Abstract
Background
IL-17A has been implicated in severe forms of asthma. However, the factors that promote IL-17A production during the pathogenesis of severe asthma remain undefined. Diesel exhaust particles (DEP) are a major component of traffic related air pollution and are implicated in asthma pathogenesis and exacerbation.
Objective
To determine the mechanism by which DEP exposure impacts asthma severity using human and mouse studies.
Methods
Balb/c mice were challenged with DEP +/− house dust mite extract (HDM). Airway inflammation and function, BALF cytokine levels, and flow cytometry of lung T cells were assessed. The impact of DEP exposure on frequency of asthma symptoms and serum cytokine levels was determined in children with allergic asthma.
Results
In mice, exposure to DEP alone did not induce asthma. DEP and HDM co-exposure markedly enhanced AHR compared to HDM alone and generated a mixed Th2 and Th17 response, including IL-13+IL-17A+ double producing T-cells. IL-17A neutralization prevented DEP-induced exacerbation of AHR. Among 235 high DEP-exposed children with allergic asthma, 32.2% had more frequent asthma symptoms over a 12 month period, compared to only 14.2% in the low DEP-exposed group (p=0.002). Additionally, high DEP-exposed children with allergic asthma had nearly six times higher serum IL-17A levels compared with low DEP-exposed children.
Conclusions
Expansion of Th17 cells contributes to DEP-mediated exacerbation of allergic asthma. Neutralization of IL-17A may be a useful potential therapeutic strategy to counteract the asthma promoting effects of traffic related air pollution especially in highly exposed severe allergic asthmatics.
Keywords: allergic asthma, house dust mite, diesel exhaust particle, IL17A, Treg
INTRODUCTION
Asthma is characterized by reversible allergen-induced chronic airway inflammation and mucus production resulting in airway obstruction and hyperesponsiveness (AHR). Allergic asthma is generally regarded as a Th2 disease since elevated levels of eosinophils and Th2 cytokines (IL-4, IL-5, IL-13) are often observed (1), however, different patterns of airway inflammation are observed in the lungs of individuals with asthma. Several asthma subgroups have been identified based on different inflammatory profiles including eosinophilic asthma, neutrophilic asthma and mixed neutrophilic/eosinophilic asthma (2, 3). Asthmatics with mixed inflammatory phenotypes including both eosinophils and neutrophils have the lowest lung function and the worst asthma control (3, 4).
Neutrophilic inflammation is a hallmark of Th17 responses (5). The importance of Th17 cells and their cytokines IL-17A and IL-17F, which exist as homo and heterodimers, was first demonstrated in autoimmune diseases like EAE (6). However, mounting evidence suggest a role for Th17 cells in asthma (6, 7). Immunohistochemistry on bronchial biopsies from patients with either mild, moderate or severe asthma (ATS criteria) demonstrate a marked increase in the number of IL-17A and IL-17F positive cells in severe asthmatics compared to either mild asthmatic or healthy controls (4). In mice deficient for either IL-17A or its receptor (formed by the IL-17RA/IL-17RC complex), airway neutrophilia and AHR are attenuated following allergen exposure further supporting a key role for IL-17A in asthma. However, IL-17A delivery or transfer of Th17 cells is not sufficient to induce AHR in these mice (8, 9). Thus, Th17 cells appear to promote disease in the context of allergen-induced Th2 responses. Hence it is important to delineate the signals that promote Th17 responses in the context of allergic asthma.
The association between air pollution, including traffic emissions, and increased risk for respiratory disease is now widely accepted and may contribute to the dramatic increase in the incidence of asthma in industrialized countries (10, 11). Recently, particulate matter has been shown to induce IL-17A in the lungs of exposed mice (12, 13), prompting us to investigate the role of diesel exhaust particles (DEP), a major component of particulate matter in traffic related air pollution, on Th17 responses in allergic asthma.
The effects of DEP exposure have been examined in healthy subjects and asthmatics (14–16). Fine and ultra-fine DEP particles (diameter < 2.5µm) can reach small airways including the alveolar/gas exchange regions of the lung exacerbating asthma symptoms (14, 15). Bronchial inflammation and airway resistance increased in normal healthy subjects after exposure to DEP. Even short-term exposure to streets with high diesel traffic reduced airway function (FEV1) in mild to moderate asthmatics compared to subjects who walked for a similar time in an area not exposed to traffic (17). However, the mechanisms by which DEP contributes to asthma exacerbations remain poorly understood.
Experimental studies in mice and rats have shown that DEP exposure can exacerbate allergic airway responses including allergen-specific IgE, eosinophilia and AHR (18). The adjuvant effect of DEP has been attributed to its ability to serve as an allergen carrier and/or to the different components of DEP itself. Indeed, DEP derived from different sources generate distinct immune responses in vivo (19). Co-exposure to allergen and DEP is known to increase allergen-specific IgE levels in humans and mice (15, 18). Co-exposure has also been reported to enhance local and systemic Th2 cytokine release compared to allergen alone (15, 18, 19). However, little is known about the effects of DEP exposure on Th17 responses in the context of allergic asthma.
Herein, we demonstrate that exposure of mice to DEP induces Treg and Th17 cell accumulation in their lungs. Co-exposure to DEP and a common allergen (house dust mite) caused a mixed Th2/Th17 response and increased asthma severity characterized by more severe inflammation, mucus production and AHR. IL-17A blockade with a neutralizing antibody alleviated DEP-induced enhancement of AHR supporting a role for Th17 cells in severe asthma exacerbation in the context of DEP exposure. In children with allergic asthma, we also found DEP exposure to be associated with more frequent asthma symptoms and elevated IL-17A serum levels suggesting our findings can be extrapolated to human disease.
METHODS
Subjects
The 235 children included in this study are enrolled in the Greater Cincinnati Pediatric Clinic Repository (GCPCR) which is a clinical repository of over 6,500 participants including over 2,300 children with asthma (20). The 46 children with IL-17A serum data were also enrolled in the Pediatric Environmental Exposures Study (PEES), a case-control study with nearly 400 total children seeking to better understand the effects of DEP exposure on childhood asthma. Children were invited to participate in PEES from the GCPCR and public advertising. For both populations, informed consent was obtained by a trained clinical research coordinator from participants and their parents/guardians using a protocol approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board. Children ages 5–18 years with confirmed allergic asthma (diagnosed according to ATS criteria (21)) who were either skin prick test (SPT) positive to one or more aeroallergens or had a doctor diagnosis of allergic rhinitis were included in our analysis. Skin prick testing was performed using the GreerPickTM system (Greer Laboratories; Lenoir, NC) as ordered during participants’ clinical visits. Tests were read 15 minutes after placement and considered positive when the wheal around the allergen tested was greater than 3 mm and was also greater than the saline negative control. Histatrol (Center Laboratories, Port Washington, NY) 1 mg/mL was used as the positive control. Eleven aeroallergens prevalent in the Ohio River Valley were evaluated. Panels included mold mixes (n=2), grass mix, ragweed (giant and short), tree pollen mixes (n=2), weed mix, dust mite mix (Der f and Der p), cat (Fel d 1), dog (hair and dander), and cockroach mix (Periplaneta americana and Blattella germanica). 119 children were defined as allergic by both physician diagnoses and SPT. 48 children were defined as allergic by SPT only and the remaining 68 children, were considered allergic based on physician diagnosis of allergic rhinitis.
