Abstract
Spermatogenesis is a complex mechanism which allows the production of male gametes; it consists of mitotic, meiotic, and differentiation phases. Spermiogenesis is the terminal differentiation process during which haploid round spermatids undergo several biochemical and morphological changes, including extensive remodelling of chromatin and nuclear shape. Spermiogenesis is under control of endocrine, paracrine, and autocrine factors, like gonadotropins and testosterone. More recently, emerging pieces of evidence are suggesting that, among these factors, estrogens may have a role. To date, this is a matter of debate and concern because of the agonistic and antagonistic estrogenic effects that environmental chemicals may have on animal and human with damaging outcome on fertility. In this review, we summarize data which fuel this debate, with a particular attention to our recent results, obtained using type 1 cannabinoid receptor knockout male mice as animal model.
1. Introduction
Spermatogenesis occurs in the testis in a stepwise fashion so that committed spermatogonia proliferate and develop into spermatocytes (SPC) to enter meiosis and produce round spermatids (SPT). These undergo a morphological transformation (spermiogenesis) into mature SPT (i.e., spermatozoa), which are differentially released from Sertoli cells (spermiation) depending on the species. In mammals, further transformations occur in the epididymis to form mature spermatozoa (SPZ) suitable for fertilization [1–4]. Spermatogenesis is a process highly conserved throughout vertebrate species and it is mainly under hypothalamic-pituitary control [5–17]. Indeed, it is a hormonally controlled mechanism; apart from gonadotropins and androgens, numerous endocrine, paracrine, or autocrine factors converge in a complex stage-specific multifactorial control of spermatogenesis [10, 18–24].
Spermiogenesis is the terminal differentiation process of male germ cells, during which haploid round SPT undergo extensive biochemical and morphological changes including acrosome formation, flagellar development, chromatin condensation, and severe nuclear and cellular reorganization [25, 26]. In mouse, morphological criteria have been used to classify spermiogenesis in 16 developmental steps [27]; in particular, round (steps 5–8), elongating (steps 9–11), condensing (steps 12–14), and condensed (steps 14–16) SPT are differentially characterized by acrosomal and flagellar development, as well as by cellular and nuclear shape. During the early steps, round SPT are transcriptionally active; in elongating SPT, transcriptional activity decreases and then turns off; later, in condensing SPT, an extensive chromatin reorganization occurs at molecular and morphological levels [28].
The underlying events that lead to this extensive chromatin reorganization and packaging have been reported in several excellent reviews and here summarized, but very little is known about the molecular mechanisms involved [26, 29–32]. Interestingly, in recent overviews about estrogens (E2) and spermatogenesis in mammals, the presence of E2 receptor (ER) and aromatase in somatic and germ cells has been underlined to suggest a possible involvement of this traditionally female hormone in spermiogenesis [2, 33–36].
In this review, we focus on the recent advances in our laboratory about the emerging role of E2 in SPT chromatin reorganization during spermiogenesis. In particular, new insight has come out from the study of type 1 cannabinoid receptor knockout (Cnr1−/−) mice.
2. Chromatin Reorganization in SPT
Spermatozoa are highly differentiated haploid cells with a particular chromatin organization that results from remodeling events occurring during meiotic and postmeiotic phases of spermatogenesis [26].
Indeed, when cells enter the meiotic prophase, all the somatic histones, except H4 (i.e., H4t-gene protein), are replaced by testis-specific (TH2A, TH2B, TH3, and H1t) or testis-enriched (H2AX, H1a) histone variants [37–39]. Testicular variants of H1 linker are H1t, H1t2, and HILS1 [40–42]. Among these, H1t has been reported to exert the lowest condensing effect on rat testis oligonucleosomes [43]. The high levels of H1t (about 55% of the linker histones) during the pachytene phase until the stage of elongating SPT suggest a role in keeping chromatin in a relatively decondensed state which enables nuclear events. Indeed, during the early steps, round SPT are transcriptionally active and contain H1t-enriched nucleosomal chromatin. In elongated SPT, H1t persists until the transcriptional activity of the genome is still detectable [37]. This histone-variant incorporation step, together with histone posttranslational modifications, such as acetylation, methylation, ubiquitination, and phosphorylation, creates specific chromatin domains characterized by quickly disassembling nucleosomes and by a new “histone code,” both facilitating histone displacement [39, 44, 45].
During the postmeiotic stage of spermatogesis, when round SPT are extensively remodelled to form mature SPZ, a gradual and radical change in the chromatin cytoarchitecture is observed (the main events are summarized in Figure 1) [46]. This extensive chromatin reorganization requires (i) expression and storage of specific proteins involved in condensation, such as transition proteins (TNP) and protamines (PRM), (ii) transient DNA strand-breaks which require the topoisomerase enzyme, (iii) displacement and degradation of the nucleosomal structure, (iv) sequential histone replacement, firstly by TNP and then by PRM, (v) transcriptional silencing and DNA repair, and (vi) repackaging of protaminated chromatin into toroidal structures [47, 48]. However, many species retain a small fraction (1% in mouse, 15% in human) of their chromatin in the more relaxed nucleosomal configuration [49] so that SPZ contain at least two differentially packaged chromatin domains: (1) the PRM-based chromatin that organizes the bulk of DNA in a highly compact toroidal configuration, suitable to arrest transcription and mask genome from exogenous and endogenous damage until fertilization [29]; (2) the nucleosome-based chromatin that organizes epigenetically marked developmental loci in a potentially dynamic transcriptional configuration, useful after fertilization [30, 50]. Interestingly, early after fertilization, before activation of the embryonic genome, the paternal pronucleus becomes highly transcriptionally active compared with the female pronucleus [51].
Figure 1.
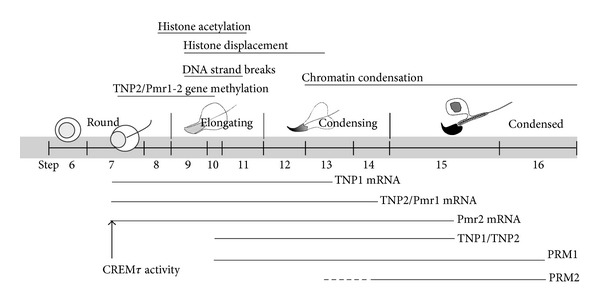
Timing of the main chromatin remodeling events in round, elongating, condensing, and condensed spermatids (the related references are reported in the text).
At molecular level, histone-to-PRM exchange requires the expression and storage of specific mRNA involved in condensation. Indeed, transcription and translation are temporally uncoupled. Tnp1/2 and Pmr1/2 mRNAs are synthesized and stored for some days in SPT and later translated, implying a timely controlled process of haploid-regulated transcription and translation [28, 52, 53]. In particular, Tnp1 and Tnp2 mRNA are preserved in translationally inert ribonucleoprotein particles; afterward, they are translated in elongating-condensing SPT (steps 10–15 of spermiogenesis) and then degraded after translation [52, 53]. Temporal and stage-specific appearance of TNP and PRM is strictly regulated and is prerequisite for the correct differentiation of round SPT into mature and motile SPZ with fertilizing capability [54].
Transcriptional regulation of haploid genes depends on potentiation of gene via association with nuclear matrix attachment regions (MARs) [55]. It also depends on DNA methylation and recruitment of transacting factors like TATA-box protein (TBP), Y-box proteins, and cAMP-responsive element modulator (CREM). The latter is a transcription factor which binds as homo- and heterodimers to the regulatory sequence CRE (cAMP-responsive element) [29, 56]. The CREM-encoding gene is highly expressed in the adult testis and shows multiple site of alternative splicing [57]. Levels of CREM transcripts are low in prepubertal testis and only the repressor isoforms (α, β) are detected. However, during puberty, transcripts encoding the activator form CREMtau (CREMτ) appear abundantly expressed only in germ cells, from the pachytene SPC stage onward, while CREMτ protein is not detected in SPC but only in haploid SPT at very high levels [57, 58]. The CREM switch (repressor versus activator) is regulated by the gonadotropin FSH which, acting through Sertoli cells, paracrinally directs the use of an alternative polyadenylation site in SPT, resulting in a more stable CREMt transcript [59]. Many haploid genes have been identified as potential CREM targets since they contain CREs or half CRE in their promoters. Indeed, CREMt regulates gene expression of Tnp and Prm [29, 56].
