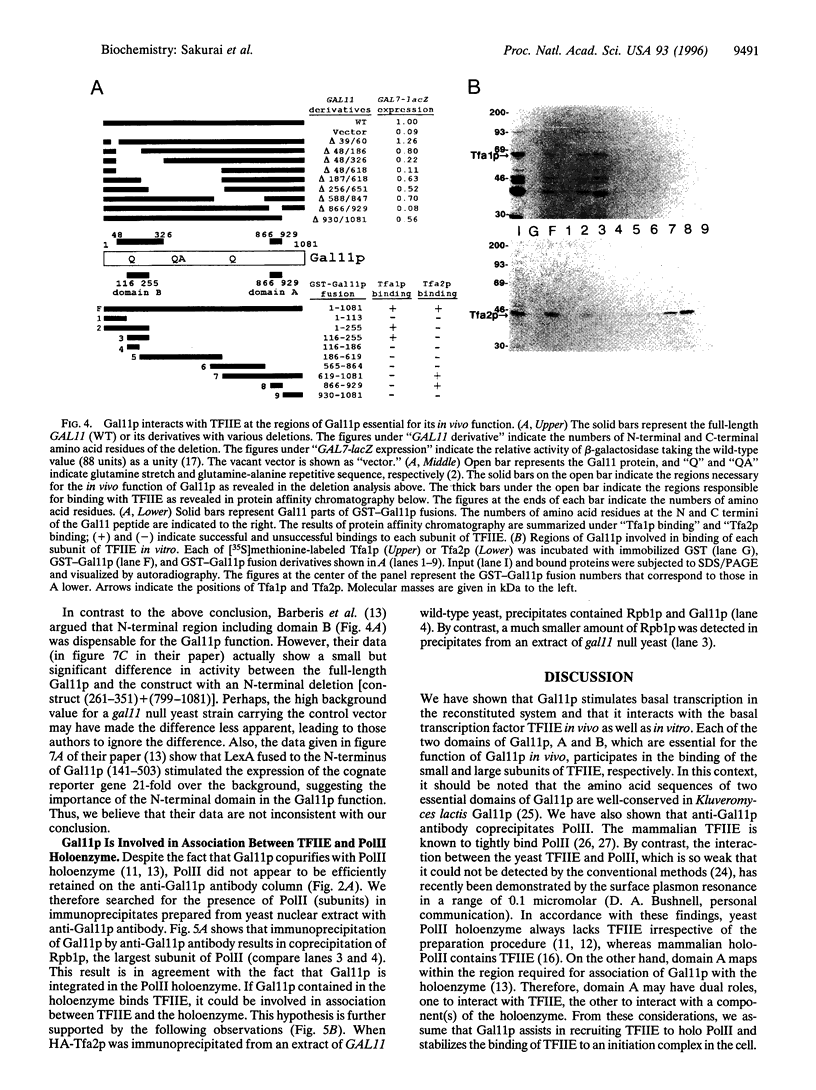
Abstract
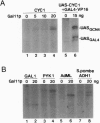
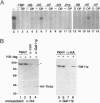
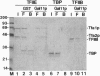
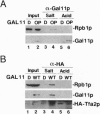
The GAL11 gene encodes an auxiliary transcription factor required for full expression of many genes in yeast. The GAL11-encoded protein (Gal11p) has recently been shown to copurify with the holoenzyme of RNA polymerase II. Here we report that Gal11p stimulates basal transcription in a reconstituted transcription system composed of recombinant or highly purified transcription factors, TFIIB, TFIIE, TFIIF, TFIIH, and TATA box-binding protein and core RNA polymerase II. We further demonstrate that each of the two domains of Gal11p essential for in vivo function respectively participates in the binding to the small and large subunits of TFIIE. The largest subunit of RNA polymerase II was coprecipitated by anti-hemagglutinin epitope antibody from crude extract of GAL11 wild type yeast expressing hemagglutinintagged small subunit of TFIIE. Such a coprecipitation of the RNA polymerase subunit was seen but in a greatly reduced amount, if extract was prepared from gal11 null yeast. In light of these findings, we suggest that Gal11p stimulates promoter activity by enhancing an association of TFIIE with the preinitiation complex in the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barberis A., Pearlberg J., Simkovich N., Farrell S., Reinagel P., Bamdad C., Sigal G., Ptashne M. Contact with a component of the polymerase II holoenzyme suffices for gene activation. Cell. 1995 May 5;81(3):359–368. doi: 10.1016/0092-8674(95)90389-5. [DOI] [PubMed] [Google Scholar]
- Carey M. Transcription. Simplifying the complex. Nature. 1994 Mar 31;368(6470):402–403. doi: 10.1038/368402a0. [DOI] [PubMed] [Google Scholar]
- Drapkin R., Reardon J. T., Ansari A., Huang J. C., Zawel L., Ahn K., Sancar A., Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994 Apr 21;368(6473):769–772. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- Fassler J. S., Winston F. The Saccharomyces cerevisiae SPT13/GAL11 gene has both positive and negative regulatory roles in transcription. Mol Cell Biol. 1989 Dec;9(12):5602–5609. doi: 10.1128/mcb.9.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaver W. J., Henry N. L., Bushnell D. A., Sayre M. H., Brickner J. H., Gileadi O., Kornberg R. D. Yeast TFIIE. Cloning, expression, and homology to vertebrate proteins. J Biol Chem. 1994 Nov 4;269(44):27549–27553. [PubMed] [Google Scholar]
- Flores O., Maldonado E., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. Factors IIE and IIF independently interact with RNA polymerase II. J Biol Chem. 1989 May 25;264(15):8913–8921. [PubMed] [Google Scholar]
- Goodrich J. A., Tjian R. Transcription factors IIE and IIH and ATP hydrolysis direct promoter clearance by RNA polymerase II. Cell. 1994 Apr 8;77(1):145–156. doi: 10.1016/0092-8674(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Hengartner C. J., Thompson C. M., Zhang J., Chao D. M., Liao S. M., Koleske A. J., Okamura S., Young R. A. Association of an activator with an RNA polymerase II holoenzyme. Genes Dev. 1995 Apr 15;9(8):897–910. doi: 10.1101/gad.9.8.897. [DOI] [PubMed] [Google Scholar]
- Holstege F. C., Tantin D., Carey M., van der Vliet P. C., Timmers H. T. The requirement for the basal transcription factor IIE is determined by the helical stability of promoter DNA. EMBO J. 1995 Feb 15;14(4):810–819. doi: 10.1002/j.1460-2075.1995.tb07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. W., Stillman D. J. Involvement of the SIN4 global transcriptional regulator in the chromatin structure of Saccharomyces cerevisiae. Mol Cell Biol. 1992 Oct;12(10):4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. J., Björklund S., Li Y., Sayre M. H., Kornberg R. D. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994 May 20;77(4):599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Koleske A. J., Young R. A. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994 Mar 31;368(6470):466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- Koleske A. J., Young R. A. The RNA polymerase II holoenzyme and its implications for gene regulation. Trends Biochem Sci. 1995 Mar;20(3):113–116. doi: 10.1016/s0968-0004(00)88977-x. [DOI] [PubMed] [Google Scholar]