The ArcView Geographic Information System software (Esri, Inc., Redlands, CA) was used to obtain longitude and latitude coordinates from each child’s primary residential address. Estimates of elemental carbon attributable to traffic (ECAT) exposure was derived using a land-use regression model from the resulting coordinates using previously established methods (22). Single estimates of DEP exposure were determined for each child. The range of estimates were 0.25 ug/m3 to 0.85 ug/m3 across the entire study population. As previous studies have indicated that the highest risk group are those children in the highest quartile of exposure (23), high DEP exposure was defined as those with values greater than 0.46 ug/m3. The frequency of asthma symptoms was determined by parent-report questionnaire over the last 12 months prior to children’s visit. Children must have had at least one respiratory symptom frequency score to be included. For children with multiple symptom scores (from multiple questionnaires completed over time), the highest symptom score was included in this analysis. The symptom frequency scores were determined from symptom frequency questions for wheezing, coughing, shortness of breath, and chest tightness. Possible frequency answer choices included 0/never having symptoms on average over the last 12 months (score 0), having symptoms less than 1 time a week on average over the last 12 months (score 0), or having symptoms on average 1–2 (score 1), 3–5 (score 2), or 6–7 (score 3) times a week over the past 12 months. For the purposes of our study, children with severe asthma are those with 1 or more symptoms that occur on average at least 6–7 days a week over the past 12 months (maximum score of 4 for one or more symptoms). IL-17A levels were determined from serum samples collected from PEES participants. Of the nearly 400 total PEES participants, only the first 120 participants have cytokine data available and only 46 of 82 childhood asthmatics met our inclusion criteria for this study. There were no significant differences in demographic or exposure variables between the 46 included in our analysis and the other children. Briefly, 25 uL of serum was tested using the premixed 39-Plex MILLIPLEX MAP Human Cytokine/Chemokine assay (Millipore Corporation, Billerica, MA) including IL-17A, and analyzed on a Luminex 100TM (BioRad; Hercules, CA). Concentrations were determined with a 5 parametric curve fitting algorithm using MasterPlex QT Quantitation Software (MiraiBio; Alameda, CA). IL-17A values ranged from 0.69 to 646.56 pg/ml. The lower limit for detection was 0.5 pg/ml and 5 of the 46 children had undetectable values. As we observed a non-normal distribution for IL-17A that was not remedied by typical transformation, we used an unbiased approach and deemed those children with levels in the top quartile (>24pg/mL) as having “high” IL-17A levels. As several children had equal levels of IL-17A at this level, we chose to include all of them in the higher category thus resulting in 30% of the children having ‘high’ IL-17A levels. Similarly, for IL-4, IL-5 and IL-13, binary variables were made defining the top quartile as ‘high’ and the bottom 3 quartiles as ‘low’. Correlations (Pearson Correlation Coefficients) were determined and univariate and multivariate logistic regression was performed using these binary variables as well as continuous variables to examine associations between these cytokines and DEP exposure. Only the analysis of binary variables are provided.
Murine asthma model and anti-IL17A treatment
Wild type 6–8 week old Balb/c mice were purchased from Jackson Labs (Bar Harbor, ME). House dust mite extract (Dermatophagoides pteronyssinus) was purchased from Greer Laboratories (Lenoir, NC). DEP was generated from a 4-cylinder Deutz diesel engine at the EPA (Research Triangle Park, NC); detailed characterization of this compressor DEP has been described previously and compared to other sources of DEP (19). Mice received either 50µl of saline, 150µg of DEP, 10µg of HDM extract (representing 3.3µg of protein; 1.1µg of Der p1; 0.5 EU of endotoxin) or both intratracheally 3 times a week for 3 weeks and were sacrificed 1 day after the last exposure (Figure 2A). In some experiments, mice were given a rat anti murine IL-17A antibody (100–200µg; M210 provided by Amgen Inc) or an IgG1 control antibody intratracheally during HDM±DEP challenges in a final volume of 50µl (Figure 7A). All animal protocols were approved by the IACUC.
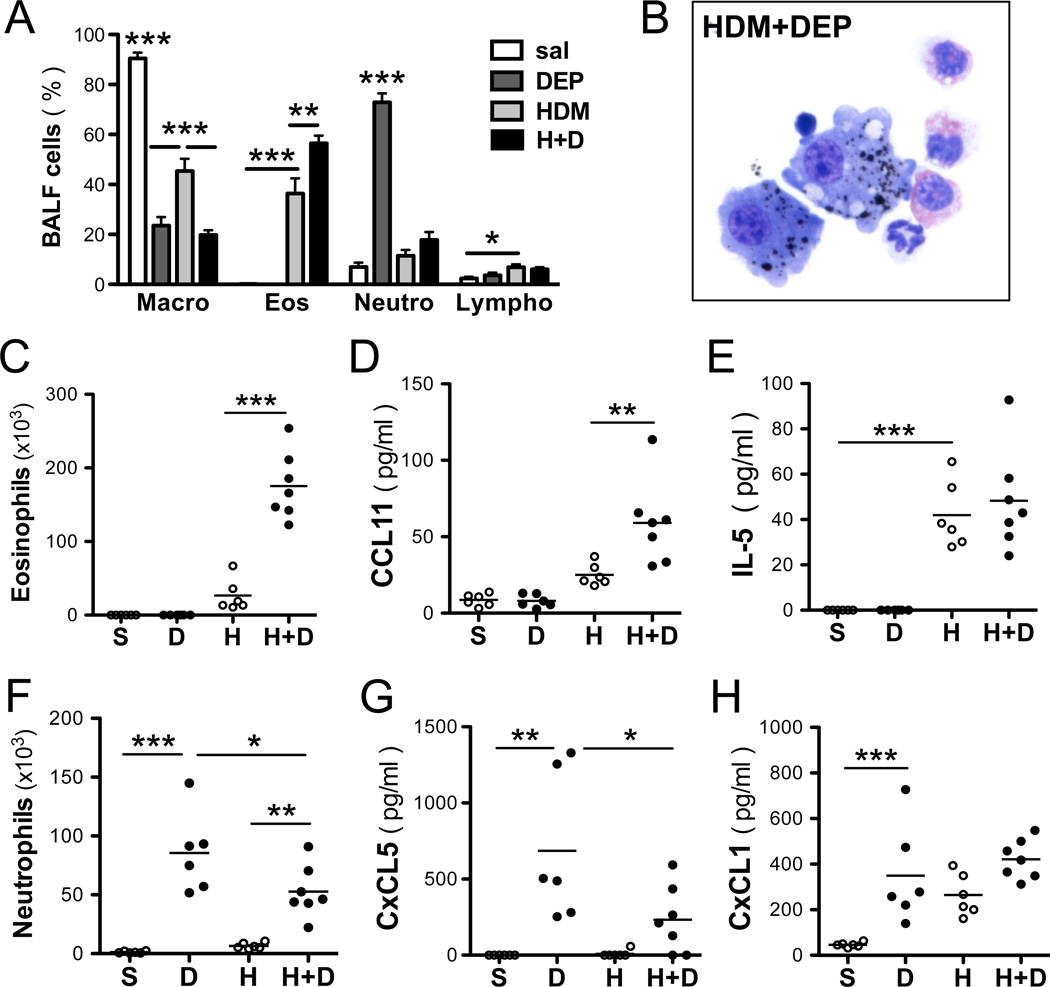
Figure 2. DEP induces neutrophilia and exacerbates HDM-induced eosinophilia.
(A) Differential counts. (B) BALF cell counts. Total BALF eosinophils (C) and neutrophils (F) assessed 24h after the last intratracheal exposure. BALF levels of (B) CCL11 (eotaxin-1), (C) IL5, (E) CxCL5 (ENA78) and (F) CxCL1 (KC) were assessed by ELISA (representative experiment with 6–7 mice / group; 1way ANOVA, ***p < 0.001, **p< 0.01, *p<0.05).
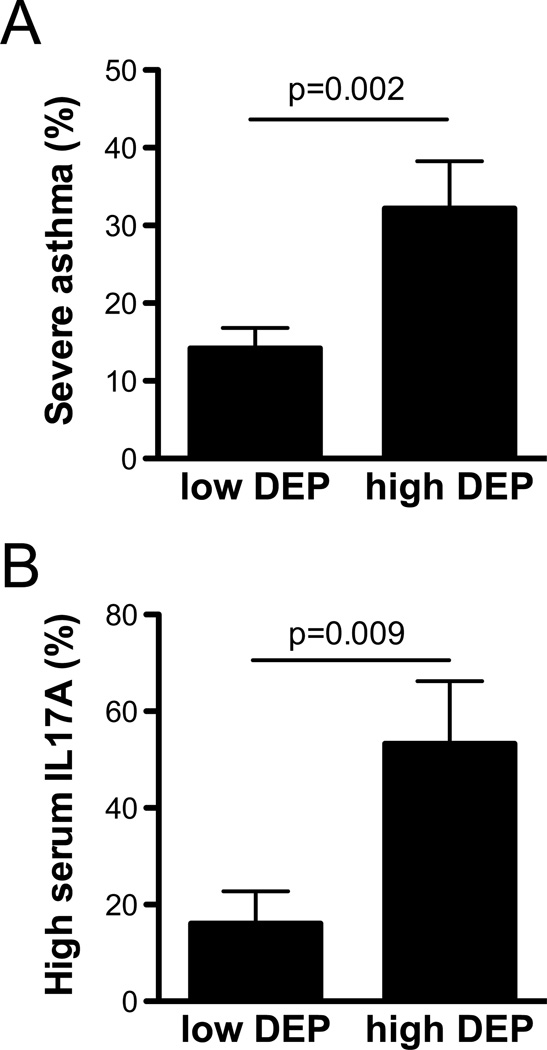
Figure 7. DEP exposure exacerbates asthma severity and increases IL-17A in childhood allergic asthma.