In mouse, Pmr1, Pmr2, and Tnp2 genes are clustered on chromosome 16 and, contrary to the usual paradigm, they are fully methylated when actively transcribed. In contrast, the Tnp1 gene shows demethylation in the 5′ region associated with gene activity [38]. In humans, the DNA methyltransferase 1 (DNMT1) is restricted to male germ cells (pachytene SPC and round SPT), and infertile patients showing round SPT maturation arrest also show a specific DNMT1 loss in these cells [60]. Similarly, Crem-null mice show round SPT maturation arrest [61, 62]. These observations suggest that methylation and CREM are master controllers of SPT differentiation.
In mouse, histone displacement starts at step 9. Main events promoting histone displacement are phosphorylation of histone H1t [63] and hyperacetylation of H4. The latter process has been largely studied in germ cells of several species [64–66] and is largely conserved during evolution. It has been demonstrated that hyperacetylation of histone tails (steps 8–11 SPT) relaxes nucleosomal DNA-histone interaction, and precedes and overlaps either histone displacement or TNP1/TNP2 presence at nuclear level (step 10–early 15 SPT). Accordingly, core-histones are displaced in their acetylated state [66]. Available reports suggest that the process of histone displacement requires (i) DNA nick/repair induced by topoisomerase which relieves torsional stress associated with histone-to-PRM exchange; (ii) hyperacetylation of histone tails by histone acetyl transferase with a concerted down regulation of histone deacetylase; (iii) histone removal mediated by the recruiting protein BRDT (testis-specific bromodomain protein) able to bind histone acetylated lysines, and (iv) acetylated histone degradation through polyubiquitylation of N-terminal lysines [31]. Histone displacement ends at step 13 SPT [47].
Concurrently to the aforementioned steps, a DNA-binding competition mechanism leads to histone-to-TNP exchange (TNP1-4 in mice, rats, boars, bulls, and men; the best characterized are TNP1 and TNP2) and to final TNP-to-PRM transition (PRM1 in rats; PRM1 and PRM2 in stallions and mice; PRM 4 in humans) [39, 47]. Phosphorylation and dephosphorylation of TNP and PRM trigger their nuclear translocation, their binding to DNA, and eventually chromatin condensation [28, 67]. It has been demonstrated that TNP1 has important DNA-nucleosome core destabilizing properties because it decreases the melting temperature of DNA and relaxes DNA in nucleosomal core particles in vitro [68]. In contrast, TNP2 seems to be a DNA-condensing protein [69] and its phosphorylation by protein kinase A greatly reduces its condensation property [70]. Although TNP1 and TNP2 apparently show distinct functions, together with PRM, both are involved in DNA strand-break repair [53, 71–74] and male mice with single tnp1 or tnp2 gene deletion demonstrate that TNP1/2 partially complement each other and both affect PRM2 processing before its binding to DNA [75]. Interestingly, in double Tnp1/Tnp2-null mice, histone displacement and PRM deposition proceeded relatively normally, chromatin condensation occurs irregularly, and many SPT show DNA breaks, thus demonstrating that although TNP are required for a normal chromatin condensation, they are not essential for the process [76].
Protamines are the most basic DNA-condensing proteins. Most likely, they arise from an ancestral histone H1 gene [77], but, differently from histones, they are characterized by arginine (in eutherians, arginine, and cysteine) rather than lysine residues [78]. This biochemical difference explains PRM greater affinity for DNA, due to a higher hydrogen binding potential of arginine over lysine [79]. These proteins may bind to the major and minor groove of DNA or to the DNA surface by interacting electrostatically with phosphate residues. It has been demonstrated that PRM allow chromatin condensation through arginine residues into toroidal structures at testicular level [29], and further through cysteine residues along the epididymal transit, when inter- and intraprotamines disulphide bonds are formed [80]. In concert with thiol oxidations, PRM also undergo tyrosine phosphorylation during caput-to-cauda transit [81].
At morphological level, when histone-to-PRM transition occurs, an extraordinary event is observed in the nucleus of differentiating germ cells: flocculent densities of chromatin coalesce into a coarsely granulofibrillar chromatin, which gradually extends in a centripetal and rostral-to-caudal direction and becomes dense and homogeneous at the end of spermiogenesis [28]. This chromatin condensation in toroidal structures modifies the shape of the whole nuclear compartment and strongly reduces its size promoting development of the peculiar elongated, small, and hydrodynamic sperm head that supports swimming ability. Indeed, by stacking these toroids, the sperm nucleus achieves a higher efficiency in packaging the paternal genome and therefore in reducing its size to an absolute minimum [30]. The mechanism by which PRM induce the conformational change in chromatin packaging is not well understood, but it is probably related to PRM properties and to enzymes involved in chromatin remodeling [29, 56].
Abnormal sperm histone or PRM content can disrupt chromatin organization [82–84]. Indeed, histone retention decreases nucleoprotamine-based chromatin and exposes a more relaxed chromatin to damage [56, 85]. In both humans and animals, abnormal DNA damage is associated with compromised fertility and increased miscarriage rates [56, 76, 86]. Therefore, chromatin quality is an objective marker of sperm function that provides a significant prognostic factor for male infertility [87–89].
3. Estrogens and Spermiogenesis
The presence of intracellular (ERα and ERβ) and transmembrane (GPR30) E2 receptors in the testis and in particular the expression of ERβ, GPR30, and aromatase in germ cells have highlighted the physiological role of the E2 in spermatogenesis [35, 90]. Aromatase knockouts (ArKO) or ERα knockouts (αERKO) have further accentuated the role of E2 in germ cell progression and maturation. Indeed, the specific phenotypes have demonstrated that in αERKO mice disruption of spermatogenesis appears to be primarily linked to mechanical defect in the epididymis [1], whereas in ArKO mice a specific depletion in developing SPT seems to occur [91]. To date, no helpful information came from ERβ or GPR30 knockout mice [2, 33, 34].
Traditionally, testosterone and E2 were considered male only and female only hormones, respectively. However, at the beginning of the 1930s, the developmental exposure to high doses of E2 was reported to induce malformation of the male reproductive tract in mammals [92], thus suggesting that E2 might regulate male reproduction [2, 93]. It is now accepted that E2 regulate spermatogenesis (gonocyte and spermatogonia proliferation, meiosis, Sertoli cell function) as well as spermiation, sperm transport, and epididymal sperm maturation. Some of these functions are evolutionarily conserved since they have been observed in mammalian and nonmammalian species [1, 7, 8, 12, 14, 19, 36, 94–99].
The first evidence that E2 affect spermiogenesis came in 1987, when adult male rats were treated with an ovarian protein able to inhibit aromatase activity. After treatment, animals showed degeneration of round SPT and a massive decrease of elongated SPT [100, 101]. Accordingly, a significant decrease in round and elongated SPT, but not in earlier germ cells, was found in adult male bonnet monkeys treated with an aromatase inhibitor for 150 days [102]. These data are not surprising, given the more intense immunostaining and higher aromatase activity in SPT than in any other testicular cells [103, 104].
To well define the role of E2 in male germ cell development, mice with a targeted disruption of the cyp19A1 aromatase gene (ArKO mice) were generated [91]. These animals were initially fertile but developed progressive infertility between 4.5 months and 1 year. Spermatogenesis is primarily arrested at early spermiogenic stages, with the appearance of multinucleated cell into the tubular lumen. Furthermore, an abnormal acrosome development with multiple acrosomal vesicles and uneven spreading over the nuclear surface is also observed [91]. This observation suggests that acrosome biogenesis may be an E2-dependent process. Accordingly, aromatase is detected at high levels in the Golgi complex of developing SPT [34]. The progressive nature of the phenotype may be intrinsic to the mechanism of E2 action in adult seminiferous epithelium, as observed also in female ArKO mice, characterized by a progressive phenotype too [91]. Alternatively, the delayed phenotype may be explained by the high content of phytoestrogens in the diet, which may supply sufficient exogenous E2 to maintain normal spermatogenesis in young animals. Indeed, in young ArKO mice on a phytoestrogens-free diet, the phenotype was more severe than in mice on normal diet [105, 106].
However, ArKO mice are not an ideal model to study E2-regulated events during spermatogenesis in adulthood because of developmental absence of E2. Therefore, using rat and mouse, several attempts were carried out to create conditions of high intratesticular E2 levels and study its effect on spermatogenesis.