- Li Y., Bjorklund S., Jiang Y. W., Kim Y. J., Lane W. S., Stillman D. J., Kornberg R. D. Yeast global transcriptional regulators Sin4 and Rgr1 are components of mediator complex/RNA polymerase II holoenzyme. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon M. E., Goodrich J. A., Tjian R. Transcription factor IIE binds preferentially to RNA polymerase IIa and recruits TFIIH: a model for promoter clearance. Genes Dev. 1994 Mar 1;8(5):515–524. doi: 10.1101/gad.8.5.515. [DOI] [PubMed] [Google Scholar]
- Mylin L. M., Gerardot C. J., Hopper J. E., Dickson R. C. Sequence conservation in the Saccharomyces and Kluveromyces GAL11 transcription activators suggests functional domains. Nucleic Acids Res. 1991 Oct 11;19(19):5345–5350. doi: 10.1093/nar/19.19.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma Y., Roeder R. G. Regulation of TFIIH ATPase and kinase activities by TFIIE during active initiation complex formation. Nature. 1994 Mar 10;368(6467):160–163. doi: 10.1038/368160a0. [DOI] [PubMed] [Google Scholar]
- Ossipow V., Tassan J. P., Nigg E. A., Schibler U. A mammalian RNA polymerase II holoenzyme containing all components required for promoter-specific transcription initiation. Cell. 1995 Oct 6;83(1):137–146. doi: 10.1016/0092-8674(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Parvin J. D., Sharp P. A. DNA topology and a minimal set of basal factors for transcription by RNA polymerase II. Cell. 1993 May 7;73(3):533–540. doi: 10.1016/0092-8674(93)90140-l. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Sakai A., Shimizu Y., Kondou S., Chibazakura T., Hishinuma F. Structure and molecular analysis of RGR1, a gene required for glucose repression of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4130–4138. doi: 10.1128/mcb.10.8.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H., Hiraoka Y., Fukasawa T. Yeast GAL11 protein is a distinctive type transcription factor that enhances basal transcription in vitro. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8382–8386. doi: 10.1073/pnas.90.18.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H., Ohishi T., Amakasu H., Fukasawa T. Yeast GAL11 protein stimulates basal transcription in a gene-specific manner by a mechanism distinct from that by DNA-bound activators. FEBS Lett. 1994 Sep 5;351(2):176–180. doi: 10.1016/0014-5793(94)80098-7. [DOI] [PubMed] [Google Scholar]
- Sakurai H., Ohishi T., Fukasawa T. Two alternative pathways of transcription initiation in the yeast negative regulatory gene GAL80. Mol Cell Biol. 1994 Oct;14(10):6819–6828. doi: 10.1128/mcb.14.10.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre M. H., Tschochner H., Kornberg R. D. Purification and properties of Saccharomyces cerevisiae RNA polymerase II general initiation factor a. J Biol Chem. 1992 Nov 15;267(32):23383–23387. [PubMed] [Google Scholar]
- Sayre M. H., Tschochner H., Kornberg R. D. Reconstitution of transcription with five purified initiation factors and RNA polymerase II from Saccharomyces cerevisiae. J Biol Chem. 1992 Nov 15;267(32):23376–23382. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nogi Y., Abe A., Fukasawa T. GAL11 protein, an auxiliary transcription activator for genes encoding galactose-metabolizing enzymes in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4991–4999. doi: 10.1128/mcb.8.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L. G., Carlson M. New SNF genes, GAL11 and GRR1 affect SUC2 expression in Saccharomyces cerevisiae. Genetics. 1991 Nov;129(3):675–684. doi: 10.1093/genetics/129.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet T., Dignard D., Thomas D. Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Zawel L., Kumar K. P., Reinberg D. Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 1995 Jun 15;9(12):1479–1490. doi: 10.1101/gad.9.12.1479. [DOI] [PubMed] [Google Scholar]