(A) Among 235 GCPCR participants with allergic asthma, those with high DEP exposure (N=44) had more frequent asthma symptoms compared with those with low exposure. The observed association remained significant even after adjusting for age, race, sex, annual family income, type of health insurance, maternal education, being prescribed asthma controllers, adherence to asthma medication over the prior two weeks, and second-hand smoke exposure in multivariate logistic models (p=0.01). (B) Among 46 PEES asthmatics, 53% of children with high DEP exposure also had high levels of IL-17A. The association remained significant after adjustment for the above covariates and asthma symptom frequency.
Airway hyperresponsiveness (AHR)
Invasive measurements of airway responsiveness were made on a flexiVent apparatus (Scireq, Montreal, Canada). Mice were anesthetized with Ketamaine, Xylazine, and Acepromazine (respectively 100, 20 and 10mg/ml mixed at a ratio of 4:1:1). Mouse tracheas were cannulated with a 20-gauge blunt needle and the mice were ventilated at 150 breaths/min, 3.0 cm water positive end expiratory pressure. Two total lung capacity perturbations were then performed for airway recruitment before baseline measurement and subsequent methacholine challenges were performed. Dynamic resistance (R) and compliance (C) were determined by fitting the data to a single compartment model of airway mechanics where Ptr = RV + EV + Po, and Ptr = tracheal pressure, V = volume, E = elastance, Po is a constant and C = 1/E. Measurements were made using a 1.25s, 2.5Hz volume-driven oscillation applied to the airways by a computer-controlled piston (SnapShot perturbation). PBS or methacholine was aerosolized for 15 s (Aeroneb ultrasonic nebulizer) followed by 15 s of ventilation and a SnapShot perturbation was performed followed by 10 s of ventilation. Twelve SnapShot/ventilation cycle measurements were made. The procedure was repeated for 0, 6.25, 12.5, 25, 50 mg/ml concentrations of methacholine. The maximum R value and minimum C value with a coefficient of determination of 0.9 or greater (as determined by the flexiVent software) was used to determine the dose-response curve
BALF collection and analysis
Bronchoalveolar lavage was performed by cannulation of the trachea. The lungs were lavaged with 1 ml PBS + 0.5% BSA + 2mM EDTA. The collected BALF was centrifuged and the total cell numbers counted with a hemacytometer. Cells were spun onto slides and stained with the HEMA3 stain set (Fisher Scientific, Kalamazoo, MI). 400 cells were counted and the total number of each cell type calculated.
Histology and immunohistochemistry
The left lobe of the lung was fixed in formalin, paraffin embedded and cut into 5µm sections. Sections were stained with hematoxylin and eosin (H&E) or PAS according to the manufacturer’s recommendations (PolyScientific).
ELISA
For measurement of HDM-specific IgE and IgG1 levels, wells were coated with 0.01% HDM (Greer Laboratories) overnight. Plasma was diluted 1:5 for IgE and 1:2000 for IgG1. After 2h of incubation, plates were washed and either HRP-conjugated anti-mouse IgG1 (X56; 1:1000; BD Biosciences-Pharmingen) or Biotin-anti-mouse IgE (R35–118; 1:250; Pharmingen) were added for 1h, followed by an incubation with streptavidin-HRP (R&D DY998; 1:200) in the case of IgE. BALF cytokines levels were assessed by Luminex xMAP technology (Millipore, Billerica, MA), using Cytokine/Chemokine Panel I and following the manufacturer’s instructions. IL-23p19 was assessed in lung lysates (homogenized in 1% Triton X) by ELISA according to the manufacturer’s instructions (BioLegend, San Diego, CA). To assess total BALF IL-13 levels (free and IL-13Rα2-bound), the IL-13 standard and samples were preincubated with 10 ng/ml murine IL-13Rα2-Fc (R&D Systems) at 37C for 1 hour. Anti-mouse IL-13 polyclonal antibody (1 mg/ml) was used for capture and biotinylated anti-mouse IL-13Rα2 polyclonal antibody (0.5 mg/ml) for detection (R&D Systems).
Real time PCR
Total RNA was isolated from homogenized mouse skin using Trizol (Invitrogen) according to manufacturer’s instructions and DNase treated (Qiagen, Valencia, CA) before being reverse transcribed with First Strand Superscript Synthesis kit (Invitrogen). Quantitative real-time PCR analysis of murine skin was done using LightCycler FastStart DNA master SYBR green I as a ready-to-use reaction mixture (Roche). cDNA were amplified using the primers listed in supplementary Table E1 and gene expression was normalized to HPRT.
Isolation of lung cells and staining for flow cytometry
Lungs were removed and the upper right lobe was minced and incubated at 37C for 25–30 min in 2ml of RPMI 1640 containing Liberase DL (0.5 mg/ml; Roche Diagnostics, Indianapolis, IN) and DNAse I (0.5 mg/ml; Sigma, St Louis, MO). Lung cells were passed through a 70µm cell strainer with a syringe rubber and the strainer washed with 5ml of RPMI + DNAse I media. Cells were centrifuged and resuspended in 2ml of RPMI before counting with a haematocytometer and cell viability confirmed by trypan blue exclusion. Around 500,000 lung cells were transferred in to a 96 well plate with V shaped wells on ice, centrifuged and resuspended in PBS containing FcBlock (2.4G2 mAb). T cells were stained with CD4-FITC, CD3-PE/Cy7, CD69-PE, CD25-AF647, CD8-APC/Cy7, CD44-Pacific Blue (BioLegend, San Diego, CA). Cells were labeled with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit according to manufacturer’s instructions (Invitrogen by Life Technologies, Carlsbad, CA). Intracellular staining for IL13-PE, IL17A–AF647, IFNγ-PerCP5.5 and Foxp3-PerCP5.5 were conducted according to manufacturer’s instructions (eBioscience, San Diego, CA). Acquisition was done on a FACS Canto III (Becton Dickinson, Mountain View, CA) and analyzed used FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
For our studies of childhood allergic asthma, a Chi-squared test and logistic regression was used to evaluate differences in proportion of more severe asthma symptoms between children having low and high DEP exposure. Second-hand smoke exposure (yes/no), age, race (African American, Caucasian, other/mixed race), sex, annual family income (<40K/>=40K), type of health insurance (public/private/self-pay), maternal education (<high school or GED/>=high school or GED), whether or not asthma controllers had been prescribed (yes/no), and parent reported imperfect adherence over the previous 2 weeks (yes/no) were evaluated as possible covariates in our models and fitted using backwards elimination. Pearson correlation coefficients were used to examine correlations between IL-17A and other Th2 cytokines. Analyses were performed in SAS Software version 9.2 (SAS Institute, Inc., Cary, NC). All mouse-related statistical analyses were done using PRISM software (GraphPad Software Inc., La Jolla, CA). Statistical significance was assessed using one-way ANOVA followed by a Bonferroni post-test, except when mentioned otherwise in the figure legend.