Earlier studies on long-term exposure to pharmacological doses of E2 in adult male rats demonstrated that this treatment suppressed both gonadotropins and testosterone releases and induced complete azoospermia [107]. Then, a second attempt was based on the administration of different doses of exogenous E2 over a period of 10 days to increase intratesticular E2 levels with a concomitant deficiency in circulating FSH and both plasma and intratesticular testosterone. By exploiting these experimental conditions, it was suggested that, during spermiogenesis, round SPT differentiation (steps 1 to 6) was largely dependent on E2, whereas SPT elongation (steps 8 to 19) was androgen dependent [108]. These data were supported by the observation that high intratesticular E2 levels preserved round SPT steps 1–6 whereas testosterone deficiency, induced by E2 treatment via a negative central feedback, in turn originated pyknotic bodies in elongated/condensed SPT steps 8–19 [108]. In a further study, a similar treatment in rats significantly decreased testicular levels of CREMτ protein, as well as the CREMτ-inducible TNP1/2 and PRM1 proteins, while the relative mRNA levels were not changed [109]. In the same article, the E2 treatment was also reported to significantly increase testicular androgen binding protein (ABP) mRNA levels, thus suggesting a specific stimulatory effect of E2 on ABP gene regulation or RNA stability. Authors concluded that E2 suppressed the appropriate translation of the spermatidal proteins through an ABP-dependent posttranscriptional mechanism. Surprisingly, no information about testicular morphology was reported [109]. A further confirmation of E2 activity on SPT came from the observation that GPR30 regulates expression of apoptotic markers in rat pachytene SPC and round SPT [90, 110]. More interestingly, growing pieces of evidence reveal that endocrine disruptors, that is, environmental pollutants able to interfere with endogenous endocrine system, have been demonstrated to negatively affect spermatogenesis; among these, there are disruptors with agonistic and antagonistic estrogenic effects. To date, this is a matter of debate and concern because these compounds may have damaging outcome on animal and human fertility [111].
Recent findings reveal that E2 restore spermatogenesis in hypogonadal (hpg) mice. Due to a natural Gnrh gene deletion, the hpg mice are functionally deficient in gonadotropins and sex steroids and show meiosis arrest at pachytene stage. Treatment with E2 or ERα agonist restores meiosis in these animals which, in absence of testosterone, produce haploid elongated SPT, likely via a mechanism involving a weak neuroendocrine activation of FSH secretion [112–114]. Qualitatively complete spermatogenesis could be also restored in hpg mice by administration of either testosterone or its metabolite, the potent nonaromatizable androgen dihydrotestosterone (DHT), in absence of FSH stimulation [115]. However, in this case, a possible E2 involvement cannot be excluded. Indeed, it has been reported that DHT can be converted in 3β-diol by 3β-hydroxysteroid dehydrogenase (3β-HSD), which preferentially binds ER rather than androgen receptor [116]. Interestingly, in addition to th results obtained in hpg mice [112–114], studies on FSH-receptor knockout mice (FORKO) support an E2 involvement in spermiogenesis, likely in a synergistic or independent way with FSH and/or androgens [117]. Indeed, FORKO mice show low testosterone and E2 levels [118], as well as a significant increase of spermatogonia, decrease of elongated SPT, and weight loss of testis, epididymis, and seminal vesicles. Tubular and luminal diameters of caput epididymis appear smaller than those of wild-type males with few lumina filled with SPZ [117]. At molecular level, deprivation of FSH signaling greatly decreases TNP/PRM levels as well as chromatin quality of SPZ [119].
We recently characterized the reproductive phenotype of type 1 cannabinoid receptor knockout (Cnr1−/−) male mice. These mutants exhibit endocrine and phenotypic features which are useful to extend the above studies about the role of E2 in SPT differentiation and in particular in the maintenance of sperm chromatin quality. Main features are summarized in Table 1 and described below.
Table 1.
Reproductive phenotype of Cnr1−/− male mice and related references.
| Hypothalamus-pituitary-testis axis | |
| (i) Normal pituitary LH content [120] | |
| (ii) Low serum LH concentration [120] | |
| (iii) Low testicular testosterone secretion [120] | |
| (iv) Low circulating testosterone and E2 levels [120, 121] | |
| (v) High pituitary GnRH-R and low FSHβ subunit mRNA levels [121] | |
| (vi) Low testicular Fsh-R mRNA levels [121] | |
| (vii) Low testicular P450 mRNA levels [121] | |
| (viii) Low P450arom levels in Leydig cells [121] | |
| (ix) Low Tnp2 levels (both mRNA and protein) [85] | |
| Adult Leydig cell | |
| (i) Low number of adult Leydig cells [122] | |
| (ii) Normal 3-βHsd mRNA levels/Leydig cell [121] | |
| Sperm chromatin quality | |
| (i) High number of SPZ with retained histones [85, 121, 123] | |
| (ii) High number of SPZ with uncondensed chromatin [85, 123] | |
| (iii) High number of SPZ with DNA damage [85, 123] | |
| (iv) SPZ with high % of damaged DNA [85] | |
| (v) Increase of DNA damage during epididymal transit from caput to cauda [85] | |
| (vi) High mean values of sperm nuclear length [123] | |
| Epididymal sperm motility acquisition | |
| (i) High number of potentially motile SPZ in caput [4] | |
| (ii) Precocious sperm motility acquisition in caput epididymis [4] |
4. Reproductive Phenotype of Cnr1 −/− Male Mice: E2 Activity on Sperm Quality
Endocannabinoids are lipidic mediators identified in several peripheral tissues (brain, testis, and epididymis) and biological fluids (follicular fluid, maternal milk, and blood) [4, 124–129]. To date, the best characterized are arachidonoylethanolamide (AEA or anandamide) and 2-arachidonoyl-glycerol (2-AG), but other molecules have been proposed as possible cannabinoid receptor (CNR) agonists [26, 130]. Endocannabinoids regulate reproduction, in both males [16, 17, 20, 26, 122, 131–139] and females [140–145], and specific G-protein-coupled cannabinoid receptors, CNR1 and CNR2 (or CB1 and CB2, resp.), have been localized in male and female reproductive tracts [20, 146–148]. In the testis, CNR1 is present in somatic and germ cells including SPT, from round stage onward [122, 129, 131, 149–154], and recently its involvement in chromatin packaging during SPT differentiation has been reported [26, 85]. However, much remains to be clarified about a direct and/or indirect role. In vivo studies, using nonmammalian and mammalian animal models, show that CNR1 acts at both central and local level [155, 156]. In frog, an intriguing CNR1-GnRH (gonadotropin-releasing hormone) interplay occurs at a central level, with CNR1 regulating GnRH synthesis [16]; at testicular level, CNR1 also regulates GnRH1/2 and GnRH-R (GnRH-receptor) expression [157]. In rat, CNR1 regulates the release of hypothalamic GnRH [158], while in mice it increases Tnp2 expression in the testis [85]. It has been hypothesized [26] that testicular AEA, responsive to E2 and produced by somatic cells [131, 159] and/or by SPT [140], may act as paracrine/autocrine factor on SPT themselves via CNR1, by regulating Tnp2 mRNA transcription or stability.
Most information about CNR1 involvement in male reproduction came from Cnr1−/− mice. An early study reported that Cnr1−/− male efficiently synthesizes the gonadotropin LH but shows low levels of LH and testosterone in the bloodstream. Furthermore, Cnr1−/− testis produces few testosterone in vitro [120]. Recently, we have characterized the reproductive phenotype of Cnr1−/− mice and reported that males show normal progression of spermatogenesis [3, 4, 122], produce SPZ [3, 4], and are fertile [85] although they displayed a lot of abnormalities (see Table 1 and references herein) such as (i) downregulation of neuroendocrine axis [121], (ii) developmental decrease of Leydig cell number [122], (iii) low sperm chromatin quality [85, 121], and (iv) abnormal epididymal sperm motility (i.e., potential to move) acquisition [3, 4]. Some of these abnormalities well fit with the early data reported by Wenger et al. [120]. Indeed, at molecular level Cnr1 gene deletion originates a ligand-dependent downregulation of GnRH-R signaling and this may explain the LH drop originally observed in these animals. In any case, although both LH and testosterone decrease by 50% in serum of Cnr1−/− as compared with wild-type mice [120], we found that LH signaling is sufficient to regulate steroidogenesis supporting testosterone production in Leydig cells. Indeed, the 3β-HSD, a LH-responsive selective marker of Leydig cells, is synthesized at normal levels in individual single cells [121], thus suggesting that in Cnr1−/− mice the testosterone decrease, in both in vivo and in vitro systems [120], is exclusively related to a decrease of Leydig cell number. We also found that GnRH downregulation is accompanied by downregulation of Fshb, Fsh-R, Tnp2, and P450arom mRNA as well as of TNP2 and P450arom protein. The P450arom protein decrease is observed in the interstitial Leydig cells and the low levels are independent by Leydig cells number. Simultaneously, low E2 levels were detected in the bloodstream suggesting that, in the mutant mice, the downregulation of neuroendocrine axis interferes with gene expression of Tnp2 in SPT as well as of P450arom in Leydig rather than in germ cells with consequent reduction of E2 levels in the bloodstream [121].