RESULTS
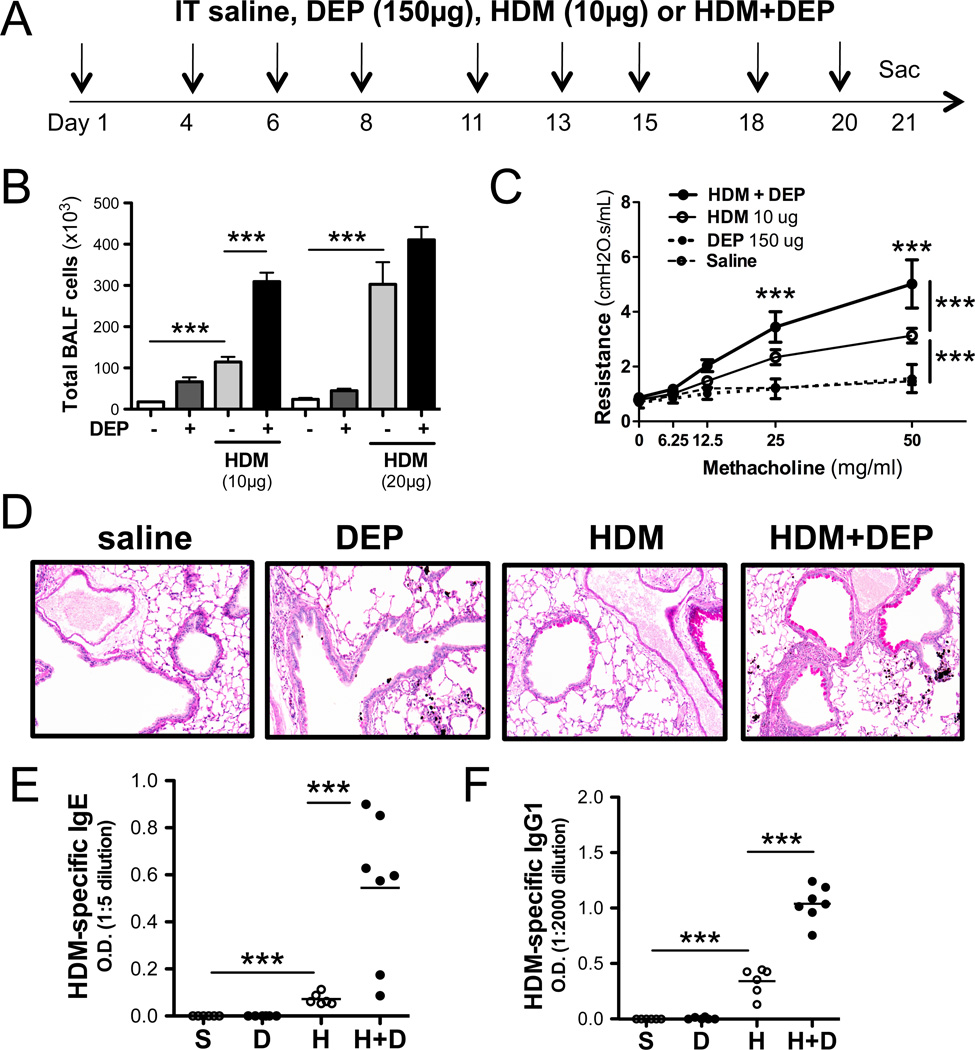
DEP exposure exacerbates HDM-induced AHR, inflammation and allergen sensitization
To determine the mechanisms underlying DEP-induced exacerbation of asthma, we utilized an experimental asthma model that did not rely on intraperitoneal sensitization in the presence of an adjuvant; Balb/c mice were exposed intratracheally to either saline, HDM, DEP or HDM+DEP 9 times over a 3 week period (Figure 1A). At low doses of allergen (10ug of total HDM extract per dose), a marked synergistic increase in BALF inflammation was observed in mice exposed to HDM+DEP compared to either DEP or HDM exposed mice (Figure 1B). This synergy was no longer observed in mice exposed to higher doses of DEP. Thus, in order to study DEP exacerbation of HDM-induced asthma, we used a submaximal dose of house dust mite extract and a dose of DEP that had previously been shown to exacerbate OVA-induced airway responses (19). Chronic allergen exposure of BALB/c mice to low doses of HDM over a 3-week period resulted in AHR, airway inflammation, goblet cell hyperplasia and mucus production (Figure 1C, 1D). DEP exposure alone also induced airway inflammation, but did not promote AHR or mucus production (Figure 1B–D). Co-exposure to DEP significantly increased HDM-induced inflammation and AHR (Figure 1B–D).
Figure 1. DEP exacerbates HDM-induced airway responses.
(A) Protocol. (B) Total BALF cell counts. (C) Airway resistance. Data from 2 separate experiments (n=10–18 mice per group; 1way ANOVA, *** p < 0.001, ** p < 0.01, * p < 0.05). (D) Representative, PAS stained airways. (E) HDM-specific IgG1 and (F) IgE titers.
DEP and HDM co-exposure exacerbates allergen sensitization
Repeated DEP exposures over a 3-week period did not result in a significant increase in total IgE or IgG1 plasma levels compared to saline treated mice (data not shown). However exposure to both HDM and DEP significantly increased total IgE and total IgG1 levels over exposure to HDM alone (data not shown). Accordingly, repeated exposures to HDM+DEP also significantly increased HDM-specific IgG1 and HDM-specific IgE levels compared to HDM exposure alone (Figure 1E, 1F) suggesting increased allergic sensitization in the presence of DEP.
DEP and HDM co-exposure result in a mixed Th17 and Th2 response
The nature of the inflammatory response differed markedly between HDM and DEP (Figure 2A). DEP was predominantly taken up by alveolar macrophages and occasionally by neutrophils (Figure 2B). Repeated HDM exposure resulted in BALF eosinophilia and increased levels of IL-5 and CCL11 (eotaxin-1) (Figure 2C–E). In contrast, exposure to DEP alone was characterized by a marked neutrophilia and elevated CXCL1 (KC) and CXCL5 (ENA-78, LIX) levels in the BALF (Figure 2F–H). CXCL5 was specifically induced by DEP, but not HDM, whereas elevated BALF levels of CXCL1 were observed after exposure to either HDM or DEP. Co-exposure to HDM and DEP did not increase neutrophilia or neutrophil chemokine levels above that observed with DEP alone (Figure 2F–H). However, HDM and DEP co-exposure increased BALF eosinophilia and eotaxin levels compared to HDM exposure alone (Figure 2C–D). BALF T-cell levels trended higher following HDM and DEP exposures and were further increased following co-exposures to HDM and DEP compared to HDM alone (Figure 2A).
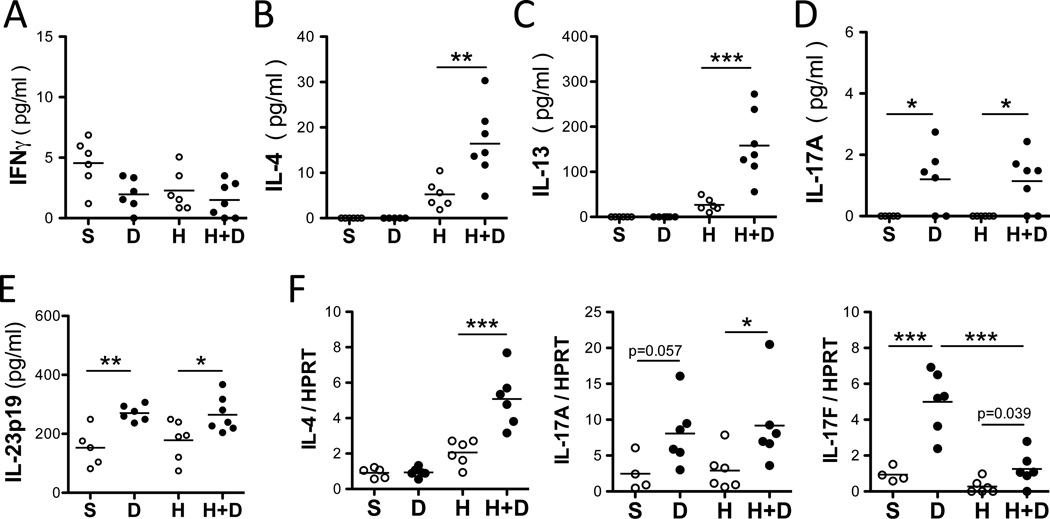
Next we assessed BALF levels of Th1, Th2 and Th17 cytokines following DEP and HDM exposure. Levels of IFNγ were unaffected following HDM and/or DEP challenge whereas Th2 cytokines (IL-4, IL-5 and IL-13) were significantly increased following exposure to HDM alone but not DEP (Figures 2E and 3A–C). Co-exposure to HDM and DEP induced a further increase in IL-4 and IL-13 levels compared to HDM alone (Figure 3B–C). Exposure to DEP alone induced IL-17A and IL-17F but not Th2 cytokines (Figures 3). Additionally, the pro-Th17 cytokine IL-23 was also increased by DEP exposure (Figure 3E). In the presence of HDM, DEP induced IL-17A, IL-17F, and IL-23, but the levels were not enhanced over those observed with DEP alone. Notably, co-exposure to HDM and DEP induced a mixed Th2 and Th17 response (Figure 3).
Figure 3. DEP induces IL-17A and exacerbate HDM-induced Th2 responses.
(A) IFNγ (B) IL-4, (C) IL-13 and (D) IL-17A BALF levels assessed 24h after the last exposure. (E) IL-23p19 in lung homogenates. (F) IL-4, IL-17A and IL-17F mRNA lung levels (representative experiment with 5–7 mice / group; 1way ANOVA, ***p < 0.001, **p< 0.01, *p<0.05).
DEP and HDM co-exposure results in increased CD44+CD62L− lung effector Th2 and Th17 cells
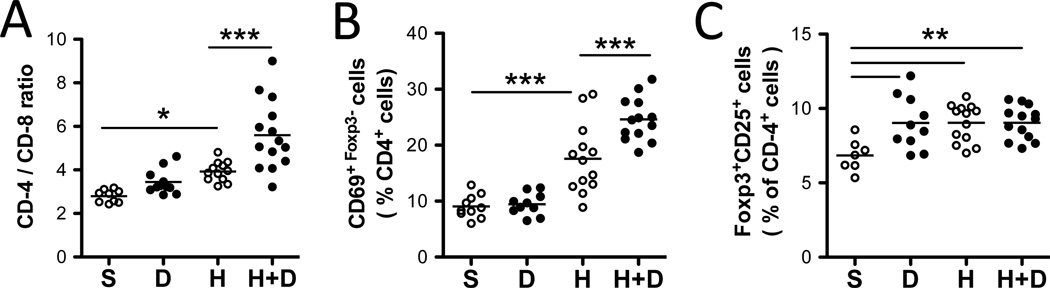
To determine the impact of HDM and DEP exposures on pulmonary T cells, lung cells were isolated and analyzed by flow cytometry. An increase in CD4+ T cells over CD8+ T cells was observed in HDM-exposed mice but not following exposure to DEP alone (Figure 4A). HDM and DEP co-exposure further increased the proportion CD4+ T cells (Figure 4A) compared to HDM alone. Among CD4+ T cells, expression of the early activation marker CD69 was increased following HDM exposure (Figure 4B). HDM and DEP co-exposure resulted in further increases in CD69 surface expression compared to HDM exposure alone (Figures 4B). The percentage of lung CD4+ T cells positive for Foxp3 and CD25 was similarly increased by DEP, HDM and HDM+DEP exposures (Figure 4C).
Figure 4. HDM+DEP exposures exacerbate HDM-induced T cell activation.
(A) Representative FACS dot plots of the proportion of CD4+ cells versus CD8+ cells among CD3+ lung T-cells. (B) Percentages of CD69+Foxp3− cells among CD4+ lung T-cells. (C) Percentages of Foxp3+CD25+ cells among CD4+ lung T-cells (n=10–14 mice/group, 2 separate experiments; 1way ANOVA, ***p < 0.001, **p< 0.01, *p<0.05).
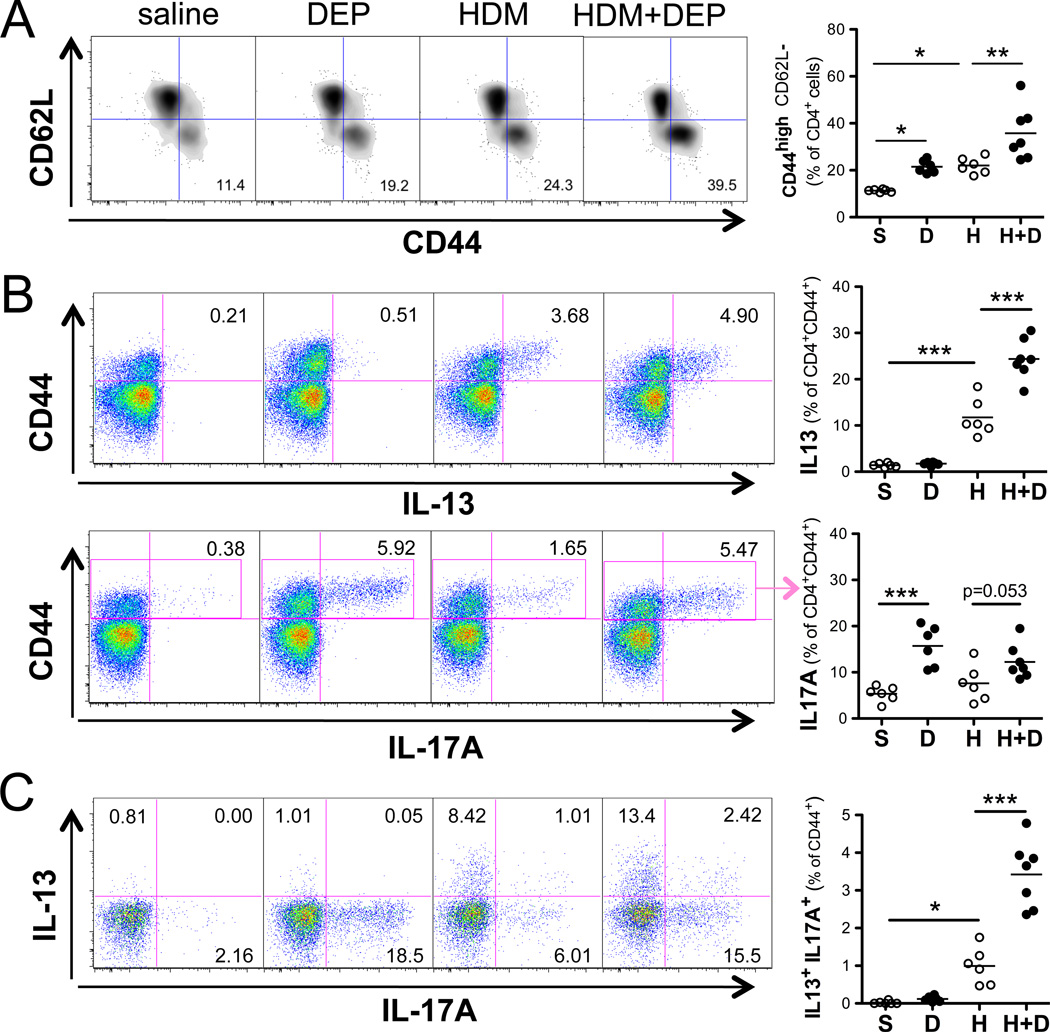
Consistent with the observed enhancement in T cell activation (Figure 4B), HDM and DEP co-exposure increased the proportion of CD44+CD62L− lung effector T cells compared to HDM alone (Figure 5A). In order to determine the phenotype of these effector T cells, lung CD4+ T cells were stimulated ex-vivo with PMA and ionomycin and then intracellular cytokine staining was performed for IL-13, IL-17A, and IFNγ (Figure 5). DEP exposure alone induced IL-17A+, but not IL-13+ or IFNγ+ effector T cells (Figure 5 and E1). In contrast, HDM exposure alone resulted in increased IL-13 producing CD44+ effector T cells. HDM and DEP co-exposure resulted in a marked increased in IL-13 producing lung effector T cells compared to HDM exposure alone (Figure 5B). The number of IL-17A producing lung effector cells was not further enhanced with co-exposure over DEP alone. However, an increase in the number of IL-13+/IL-17A+ co-producing effector T cells was observed in the DEP and HDM exposure group compared to HDM alone (Figure 5C). Thus, DEP enhances the generation of effector T-cells capable of producing both IL-13 and IL-17A in the presence of allergen.
Figure 5. Exposure to HDM+DEP generates IL-17A+IL-13+CD4+ effector T-cells.
(A) Representative FACS dot plots of lung CD44highCD62low effector T-cells. (B) Intracellular staining for CD4+ T cells producing IL-13 and IL-17A following ex vivo restimulation with PMA+ionomycin (n = 5–7 mice/goup). (C) Representative FACS dot plots of cytokine expression by CD4+CD44+effector T-cells and frequency of IL-13+ IL-17A+ double producers (n = 5–7 mice/goup; 1way ANOVA *p< 0.05, **p< 0.01, ***p<0.001).