The morphological and biochemical evaluations of epididymal Cnr1−/− sperm samples showed a lot of abnormalities. In wild-type animals, a 2-AG gradient (high level in caput versus low levels in cauda) prevents sperm motility acquisition in caput through the inhibitory activation of CNR1. In knockout animals, a high number of SPZ from caput epididymis appears motile, thus demonstrating that, in absence of CNR1, SPZ precociously acquire their potential to move [4]. The morphological and biochemical analyses of epididymal Cnr1−/− SPZ also showed poor chromatin quality [26, 85]. In particular, we found that the genetic inactivation of Cnr1 affects chromatin remodeling mechanisms that occur in SPT during spermiogenesis. Indeed, in caput epididymis from Cnr1−/− animals, the number of SPZ with histone retention as well as the number of SPZ with uncondensed chromatin or with DNA damage is higher than in Cnr1+/+ and Cnr1+/− animals [85, 121, 123] demonstrating that in these animals spermiogenesis is qualitatively inefficient. Despite that, animals retain their fertility [85], likely because of a sufficient number of SPZ with mature chromatin. Correlation analysis and morphological studies also showed that abnormal histone retention is strictly related to uncondensed chromatin or DNA damage as well as it is associated to sperm nuclear size elongation. Intriguingly, histone displacement and chromatin condensation normally occur in a rostral-to-caudal direction [89] and then the failure of these mechanisms might be responsible of the nuclear swelling along its longitudinal axes. Recent experiments suggest that low plasma E2 levels might be the cause of the sperm chromatin imperfections observed in these animals [123]. Indeed, 24-day postpartum Cnr1−/− male mice exposed to low doses of E2, every other day for a complete cycle of spermatogenesis, showed a weak upregulation of neuroendocrine axis (no effect on GnRH mRNA levels; strong increase of GnRH-R mRNA; weak increase of Fshb subunit, Fsh-R, and P450arom mRNA; weak increase, about 20%, of P450arom protein in Leydig cells; no effect on Tnp2 mRNA) and the rescue of sperm chromatin quality indices (histone content, chromatin condensation, DNA damage, and nuclear size), via an ER mediated mechanism [121]. Several studies propose that chromatin condensation and DNA damage are related to each other and are secondary effects associated to disrupted histone displacement [29, 56]. Therefore, it is plausible to conclude that E2 treatment, through a TNP2-independent effect, primarily affects histone displacement and sequentially induce the rescue of sperm chromatin quality indices to physiological values. In agreement, caput SPZ from rat chronically injected with E2 showed a TNP/PRM1-independent chromatin hypercompaction [109]. In addition, serum concentration of E2 and free T4 inversely correlates with sperm DNA damage in men from an infertility clinic [160]. Furthermore, it has been reported that E2 delay testicular cell damage, which leads to functional senescence. Therefore, E2 are helpful in protecting the reproductive functions from the adverse effects exerted by reactive oxygen species (ROS) produced in large quantities in the aged testis [161].
These results, in combination with aforementioned data, show that E2, indirectly via stimulatory effects on FSH secretion and/or directly via paracrine actions within the testis, play a key role in spermiogenesis since they preserve chromatin packaging in SPT and then sperm quality. Interestingly, sperm nuclear length, which is related to chromatin quality, appears to be an E2-responsive morphological parameter [123] and may be used as helpful tool to discriminate “in real time,” among morphologically normal SPZ, those with a good chromatin quality. To date, no tool exists to verify “in real time” sperm chromatin quality. Interestingly, in assisted reproduction technique field, abnormal nuclear size of human SPZ is commonly considered to be of poor prognosis [162]. More interestingly, a recent article describes a tight correlation between percentage of SPZ with nuclear form abnormalities, screened by MSOME (motile sperm organelle morphology examination) technique, and DNA fragmentation [163].
5. Last Considerations and Conclusions
Studies on αERKO mice led to conclude that E2 are involved in epididymal sperm maturation. Our data and those reported in the literature suggest a further and intriguing role for E2 in spermiogenesis and in maintenance of sperm chromatin quality. The main future endpoint will be the characterization of E2 mechanisms to better understand whether its action is direct and/or mediated. Indeed, it is still a matter of debate whether E2 and/or FSH affect chromatin remodeling in SPT in either a synergistic or an independent way with androgens [31]. Gene deletion animal models have revealed that both FSH and testosterone levels are implicated in the regulation of chromatin condensation during spermiogenesis [31]. However, although is emerging idea is that these hormones may act in synergy [31, 164], it has been reported in rat that the inhibition of FSH, resulted from hyperprolactinemia induction, reduces chromatin packaging in an androgen-independent way.
Apart from the divergences, FORKO mice exhibit endocrine and phenotypic features of Cnr1−/− male mice, including reduced histone displacement, enlarged sperm head size, decreased chromatin quality of SPZ (low packaging and high DNA damage), and low levels of testosterone and E2 [117–119]. In Cnr1−/−, the number of SPZ and epididymal epithelium morphology, both dependent on testosterone [165], are not affected [3] suggesting that, in mutant mice, testosterone ranges within levels sufficient to support spermatogenesis. Therefore, we speculate that, in Cnr1−/− mice, E2 action, (i.e., the rescue of histone displacement in SPT and chromatin quality in SPZ) is independent of testosterone. Our future endpoint will be to confirm if this really occurs and to establish E2 and FSH roles. The importance of these studies is corroborated by the growing pieces of evidence that E2 activity can be mimicked or antagonized by estrogenic environmental chemicals with potentially damaging effects on animal and human fertility [111].
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors are grateful to Dr. Diana Ferrara for English editing and critical analysis of the paper. This work was supported by Grants PRIN Pierantoni 2008 and PRIN Cobellis 2010-2011.
Abbreviations
- SPC:
Spermatocytes
- SPT:
Spermatids
- SPZ:
Spermatozoa
- E2:
Estrogens
- ER:
Estrogen receptor
- Cnr1−/−:
Type 1 cannabinoid receptor knockout
- TNP:
Transition proteins
- PRM:
Protamines
- MARs:
Nuclear matrix attachment regions
- TBP:
TATA-box protein
- CREM:
cAMP-responsive element modulator
- CRE:
cAMP-responsive element
- CREMτ:
CREMtau
- DNMT1:
DNA methyltransferase 1
- BRDT:
Testis-specific bromodomain protein
- GPR30:
Transmembrane E2 receptor
- ArKO:
Aromatase knockouts
- αERKO:
ERα knockouts
- ABP:
Androgen binding protein
- Hpg:
Hypogonadal
- DHT:
Dihydrotestosterone
- 3β-HSD:
3β-Hydroxysteroid dehydrogenase
- AEA:
Arachidonoylethanolamide
- 2-AG:
2-Arachidonoyl-glycerol
- CNR:
Cannabinoid receptor
- GNRH:
Gonadotropin-releasing hormone
- GNRH-R:
GnRH-receptor
- ROS:
Reactive oxygen species
- MSOME:
Motile sperm organelle morphology examination
- FORKO:
FSH-receptor knockout mice.