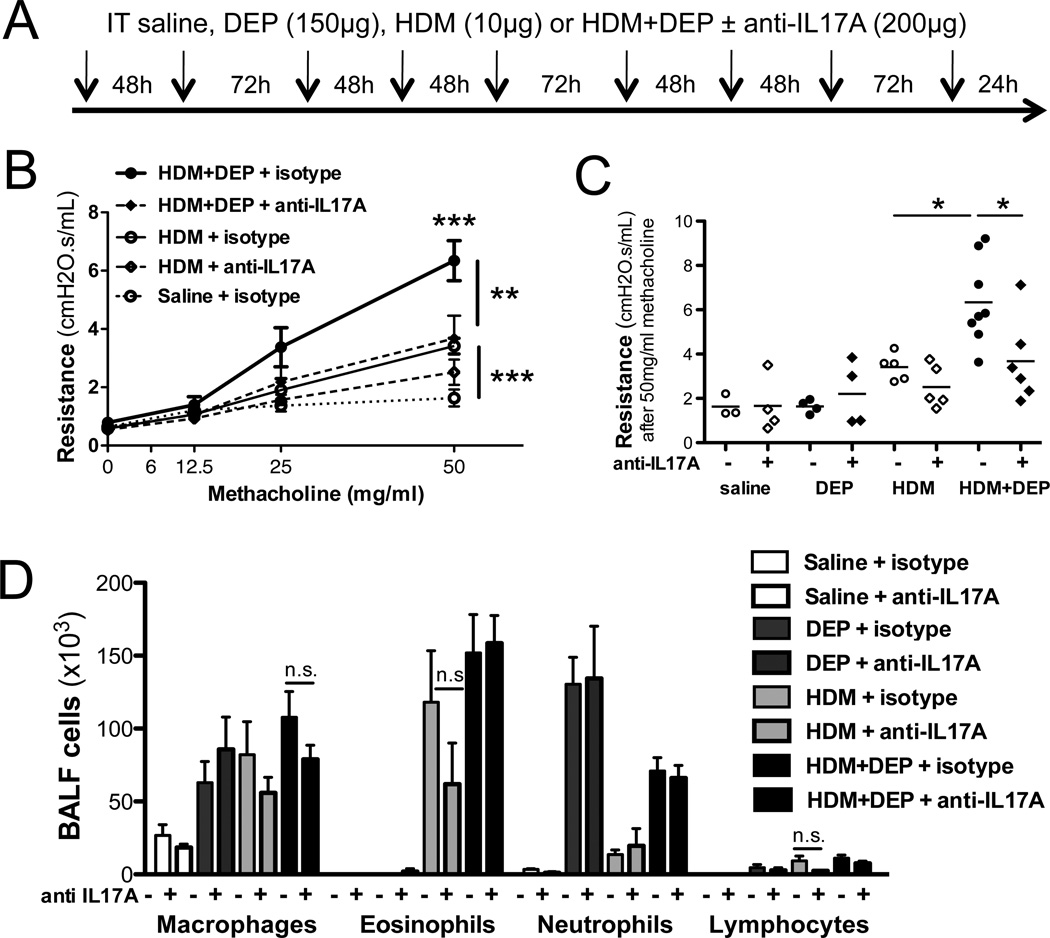
DEP exacerbation of HDM-induced allergic inflammation is partially dependent on IL-17A
It has been recently demonstrated that IL-17A can exacerbate IL-13-induced AHR (8). Our findings suggest a contribution of IL-17A to DEP exacerbation of HDM-induced allergic responses. In order to directly address this possibility, we used an anti-IL-17A antibody (Figure 6A). IL-17A neutralization in the lungs counteracted the synergistic effect of DEP on HDM-induced AHR (Figure 6B, 6C). Surprisingly, BALF inflammation and most notably BALF neutrophil levels were not significantly altered by anti-IL17A treatment (Figure 6D), suggesting that IL-17A can exacerbate AHR independent of neutrophils.
Figure 6. IL-17A neutralization alleviated DEP exacerbation of HDM-induced airway responses.
(A) Mice were given rat anti-murine IL-17A (200ug; M210) or an IgG1 control antibody intratracheally during FIDM±DEP challenges. (B) AHR was assessed 24h after the last exposure to HDM±DEP. (C) AHR following the last methacholine dose (50mg/ml). (D) Differential counts (2way ANOVA for panel B and D, 1way ANOVA for panel C, ***p < 0.001, **p<0.01, *p<0.05)
DEP exposure exacerbates asthma symptoms and increases IL-17A serum levels in childhood allergic asthma
In order to translate our mouse model findings and determine if asthma severity (based on asthma symptom frequency) is altered in DEP-exposed children with allergic asthma, we identified 235 children with allergic asthma in the Greater Cincinnati Pediatric Clinic Repository (GCPCR) between the ages of 5–18. We compared the proportion of children with one or more symptoms of wheeze, cough, shortness of breath or chest tightness 6–7 days per week over the past 12 months exposed to low vs. high DEP (> 0.464 ug/m3) (23). Our analysis revealed a significant association between asthma symptom frequency and DEP exposure (OR=2.9, CI=1.4–5.7). Indeed, among the highly exposed group, 32.2% of the children had more frequent asthma symptoms compared to only 14.2% in the low exposure group (Figure 7A). To account for potential confounders, we fit a multivariate logistic model using backward elimination adjusting for age, race, sex, annual family income, type of health insurance, maternal education, being prescribed asthma controllers, adherence to asthma medication over the prior two weeks and second-hand smoke exposure. With only race and annual family income remaining in the final model, we continued to observe a significant association between severe asthma and high DEP exposure (OR=2.9, CI=1.3–6.9).
In order to assess if DEP exposure was similarly associated with IL-17A production in children with allergic asthma, we evaluated IL-17A levels as well as Th2 cytokines in a subset of 46 Pediatric Environmental Exposures Study (PEES) participants with available serum (Table 1). We examined the association between DEP exposure and serum IL-17A levels. Serum IL-17A levels in the children ranged from 0 to 646.56 pg/ml (0.5g pg/ml lower limit of detection) and were not normally distributed. Children in the top quartile (30% after considering children with equal levels at the 25% level) of IL-17A values were defined as ‘high’ (>24pg/mL). Using this binary variable, we found that high DEP exposure was associated with high IL-17A levels (OR=5.9, CI=1.5–24.0; Figure 7B). After using backwards elimination to fit a logistic model, only DEP exposure, sex and prescribed asthma controllers remained in the model and the association between DEP exposure and IL-17A was strengthened as a result (OR=12.1, CI=1.2–125.6). Taken together with the data presented in Figure 7A, our findings support that among children with allergic asthma, high DEP exposure is associated with both greater asthma symptoms and elevated serum IL-17A levels. Importantly, no other cytokine evaluated (IL-4, IL-5, IL-13) was associated with DEP exposure (Table 2). IL-17A was the only cytokine significantly associated with DEP exposure. No other covariate was associated with IL-17A or significantly modified the association between IL-17A and DEP exposure (Figure 7B).
Table 1.
Population Characteristics of Study Participants
| Variables | GCPCR (n=235) | PEEPS (N=46) | ||
|---|---|---|---|---|
| N= | % of total | N= | % of total | |
| Higher annual asthma symptom frequency | 44 | 18.7% | 7 | 15.2% |
| Black/African American | 116 | 49.4% | 34 | 73.9% |
| Caucasian/white | 98 | 41.7% | 12 | 26.0% |
| Other/mixed race | 21 | 8.9% | 0 | 0.0% |
| Male sex | 141 | 60.0% | 31 | 67.3% |
| High DEP exposure (>0.464ug/m3) | 59 | 25.1% | 15 | 32.6% |
| Second-hand smoke exposure | 114 | 48.5% | 34 | 73.9% |
| Diagnosis of allergic rhinitis | 187 | 79.6% | 35 | 76.1% |
| Any positive SPT | 166 | 70.6% | 37 | 92.5% |
| Public insurance | 114 | 48.5% | 27 | 58.7% |
| Annual family income < $40K | 135 | 57.4% | 34 | 75.6% |
| Maternal education < high school | 21 | 8.9% | 7 | 15.6% |
| Currently prescribed ICS or leukotrienes | 166 | 70.6% | 33 | 71.4% |
| Imperfect adherence over last 2 weeks | 86 | 44.8% | 21 | 53.9% |
| Mean | Std Dev | Mean | Std Dev | |
| Age (range 5–18 years) | 10.0 | 3.1 | 9.8 | 2.7 |
Children with allergic asthma from the GCPCR and PEES are included. Diagnosis of allergic rhinitis (yes/no), skin prick test (+/−), age at study consent, race, sex, annual family income, type of health insurance (public vs. private/self-pay), maternal education, being prescribed asthma controllers, adherence to asthma medication over the prior two weeks and second-hand smoke exposure were determined by questionnaire or electronic medical record. Severe asthma was defined as having 1 or more symptoms that occur at least 6−7 days a week compared to others with less frequent symptoms. High DEP is based on the highest quartile of elemental carbon attributable to traffic (ECAT; 0.464ug/m3) estimated at the children’s current home address. Children with >24 pg/ml serum IL-17A were considered as having high levels of IL-17A.