References
- 1.Hess RA, Bunick D, Lee K-H, et al. A role for oestrogens in the male reproductive system. Nature. 1997;390(6659):509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocrine Reviews. 2001;22(3):289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- 3.Ricci G, Cacciola G, Altucci L, et al. Endocannabinoid control of sperm motility: the role of epididymus. General and Comparative Endocrinology. 2007;153(1–3):320–322. doi: 10.1016/j.ygcen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Cobellis G, Ricci G, Cacciola G, et al. A gradient of 2-arachidonoylglycerol regulates mouse epididymal sperm cell start-up. Biology of Reproduction. 2010;82(2):451–458. doi: 10.1095/biolreprod.109.079210. [DOI] [PubMed] [Google Scholar]
- 5.Fasano S, D’Antonio M, Chieffi P, Cobellis G, Pierantoni R. Chicken GnRH-II and salmon GnRH effects on plasma and testicular androgen concentrations in the male frog, Rana esculenta, during the annual reproductive cycle. Comparative Biochemistry and Physiology C. 1995;112(1):79–86. doi: 10.1016/0742-8413(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 6.Fasano S, Chieffi P, Cobellis G, Pierantoni R. Neuroendocrine and local control of the frog testis. Annals of the New York Academy of Sciences. 1998;839:260–264. doi: 10.1111/j.1749-6632.1998.tb10771.x. [DOI] [PubMed] [Google Scholar]
- 7.Cobellis G, Vallarino M, Meccariello R, et al. Fos localization in cytosolic and nuclear compartments in neurones of the frog, Rana esculenta, brain: an analysis carried out in parallel with GnRH molecular forms. Journal of Neuroendocrinology. 1999;11(9):725–735. doi: 10.1046/j.1365-2826.1999.00390.x. [DOI] [PubMed] [Google Scholar]
- 8.Cobellis G, Pierantoni R, Minucci S, Pernas-Alonso R, Meccariello R, Fasano S. c-fos Activity in Rana esculenta testis: seasonal and estradiol-induced changes. Endocrinology. 1999;140(7):3238–3244. doi: 10.1210/endo.140.7.6790. [DOI] [PubMed] [Google Scholar]
- 9.Minucci S, De Rienzo G, Di Sena R, et al. Effects of multiple injections of ethane 1, 2-dimethane sulphonate (EDS) on the frog, Rana esculenta, testicular activity. Journal Experimental Zoology. 2000;287(5):384–393. doi: 10.1002/1097-010x(20001001)287:5<384::aid-jez6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Pierantoni R, Cobellis G, Meccariello R, Fasano S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. International Review of Cytology. 2002;218:69–141. doi: 10.1016/s0074-7696(02)18012-0. [DOI] [PubMed] [Google Scholar]
- 11.Pierantoni R, Cobellis G, Meccariello R, et al. The amphibian testis as model to study germ cell progression during spermatogenesis. Comparative Biochemistry and Physiology B. 2002;132(1):131–139. doi: 10.1016/s1096-4959(01)00543-7. [DOI] [PubMed] [Google Scholar]
- 12.Cobellis G, Meccariello R, Fienga G, Pierantoni R, Fasano S. Cytoplasmic and nuclear Fos protein forms regulate resumption of spermatogenesis in the frog, Rana esculenta. Endocrinology. 2002;143(1):163–170. doi: 10.1210/endo.143.1.8567. [DOI] [PubMed] [Google Scholar]
- 13.Meccariello R, Mathieu M, Cobellis G, et al. Jun localization in cytosolic and nuclear compartments in brain-pituitary system of the frog, Rana esculenta: an analysis carried out in parallel with GnRH molecular forms during the annual reproductive cycle. General and Comparative Endocrinology. 2004;135(3):310–323. doi: 10.1016/j.ygcen.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Cobellis G, Lombardi M, Scarpa D, et al. Fra-1 activity in the frog, Rana esculenta, testis. Annals of the New York Academy of Sciences. 2005;1040:264–268. doi: 10.1196/annals.1327.039. [DOI] [PubMed] [Google Scholar]
- 15.Meccariello R, Chianese R, Scarpa D, et al. UBPy/MSJ-1 system during male germ cell progression in the frog, Rana esculenta. General and Comparative Endocrinology. 2007;153(1–3):275–279. doi: 10.1016/j.ygcen.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Meccariello R, Franzoni MF, Chianese R, et al. Interplay between the endocannabinoid system and GnRH-I in the forebrain of the anuran amphibian Rana esculenta. Endocrinology. 2008;149(5):2149–2158. doi: 10.1210/en.2007-1357. [DOI] [PubMed] [Google Scholar]
- 17.Chianese R, Chioccarelli T, Cacciola G, et al. The contribution of lower vertebrate animal models in human reproduction research. General and Comparative Endocrinology. 2011;171(1):17–27. doi: 10.1016/j.ygcen.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Cobellis G, Meccariello R, Pierantoni R, Fasano S. Intratesticular signals for progression of germ cell stages in vertebrates. General and Comparative Endocrinology. 2003;134(3):220–228. doi: 10.1016/s0016-6480(03)00281-8. [DOI] [PubMed] [Google Scholar]
- 19.Cobellis G, Meccariello R, Minucci S, Palmiero C, Pierantoni R, Fasano S. Cytoplasmic versus nuclear localization of fos-related proteins in the frog, Rana esculenta, testis: in vivo and direct in vitro effect of a gonadotropin-releasing hormone agonist. Biology of Reproduction. 2003;68(3):954–960. doi: 10.1095/biolreprod.102.008938. [DOI] [PubMed] [Google Scholar]
- 20.Pierantoni R, Cobellis G, Meccariello R, et al. Testicular gonadotropin-releasing hormone activity, progression of spermatogenesis, and sperm transport in vertebrates. Annals of the New York Academy of Sciences. 2009;1163:279–291. doi: 10.1111/j.1749-6632.2008.03617.x. [DOI] [PubMed] [Google Scholar]
- 21.Cacciola G, Chianese R, Chioccarelli T, et al. Cannabinoids and reproduction: a lasting and intriguing history. Pharmaceuticals. 2010;3(10):3275–3323. [Google Scholar]
- 22.Meccariello R, Berruti G, Chianese R, et al. Structure of msj-1 gene in mice and humans: a possible role in the regulation of male reproduction. General and Comparative Endocrinology. 2008;156(1):91–103. doi: 10.1016/j.ygcen.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Chianese R, Scarpa D, Berruti G, et al. Expression and localization of the deubiquitinating enzyme mUBPy in wobbler mouse testis during spermiogenesis. General and Comparative Endocrinology. 2010;166(2):289–295. doi: 10.1016/j.ygcen.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Meccariello R, Cobellis G, Berruti G, et al. Mouse sperm cell-specific DnaJ first homologue: an evolutionarily conserved protein for spermiogenesis. Biology of Reproduction. 2002;66(5):1328–1335. doi: 10.1095/biolreprod66.5.1328. [DOI] [PubMed] [Google Scholar]
- 25.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Archives of Histology and Cytology. 2004;67(4):271–284. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 26.Battista N, Meccariello R, Cobellis G, et al. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Molecular and Cellular Endocrinology. 2012;355(1):1–14. doi: 10.1016/j.mce.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. The American Journal of Anatomy. 1956;99(3):507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 28.Dadoune J-P. Expression of mammalian spermatozoal nucleoproteins. Microscopy Research and Technique. 2003;61(1):56–75. doi: 10.1002/jemt.10317. [DOI] [PubMed] [Google Scholar]
- 29.Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Human Reproduction Update. 2007;13(3):313–327. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- 30.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010;139(2):287–301. doi: 10.1530/REP-09-0281. [DOI] [PubMed] [Google Scholar]
- 31.Gill-Sharma MK, Choudhuri J, D’Souza S. Sperm chromatin protamination: an endocrine perspective. Protein and Peptide Letters. 2011;18(8):786–801. doi: 10.2174/092986611795714005. [DOI] [PubMed] [Google Scholar]
- 32.Johnson GD, Lalancette C, Linnemann AK, Leduc F, Boissonneault G, Krawetz SA. The sperm nucleus: chromatin, RNA, and the nuclear matrix. Reproduction. 2011;141(1):21–36. doi: 10.1530/REP-10-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carreau S, Bouraima-Lelong H, Delalande C. Estrogens: new players in spermatogenesis. Reproductive Biology. 2011;11(3):174–193. doi: 10.1016/s1642-431x(12)60065-5. [DOI] [PubMed] [Google Scholar]
- 34.Carreau S, Bouraima-Lelong H, Delalande C. Estrogens in male germ cells. Spermatogenesis. 2011;1(2):90–94. doi: 10.4161/spmg.1.2.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carreau S, Bouraima-Lelong H, Delalande C. Estrogens, a female hormone concerned in spermatogenesis. Advances in Medical Sciences. 2012;57(1):31–36. doi: 10.2478/v10039-012-0005-y. [DOI] [PubMed] [Google Scholar]