Table 2.
DEP exposure is associated with serum IL-17A but not Th2 cytokine levels
| N=46 | DEP | |
|---|---|---|
| OR | 95% CI | |
| IL-4 | 0.86 | 0.19–3.92 |
| IL-5 | 1.44 | 0.38–5.50 |
| IL-13 | 1.25 | 0.30–5.16 |
| IL-17A | 5.94 | 1.47–23.97 |
Cytokine levels were determined from serum samples collected from 46 PEES participants using the premixed 39-Plex MILLIPLEX MAP Human Cytokine/Chemokine assay. Presented are binary variables with the top quartile indicating ‘high’ cytokine levels and the bottom 3 quartiles indicating ‘low’ cytokine levels. High DEP exposure was defined as quartile of values > 0.46 ug/m3. Logistic regression was performed and unadjusted odds ratios (OR) and 95% confidence intervals (CI) are presented. IL-17A was the only significant association observed (in bold) and inclusion of potential confounders in the models only strengthened the magnitude of the association between IL-17A and DEP (OR=12.1, CI=1.2–125.6).
DISCUSSION
The present study demonstrates that DEP exposure is associated with increased asthma symptoms and increased IL-17A levels in human and murine asthma. Further, IL-17A blockade in mice prevented DEP-induced exacerbation of allergic asthma. IL-17A has been implicated in severe forms of asthma, however, the factors that promote IL-17A production during the pathogenesis of severe asthma have remained undefined. Our data provide the first evidence that DEP exposure is a factor that contributes to severe asthma by enhancing IL-17A production. In addition, our data demonstrate that elevated serum IL-17A levels, which have been associated with severe adult allergic asthma, may also be an important marker of asthma severity in asthmatic children. Current asthma therapies are largely focused on decreasing Th2 responses, however, approaches targeting neutralization of IL-17A may be useful therapeutic strategies to counteract the asthma promoting effects of traffic related air pollution, especially among high DEP-exposed individuals with severe asthma.
Exposure of mice to DEP induced accumulation of Th17 cells and neutrophils in the lungs. Co-exposure to HDM and DEP resulted in a mixed neutrophil and eosinophil lung infiltrate, a mixed Th2 and Th17 response, and enhanced AHR. The synergy was not evident when large doses of DEP or allergen were used, likely because the effect of the single high dose exposure masked the synergy response. Similarly, among children with allergic asthma, those exposed to high levels of DEP had increased asthma severity and increased serum IL-17A levels compared to those with low DEP exposure. Unlike the mouse model where absent, low, and high DEP exposure can be defined and controlled, there are no children with absent DEP exposure. It is not possible to directly compare the low and high groups between the human and mouse studies. We defined low and high exposure groups of children based on published data from the Cincinnati Childhood Allergy and Air Pollution Study birth cohort (22). All of the children were atopic (ie allergen exposed). The association between high DEP exposure and high serum IL-17A was not observed with Th2 cytokines, and remained significant even after adjusting for age, race, sex, annual family income, type of health insurance, maternal education, being prescribed asthma controllers, self-reported adherence to asthma medication over the prior two weeks, and second-hand smoke exposure in multivariate logistic models. These findings suggest that children with allergic asthma and concomitant exposure to DEP may have a Th17-driven component to their airways disease.
The murine findings are further supported by previous studies that have found that exposure to DEP induces pro-inflammatory cytokines (IL-1β, IL-6, TNFα), as well as neutrophil chemokines in vivo (24, 25). We have observed that IL-1β, IL-6 which induce Th17 differentiation (26), are secreted in the BALF 3h after DEP exposure and return to baseline within 24h (data not shown). IL-23 is not necessary for Th17 differentiation but is required to maintain and expand Th17 cells (26). Accordingly, our data demonstrated that DEP-induction of IL-17A was associated with elevated lung levels of IL-23. Finally, we show that DEP induces release of neutrophil chemokines (CXCL1 and CXCL5) into the lungs of exposed mice. While IL-17A promotes secretion of neutrophil chemokines by epithelial cells (27), DEP can directly induce secretion of Th17 related cytokines (IL-1β, IL-6) and chemokines (IL-8) by human bronchial epithelial cells (28, 29). Thus, DEP induces neutrophilia not only by promoting Th17 responses, but also by directly inducing neutrophil chemokines in exposed lung epithelial cells.
Several studies have proposed that members of the aryl hydrocarbon family, some of which are present on DEP particles, can promote the differentiation of Treg cells via the aryl hydrocarbon receptor (AhR) (30, 31). DEP induced an increase in the proportion of CD25+ Foxp3+ CD4+ T cells, similar to that induced by HDM. We did not access the functionality of these Tregs. However, the increase in effector T-cells, specifically HDM-induced Th2 cells and DEP-induced Th17 cells, suggest that these Tregs have a limited ability to control Th2 and Th17 responses. Indeed, exposure to high levels of air pollution has been associated with impaired regulatory T-cell function (32). One of the mechanisms by which Tregs control T-cell proliferation is by depleting the pool of IL-2 available to effector T-cells to sustain proliferation. Since IL-2 is a negative regulator of Th17 differentiation (33), this mechanism could favor Th17 differentiation in DEP-exposed mice.
There is mounting evidence that DEP exposure plays a significant role in the development of asthma and expression of asthma symptoms. However, the relationship is complex and depends on the timing and duration of the exposure, as well as the dose and the co-exposures (34). In one study, co-exposure of asthmatic subjects to cat allergens and DEP resulted in only mild increases in inflammation (35). The observed effect of DEP may have been much greater with a different DEP dose or in children. These investigators did not assess IL-17A production.
Two recent studies have shown that particulate matter induces IL-17A in the lungs of exposed mice (12, 13). We extend this finding by demonstrating that DEP, a component of traffic-related particulate matter, induces IL-17A (mRNA and protein) in exposed lungs. Furthermore, we demonstrated that exposure to DEP alone was able to promote accumulation of Th17 cells in the lungs of exposed mice. Finally, we demonstrated that neutralization of IL-17A alleviated DEP exacerbation of HDM-induced AHR. As is the case for DEP, IL-17A is unable to induce AHR by itself (8, 36). However, IL-17A can exacerbate IL-13-induced AHR and partially mediates HDM-induced AHR (8). Importantly, Th17 derived IL-17A has recently been demonstrated to exacerbate AHR by directly promoting airway smooth muscle contraction (37). In this study, mice lacking v 8 integrin on CD11c+ cells, fail to mount a significant Th17 response in a classic murine asthma model. Despite normal allergen-induced inflammation, Th2 responses and mucus production, the absence of IL-17A production by Th17 cells was enough to alleviate allergen-induced AHR (37). This is consistent with our finding that anti-IL-17A treatment decreased AHR without significantly affecting BALF inflammation.
It has been recently demonstrated that IL-17A levels in bronchial biopsies from asthma patients are associated with disease severity (4). Importantly, severe asthma characterized by neutrophilia is often resistant to steroid treatment (38, 39). In an experimental murine model, Th17 cells were demonstrated to mediate steroid resistant airway inflammation and AHR (40). Our data suggest that repeated DEP exposure contributes to the development of severe asthma by promoting a mixed Th2/Th17 response. Indeed, among children with allergic asthma, we found that DEP exposure was associated with increased asthma symptoms regardless of age, race, sex, annual family income, type of health insurance, maternal education, being prescribed asthma controllers, self reported adherence to asthma medication over the prior two weeks, and second-hand smoke exposure in multivariate logistic models. It is important to note that in individuals exposed to DEP are likely exposed to other components of air pollution that may also be contributing to the overall phenotype.
It is tempting to speculate that DEP promotes severe asthma by inducing development of IL-13/IL-17 double producing CD4+ T cells. We have identified a population of IL-17A and IL-13 double producing CD4+ effector T-cells in HDM and DEP co-exposed mice, similar to the recently described IL-17A and IL-13-producing T-cells (41, 42). Wang et al. demonstrated that the Th17 polarizing cytokines IL-1β, IL-6, or IL-21, are capable of inducing classical Th2 cells to produce significant amounts of IL-17. Conversely, murine and human Th17 cells have the potential to become IL-4/IL-17 dual-producing cells (42, 43). Transfer of antigen-specific, dual-producing cells into Balb/c mice triggered more severe inflammation upon allergen challenge compared to transfer of conventional Th2 or Th17 cells, highlighting the potential role of this novel cell subset in allergic asthma severity (41).
In conclusion, DEP exposure induces a mixed Th2 and Th17 response and increased AHR and lung inflammation in a mouse model of allergen-induced asthma. Similarly, in allergic children with asthma, higher DEP exposure is associated with increased asthma symptoms and higher serum IL-17A levels.
Supplementary Material
Key Messages.
-
-
In children with allergic asthma, DEP exposure was associated with more frequent asthma symptoms and increased IL-17A blood levels.
-
-
Similarly, DEP exposure worsened the allergic asthma phenotype in an experimental asthma model resulting in increased AHR, allergen sensitization, BALF Th2 and Th17 cytokines levels and pulmonary eosinophilia compared to HDM alone
-
-
Exposure to DEP alone induced a Th17 response associated with neutrophilia but did not result in AHR, eosinophilia, Th2 cytokines, or mucus production.
-
-
IL-17A neutralization alleviated DEP-induced AHR in mice.
Acknowledgements
The DEP was kindly provided by Ian Gilmour (EPA, Research Triangle Park, NC 27711) and the anti-IL17A and control IgG1 antibodies by Amgen Inc. We thank Alyssa Sproles, Stacey Bass, Brandy Day, Jiadi Xu and Carolyn Rydyznski for technical assistance and Cynthia Chappell for editorial assistance. Supported by NIEHS T32 ES010957 (EBB) and NHLBI R01HL097135 (TDLC, GKKH), and NIEHS R21016830 (MBK).
Abbreviations
- AHR
airway hyperresponsiveness
- BALF
bronchoalveolar lavage fluid
- DEP
diesel exhaust particle
- ECAT
estimates of elemental carbon attributable to traffic
- HDM
house dust mite extract
- HPRT
hypoxanthine guanine phosphoribosyl transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that there are no conflicts of interest. AL Budelsky is a paid employee and stockholder in Amgen, Inc.
REFERENCES
- 1.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 3.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036. doi: 10.1016/j.jaci.2010.02.008. e1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25:159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 6.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 8.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 10.Braback L, Forsberg B. Does traffic exhaust contribute to the development of asthma and allergic sensitization in children: findings from recent cohort studies. Environ Health. 2009;8:17. doi: 10.1186/1476-069X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, Ning Z, Kleinman MT, Sioutas C, Nel AE. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol. 2010;299:L374–L383. doi: 10.1152/ajplung.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect. 2010;118:640–646. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alessandrini F, Schulz H, Takenaka S, Lentner B, Karg E, Behrendt H, Jakob T. Effects of ultrafine carbon particle inhalation on allergic inflammation of the lung. J Allergy Clin Immunol. 2006;117:824–830. doi: 10.1016/j.jaci.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005;115:221–228. doi: 10.1016/j.jaci.2004.11.047. quiz 229. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JJ, McCreanor JE, Cullinan P, Chung KF, Ohman-Strickland P, Han IK, Jarup L, Nieuwenhuijsen MJ. Health effects of real-world exposure to diesel exhaust in persons with asthma. Res Rep Health Eff Inst: 2009:5–109. discussion 111–123. [PubMed] [Google Scholar]
- 17.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, Harrington R, Svartengren M, Han IK, Ohman-Strickland P, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 18.Maes T, Provoost S, Lanckacker EA, Cataldo DD, Vanoirbeek JA, Nemery B, Tournoy KG, Joos GF. Mouse models to unravel the role of inhaled pollutants on allergic sensitization and airway inflammation. Respir Res. 2010;11:7. doi: 10.1186/1465-9921-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens T, Cho SH, Linak WP, Gilmour MI. Differential potentiation of allergic lung disease in mice exposed to chemically distinct diesel samples. Toxicol Sci. 2009;107:522–534. doi: 10.1093/toxsci/kfn248. [DOI] [PubMed] [Google Scholar]
- 20.Butsch Kovacic M, Biagini Myers JM, Lindsey M, Patterson T, Sauter S, Ericksen MB, Ryan P, Assa’ad A, Lierl M, Fischer T, et al. The Greater Cincinnati Pediatric Clinic Repository: A Novel Framework for Childhood Asthma and Allergy Research. Pediatr Allergy Immunol Pulmonol. 2012;25:104–113. doi: 10.1089/ped.2011.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, Wright RJ. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115:1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan PH, Lemasters GK, Biswas P, Levin L, Hu S, Lindsey M, Bernstein DI, Lockey J, Villareal M, Khurana Hershey GK, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007;115:278–284. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, Villareal M, Hershey GK, Burkle J, LeMasters G. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med. 2009;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provoost S, Maes T, Pauwels NS, Vanden Berghe T, Vandenabeele P, Lambrecht BN, Joos GF, Tournoy KG. NLRP3/caspase-1-independent IL-1beta production mediates diesel exhaust particle-induced pulmonary inflammation. J Immunol. 2011;187:3331–3337. doi: 10.4049/jimmunol.1004062. [DOI] [PubMed] [Google Scholar]
- 25.Saber AT, Jacobsen NR, Bornholdt J, Kjaer SL, Dybdahl M, Risom L, Loft S, Vogel U, Wallin H. Cytokine expression in mice exposed to diesel exhaust particles by inhalation. Role of tumor necrosis factor. Part Fibre Toxicol. 2006;3:4. doi: 10.1186/1743-8977-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 27.Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 2012;33:343–349. doi: 10.1016/j.it.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Hirota JA, Hirota SA, Warner SM, Stefanowicz D, Shaheen F, Beck PL, Macdonald JA, Hackett TL, Sin DD, Van Eeden S, et al. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. J Allergy Clin Immunol. 2012;129:1116–1125. doi: 10.1016/j.jaci.2011.11.033. e1116. [DOI] [PubMed] [Google Scholar]
- 29.Totlandsdal AI, Cassee FR, Schwarze P, Refsnes M, Lag M. Diesel exhaust particles induce CYP1A1 and pro-inflammatory responses via differential pathways in human bronchial epithelial cells. Part Fibre Toxicol. 2010;7:41. doi: 10.1186/1743-8977-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 32.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, Tager I. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–852. doi: 10.1016/j.jaci.2010.08.008. e810. [DOI] [PubMed] [Google Scholar]
- 33.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, et al. Aiolos promotes T(H)17 differentiation by directly silencing II2 expression. Nat Immunol. 2012 doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delfino RJ. Do endotoxin and air pollution have a synergistic relationship to asthma onset or exacerbation? Am J Respir Crit Care Med. 2009;180:1037–1038. doi: 10.1164/rccm.200908-1285ED. [DOI] [PubMed] [Google Scholar]
- 35.Riedl MA, Diaz-Sanchez D, Linn WS, Gong H, Jr, Clark KW, Effros RM, Miller JW, Cocker DR, Berhane KT. Allergic inflammation in the human lower respiratory tract affected by exposure to diesel exhaust. Res Rep Health Eff lnst. 2012:5–43. discussion 45–64. [PubMed] [Google Scholar]
- 36.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012 doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halwani R, Al-Muhsen S, Hamid Q. T helper 17 cells in airway diseases: from laboratory bench to bedside. Chest. 2013;143:494–501. doi: 10.1378/chest.12-0598. [DOI] [PubMed] [Google Scholar]
- 39.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 40.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raymond M, Van VQ, Wakahara K, Rubio M, Sarfati M. Lung dendritic cells induce T(H)17 cells that produce T(H)2 cytokines, express GATA-3, and promote airway inflammation. J Allergy Clin Immunol. 2011;128:192–201. doi: 10.1016/j.jaci.2011.04.029. e196. [DOI] [PubMed] [Google Scholar]
- 43.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–230. doi: 10.1016/j.jaci.2009.10.012. e221–224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.