- 36.Lucas TF, Pimenta MT, Pisolato R, Lazari MF, Porto CS. 17beta-estradiol signaling and regulation of Sertoli cell function. Spermatogenesis. 2011;1(4):318–324. doi: 10.4161/spmg.1.4.18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churikov D, Zalenskaya IA, Zalensky AO. Male germline-specific histones in mouse and man. Cytogenetic and Genome Research. 2004;105(2–4):203–214. doi: 10.1159/000078190. [DOI] [PubMed] [Google Scholar]
- 38.D’Occhio MJ, Hengstberger KJ, Johnston SD. Biology of sperm chromatin structure and relationship to male fertility and embryonic survival. Animal Reproduction Science. 2007;101(1-2):1–17. doi: 10.1016/j.anireprosci.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Gaucher J, Reynoird N, Montellier E, Boussouar F, Rousseaux S, Khochbin S. From meiosis to postmeiotic events: the secrets of histone disappearance. FEBS Journal. 2010;277(3):599–604. doi: 10.1111/j.1742-4658.2009.07504.x. [DOI] [PubMed] [Google Scholar]
- 40.Drabent B, Bode C, Doenecke D. Structure and expression of the mouse testicular H1 histone gene (H1t) Biochimica et Biophysica Acta. 1993;1216(2):311–313. doi: 10.1016/0167-4781(93)90162-7. [DOI] [PubMed] [Google Scholar]
- 41.Yan W, Ma L, Burns KH, Matzuk MM. HILS1 is a spermatid-specific linker histone H1-like protein implicated in chromatin remodeling during mammalian spermiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10546–10551. doi: 10.1073/pnas.1837812100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martianov I, Brancorsini S, Catena R, et al. Polar nuclear localization of H1T2, a histone H1 variant, required for spermatid elongation and DNA condensation during spermiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(8):2808–2813. doi: 10.1073/pnas.0406060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Lucia F, Faraone-Mennella MR, D’Erme M, Quesada P, Caiafa P, Farina B. Histone-induced condensation of rat testis chromatin: testis-specific H1t versus somatic H1 variants. Biochemical and Biophysical Research Communications. 1994;198(1):32–39. doi: 10.1006/bbrc.1994.1005. [DOI] [PubMed] [Google Scholar]
- 44.Meistrich ML. Histones and basic nuclear protein transitions in mammalian spermatogenesis. In: Hnilica LS, Stein GS, Stein JL, editors. Histones and Other Basic Nuclear Proteins. Boca Raton, Fla, USA: CRC Press; 1989. pp. 165–182. [Google Scholar]
- 45.Govin J, Caron C, Lestrat C, Rousseaux S, Khochbin S. The role of histones in chromatin remodelling during mammalian spermiogenesis. European Journal of Biochemistry. 2004;271(17):3459–3469. doi: 10.1111/j.1432-1033.2004.04266.x. [DOI] [PubMed] [Google Scholar]
- 46.Marcon L, Boissonneault G. Transient DNA strand breaks during mouse and human spermiogenesis: new insights in stage specificity and link to chromatin remodeling. Biology of Reproduction. 2004;70(4):910–918. doi: 10.1095/biolreprod.103.022541. [DOI] [PubMed] [Google Scholar]
- 47.Rousseaux S, Caron C, Govin J, Lestrat C, Faure A-K, Khochbin S. Establishment of male-specific epigenetic information. Gene. 2005;345(2):139–153. doi: 10.1016/j.gene.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Oliva R, Castillo J. Proteomics and the genetics of sperm chromatin condensation. Asian Journal of Andrology. 2011;13(1):24–30. doi: 10.1038/aja.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pittoggi C, Renzi L, Zaccagnini G, et al. A fraction of mouse sperm chromatin is organized in nucleosomal hypersensitive domains enriched in retroposon DNA. Journal of Cell Science. 1999;112(20):3537–3548. doi: 10.1242/jcs.112.20.3537. [DOI] [PubMed] [Google Scholar]
- 50.Brykczynska U, Hisano M, Erkek S, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nature Structural and Molecular Biology. 2010;17(6):679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- 51.Aoki F, Worrad DM, Schultz RM. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Developmental Biology. 1997;181(2):296–307. doi: 10.1006/dbio.1996.8466. [DOI] [PubMed] [Google Scholar]
- 52.Mali P, Kaipia A, Kangasniemi M, et al. Stage-specific expression of nucleoprotein mRNAs during rat and mouse spermiogenesis. Reproduction, Fertility and Development. 1989;1(4):369–382. doi: 10.1071/rd9890369. [DOI] [PubMed] [Google Scholar]
- 53.Kistler WS, Henriksén K, Mali P, Parvinen M. Sequential expression of nucleoproteins during rat spermiogenesis. Experimental Cell Research. 1996;225(2):374–381. doi: 10.1006/excr.1996.0188. [DOI] [PubMed] [Google Scholar]
- 54.Tseden K, Topaloglu Ö, Meinhardt A, et al. Premature translation of transition protein 2 mRNA causes sperm abnormalities and male infertility. Molecular Reproduction and Development. 2007;74(3):273–279. doi: 10.1002/mrd.20570. [DOI] [PubMed] [Google Scholar]
- 55.Martins RP, Charles Ostermeier G, Krawetz SA. Nuclear matrix interactions at the human protamine domain: a working model of potentiation. Journal of Biological Chemistry. 2004;279(50):51862–51868. doi: 10.1074/jbc.M409415200. [DOI] [PubMed] [Google Scholar]
- 56.Hogeveen KN, Sassone-Corsi P. Regulation of gene expression in post-meiotic male germ cells: CREM-signalling pathways and male fertility. Human Fertility. 2006;9(2):73–79. doi: 10.1080/14647270500463400. [DOI] [PubMed] [Google Scholar]
- 57.Foulkes NS, Mellstrom B, Benusiglio E, Sassone-Corsi P. Development switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- 58.Delmas V, Van der Hoorn F, Mellstrom B, Jegou B, Sassone-Corsi P. Induction of CREM activator proteins in spermatids: down-stream targets and implications for haploid germ cell differentiation. Molecular Endocrinology. 1993;7(11):1502–1514. doi: 10.1210/mend.7.11.8114765. [DOI] [PubMed] [Google Scholar]
- 59.Foulkes NS, Schlotter F, Pevet P, Sassone-Corsi P. Pituitary hormone FSH direct the CREM functional switch during spermatogenesis. Nature. 1993;362(6417):264–267. doi: 10.1038/362264a0. [DOI] [PubMed] [Google Scholar]
- 60.Omisanjo OA, Biermann K, Hartmann S, et al. DNMT1 and HDAC1 gene expression in impaired spermatogenesis and testicular cancer. Histochemistry and Cell Biology. 2007;127(2):175–181. doi: 10.1007/s00418-006-0234-x. [DOI] [PubMed] [Google Scholar]
- 61.Nantel F, Sassone-Corsi P. CREM: a transcriptional master switch during the spermatogenesis differentiation program. Frontiers in Bioscience. 1996;1:d266–269. doi: 10.2741/a131. [DOI] [PubMed] [Google Scholar]
- 62.Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schütz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380(6570):162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- 63.Sarg B, Chwatal S, Talasz H, Linder HH. Testis-specific linker histone H1t is multiply phosphorylated during spermatogenesis: identification of phosphorylation sites. Journal of Biological Chemistry. 2009;284(6):3610–3618. doi: 10.1074/jbc.M805925200. [DOI] [PubMed] [Google Scholar]
- 64.Kurtz K, Saperas N, Ausió J, Chiva M. Spermiogenic nuclear protein transitions and chromatin condensation. proposal for an ancestral model of nuclear spermiogenesis. Journal of Experimental Zoology B. 2009;312(3):149–163. doi: 10.1002/jez.b.21271. [DOI] [PubMed] [Google Scholar]
- 65.Meistrich ML, Trostle-Weige PK, Lin R, Bhatnagar YM, Allis CD. Highly acetylated H4 is associated with histone displacement in rat spermatids. Molecular Reproduction and Development. 1992;31(3):170–181. doi: 10.1002/mrd.1080310303. [DOI] [PubMed] [Google Scholar]
- 66.Hazzouri M, Pivot-Pajot C, Faure A-K, et al. Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histone-deacetylases. European Journal of Cell Biology. 2000;79(12):950–960. doi: 10.1078/0171-9335-00123. [DOI] [PubMed] [Google Scholar]
- 67.Sassone-Corsi P. Transcription factors governing male fertility. Andrologia. 2005;37(6):228–229. doi: 10.1111/j.1439-0272.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 68.Singh J, Rao MRS. Interaction of rat testis protein, TP, with nucleosome core particle. Biochemistry International. 1988;17(4):701–710. [PubMed] [Google Scholar]
- 69.Kundu TK. DNA condensation by the rat spermatidal protein TP2 shows GC-rich sequence preference and is zinc dependent. Biochemistry. 1995;34(15):5143–5150. doi: 10.1021/bi00015a027. [DOI] [PubMed] [Google Scholar]
- 70.Meetei AR, Ullas KS, Vasupradha V, Rao MRS. Involvement of protein kinase A in the phosphorylation of spermatidal protein TP2 and its effect on DNA condensation. Biochemistry. 2002;41(1):185–195. doi: 10.1021/bi0117652. [DOI] [PubMed] [Google Scholar]
- 71.Caron N, Veilleux S, Boissonneault G. Stimulation of DNA repair by the spermatidal TP1 protein. Molecular Reproduction and Development. 2001;58(4):437–443. doi: 10.1002/1098-2795(20010401)58:4<437::AID-MRD12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 72.Sakkas D, Manicardi G, Bianchi PG, Bizzaro D, Bianchi U. Relationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoa. Biology of Reproduction. 1995;52(5):1149–1155. doi: 10.1095/biolreprod52.5.1149. [DOI] [PubMed] [Google Scholar]
- 73.Oliva R, Dixon GH. Vertebrate protamine genes and the histone-to-protamine replacement reaction. Progress in Nucleic Acid Research and Molecular Biology. 1991;40:25–94. doi: 10.1016/s0079-6603(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 74.Smith A, Haaf T. DNA nicks and increased sensitivity of DNA to fluorescence in situ end labeling during functional spermiogenesis. BioTechniques. 1998;25(3):496–502. doi: 10.2144/98253rr05. [DOI] [PubMed] [Google Scholar]
- 75.Yu YE, Zhang Y, Unni E, et al. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4683–4688. doi: 10.1073/pnas.97.9.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao M, Shirley CR, Hayashi S, et al. Transition nuclear proteins are required for normal chromatin condensation and functional sperm development. Genesis. 2004;38(4):200–213. doi: 10.1002/gene.20019. [DOI] [PubMed] [Google Scholar]
- 77.Ausió J, Eirín-López JM, Frehlick LJ. Evolution of vertebrate chromosomal sperm proteins: implications for fertility and sperm competition. Society of Reproduction and Fertility supplement. 2007;65:63–79. [PubMed] [Google Scholar]
- 78.Oliva R. Protamines and male infertility. Human Reproduction Update. 2006;12(4):417–435. doi: 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 79.Helene C, Toulme J-J, Behmoaras T, Cazenave C. Mechanisms for the recognition of chemically-modified DNA by peptides and proteins. Biochimie. 1982;64(8-9):697–705. doi: 10.1016/s0300-9084(82)80113-2. [DOI] [PubMed] [Google Scholar]
- 80.Balhorn R, Corzett M, Mazrimas JA. Formation of intraprotamine disulfides in vitro. Archives of Biochemistry and Biophysics. 1992;296(2):384–393. doi: 10.1016/0003-9861(92)90588-n. [DOI] [PubMed] [Google Scholar]
- 81.Seligman J, Zipser Y, Kosower NS. Tyrosine phosphorylation, thiol status, and protein tyrosine phosphatase in rat epididymal spermatozoa. Biology of Reproduction. 2004;71(3):1009–1015. doi: 10.1095/biolreprod.104.028035. [DOI] [PubMed] [Google Scholar]
- 82.Bench GS, Friz AM, Corzett MH, Morse DH, Balhorn R. DNA and total protamine masses in individual sperm from fertile mammalian subjects. Cytometry. 1996;23(4):263–271. doi: 10.1002/(SICI)1097-0320(19960401)23:4<263::AID-CYTO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 83.Steger K, Fink L, Failing K, et al. Decreased protamine-1 transcript levels in testes from infertile men. Molecular Human Reproduction. 2003;9(5-6):331–336. doi: 10.1093/molehr/gag041. [DOI] [PubMed] [Google Scholar]
- 84.Aoki VW, Emery BR, Liu L, Carrell DT. Protamine levels vary between individual sperm cells of infertile human males and correlate with viability and DNA integrity. Journal of Andrology. 2006;27(6):890–898. doi: 10.2164/jandrol.106.000703. [DOI] [PubMed] [Google Scholar]
- 85.Chioccarelli T, Cacciola G, Altucci L, et al. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology. 2010;151(10):5017–5029. doi: 10.1210/en.2010-0133. [DOI] [PubMed] [Google Scholar]
- 86.Lewis SEM, Agbaje IM. Using the alkaline comet assay in prognostic tests for male infertility and assisted reproductive technology outcomes. Mutagenesis. 2008;23(3):163–170. doi: 10.1093/mutage/gem052. [DOI] [PubMed] [Google Scholar]
- 87.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Human Reproduction Update. 2003;9(4):331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 88.Aoki VW, Liu L, Carrell DT. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Human Reproduction. 2005;20(5):1298–1306. doi: 10.1093/humrep/deh798. [DOI] [PubMed] [Google Scholar]
- 89.Aoki VW, Moskovtsev SI, Willis J, Liu L, Mullen JBM, Carrell DT. DNA integrity is compromised in protamine-deficient human sperm. Journal of Andrology. 2005;26(6):741–748. doi: 10.2164/jandrol.05063. [DOI] [PubMed] [Google Scholar]
- 90.Chimento A, Sirianni R, Delalande C, et al. 17β-Estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ERα . Molecular and Cellular Endocrinology. 2010;320(1-2):136–144. doi: 10.1016/j.mce.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 91.Robertson KM, O’Donnell L, Jones MEE, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolff E, Ginglinger A. Sur la transformation des Poulets males en intersexues par injection d'hormone femelle (folliculine) aux embryons. Archives Anatomie, Histologie et Embryologie. 1935;20:219–278. [Google Scholar]
- 93.Staub C, Rauch M, Ferrière F, et al. Expression of estrogen receptor ESR1 and its 46-kDa variant in the gubernaculum testis. Biology of Reproduction. 2005;73(4):703–712. doi: 10.1095/biolreprod.105.042796. [DOI] [PubMed] [Google Scholar]
- 94.Chieffi P, Minucci S, Cobellis G, Fasano S, Pierantoni R. Changes in proto-oncogene activity in the testis of the frog, Rana esculenta, during the annual reproductive cycle. General and Comparative Endocrinology. 1995;99(2):127–136. doi: 10.1006/gcen.1995.1093. [DOI] [PubMed] [Google Scholar]
- 95.Cobellis G, Pierantoni R, Fasano S. c-fos- and c-jun-like mRNA expression in frog (Rana esculenta) testis during the annual reproductive cycle. General and Comparative Endocrinology. 1997;106(1):23–29. doi: 10.1006/gcen.1996.6846. [DOI] [PubMed] [Google Scholar]
- 96.Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997;138(3):1289–1298. doi: 10.1210/endo.138.3.5021. [DOI] [PubMed] [Google Scholar]
- 97.Miura T, Miura C, Ohta T, Nader MR, Todo T, Yamauchi K. Estradiol-17β stimulates the renewal of spermatogonial stem cells in males. Biochemical and Biophysical Research Communications. 1999;264(1):230–234. doi: 10.1006/bbrc.1999.1494. [DOI] [PubMed] [Google Scholar]
- 98.Cobellis G, Lombardi M, Scarpa D, et al. Fra1 activity in the frog, Rana esculenta, testis: a new potential role in sperm transport. Biology of Reproduction. 2005;72(5):1101–1108. doi: 10.1095/biolreprod.104.036541. [DOI] [PubMed] [Google Scholar]
- 99.Cobellis G, Cacciola G, Chioccarelli T, et al. Estrogen regulation of the male reproductive tract in the frog, Rana esculenta: a role in Fra-1 activation in peritubular myoid cells and in sperm release. General and Comparative Endocrinology. 2008;155(3):838–846. doi: 10.1016/j.ygcen.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Tsutsumi I, Toppari J, Campeau JD, DiZerega GS. Reduction of fertility in the male rat by systemic treatment with follicle regulatory protein. Fertility and Sterility. 1987;47(4):689–695. doi: 10.1016/s0015-0282(16)59123-7. [DOI] [PubMed] [Google Scholar]
- 101.Tsutsumi I, Fugimori K, Nakamura RM. Disruption of seminiferous epithelial function in the rat by ovarian protein. Biology of Reproduction. 1987;36(2):451–461. doi: 10.1095/biolreprod36.2.451. [DOI] [PubMed] [Google Scholar]
- 102.Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar AS, Moudgal NR. Effect of long-term treatment with aromatase inhibitor on testicular function of adult male bonnet monkeys (M. radiata) Steroids. 1998;63(7-8):414–420. doi: 10.1016/s0039-128x(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 103.Nitta H, Bunick D, Hess RA, et al. Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993;132(3):1396–1401. doi: 10.1210/endo.132.3.8440194. [DOI] [PubMed] [Google Scholar]
- 104.Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome p450 aromatase in mature rat testicular cells. Biology of Reproduction. 1998;58(4):919–926. doi: 10.1095/biolreprod58.4.919. [DOI] [PubMed] [Google Scholar]
- 105.Robertson KM, Simpson ER, Lacham-Kaplan O, Jones MEE. Characterization of the fertility of male aromatase knockout mice. Journal of Andrology. 2001;22(5):825–830. [PubMed] [Google Scholar]
- 106.Robertson KM, O’Donnell L, Simpson ER, Jones MEE. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology. 2002;143(8):2913–2921. doi: 10.1210/endo.143.8.8957. [DOI] [PubMed] [Google Scholar]
- 107.Gill-Sharma MK, D’Souza S, Padwal V, et al. Antifertility effects of estradiol in adult male rats. Journal of Endocrinological Investigation. 2001;24(8):598–607. doi: 10.1007/BF03343900. [DOI] [PubMed] [Google Scholar]
- 108.D’Souza R, Gill-Sharma MK, Pathak S, Kedia N, Kumar R, Balasinor N. Effect of high intratesticular estrogen on the seminiferous epithelium in adult male rats. Molecular and Cellular Endocrinology. 2005;241(1-2):41–48. doi: 10.1016/j.mce.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 109.Aleem M, Padwal V, Choudhari J, Balasinor N, Parte P, Gill-Sharma MK. Estradiol affects androgen-binding protein expression and fertilizing ability of spermatozoa in adult male rats. Molecular and Cellular Endocrinoogy. 2006;253(1-2):1–13. doi: 10.1016/j.mce.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 110.Chimento A, Sirianni R, Zolea F, et al. Gper and ESRs are expressed in rat round spermatids and mediate oestrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax. International Journal of Andrology. 2011;34(5):420–429. doi: 10.1111/j.1365-2605.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- 111.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philosophical Transactions of the Royal Society B. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ebling FJP, Brooks AN, Cronin AS, Ford H, Kerr JB. Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology. 2000;141(8):2861–2869. doi: 10.1210/endo.141.8.7596. [DOI] [PubMed] [Google Scholar]
- 113.Baines H, Nwagwu MO, Hastie GR, Wiles RA, Mayhew TM, Ebling FJP. Effects of estradiol and FSH on maturation of the testis in the hypogonadal (hpg) mouse. Reproductive Biology and Endocrinology. 2008;6, article 4 doi: 10.1186/1477-7827-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Allan CM, Couse JF, Simanainen U, et al. Estradiol induction of spermatogenesis is mediated via an estrogen receptor-α mechanism involving neuroendocrine activation of follicle-stimulating hormone secretion. Endocrinology. 2010;151(6):2800–2810. doi: 10.1210/en.2009-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Singh J, O’Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136(12):5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- 116.Pak TR, Chung WCJ, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5α-androstane-3β, 17β-diol, is a potent modulator of estrogen receptor-β1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146(1):147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- 117.Krishnamurthy H, Danilovich N, Morales CR, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle- stimulating hormone receptor knockout (FORKO) mouse. Biology of Reproduction. 2000;62(5):1146–1159. doi: 10.1095/biolreprod62.5.1146. [DOI] [PubMed] [Google Scholar]
- 118.Javeshghani D, Touyz RM, Sairam MR, Schiffrin EL. Gender differences in blood pressure in estrogen-deficient FORKO mice. The American Journal of Hypertension. 2002;15(S3):152A–153A. [Google Scholar]
- 119.Xing W, Krishnamurthy H, Sairam MR. Role of follitropin receptor signaling in nuclear protein transitions and chromatin condensation during spermatogenesis. Biochemical and Biophysical Research Communications. 2003;312(3):697–701. doi: 10.1016/j.bbrc.2003.10.177. [DOI] [PubMed] [Google Scholar]
- 120.Wenger T, Ledent C, Csernus V, Gerendai I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochemical and Biophysical Research Communications. 2001;284(2):363–368. doi: 10.1006/bbrc.2001.4977. [DOI] [PubMed] [Google Scholar]
- 121.Cacciola G, Chioccarelli T, Altucci L, et al. Low 17beta-estradiol levels in Cnr1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biology of Reproduction. 2013;88(6):1–12. doi: 10.1095/biolreprod.112.105726. [DOI] [PubMed] [Google Scholar]
- 122.Cacciola G, Chioccarelli T, Mackie K, et al. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: possible involvement in adult leydig cell differentiation. Biology of Reproduction. 2008;79(4):758–765. doi: 10.1095/biolreprod.108.070128. [DOI] [PubMed] [Google Scholar]
- 123.Cacciola G, Chioccarelli T, Viggiano A, Fasano S, Pierantoni R, Cobellis G. Nuclear size as estrogen-responsive chromatin quality parameter of mouse spermatozoa. General and Comparative Endocrinology. 2013;193:201–209. doi: 10.1016/j.ygcen.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 124.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 125.Mechoulam R, Ben-Shabat S, Hanuš L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 126.Sugiura T, Kondo S, Sukagawa A, et al. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis: involvement of Ca2+-dependent transacylase and phosphodiesterase activities. Biochemical and Biophysical Research Communications. 1996;218(1):113–117. doi: 10.1006/bbrc.1996.0020. [DOI] [PubMed] [Google Scholar]
- 127.Schuel H, Burkman LJ, Lippes J, et al. N-Acylethanolamines in human reproductive fluids. Chemistry and Physics of Lipids. 2002;121(1-2):211–227. doi: 10.1016/s0009-3084(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 128.Habayeb OMH, Taylor AH, Evans MD, et al. Plasma levels of the endocannabinoid anandamide in women: a potential role in pregnancy maintenance and labor? Journal of Clinical Endocrinology and Metabolism. 2004;89(11):5482–5487. doi: 10.1210/jc.2004-0681. [DOI] [PubMed] [Google Scholar]
- 129.Cobellis G, Cacciola G, Scarpa D, et al. Endocannabinoid system in frog and rodent testis: type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biology of Reproduction. 2006;75(1):82–89. doi: 10.1095/biolreprod.106.051730. [DOI] [PubMed] [Google Scholar]
- 130.Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chemistry and Biology. 2007;14(7):741–756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 131.Maccarrone M, Cecconi S, Rossi G, Battista N, Pauselli R, Finazzi-Agrò A. Anandamide activity and degradation are regulated by early postnatal aging and follicle-stimulating hormone in mouse Sertoli cells. Endocrinology. 2003;144(1):20–28. doi: 10.1210/en.2002-220544. [DOI] [PubMed] [Google Scholar]
- 132.Maccarrone M, Barboni B, Paradisi A, et al. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. Journal of Cell Science. 2005;118(19):4393–4404. doi: 10.1242/jcs.02536. [DOI] [PubMed] [Google Scholar]
- 133.Chianese R, Cobellis G, Pierantoni R, Fasano S, Meccariello R. Non-mammalian vertebrate models and the endocannabinoid system: relationships with gonadotropin-releasing hormone. Molecular and Cellular Endocrinology. 2008;286(1-2):S46–S51. doi: 10.1016/j.mce.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 134.Cacciola G, Chioccarelli T, Ricci G, et al. The endocannabinoid system in vertebrate male reproduction: a comparative overview. Molecular and Cellular Endocrinology. 2008;286(1-2):S24–S30. doi: 10.1016/j.mce.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 135.Sun X, Wang H, Okabe M, et al. Genetic loss of Faah compromises male fertility in mice. Biology of Reproduction. 2009;80(2):235–242. doi: 10.1095/biolreprod.108.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Francavilla F, Battista N, Barbonetti A, et al. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology. 2009;150(10):4692–4700. doi: 10.1210/en.2009-0057. [DOI] [PubMed] [Google Scholar]
- 137.Lewis SE, Maccarrone M. Endocannabinoids, sperm biology and human fertility. Pharmacological Research. 2009;60(2):126–131. doi: 10.1016/j.phrs.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 138.Meccariello R, Chianese R, Cacciola G, Cobellis G, Pierantoni R, Fasano S. Type-1 cannabinoid receptor expression in the frog, Rana esculenta, tissues: a possible involvement in the regulation of testicular activity. Molecular Reproduction and Development. 2006;73(5):551–558. doi: 10.1002/mrd.20434. [DOI] [PubMed] [Google Scholar]
- 139.M Lewis SE, Paro R, Borriello L, et al. Long-term use of HU210 adversely affects spermatogenesis in rats by modulating the endocannabinoid system. International Journal of Andrology. 2012;35(5):731–740. doi: 10.1111/j.1365-2605.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- 140.Lazzarin N, Valensise H, Bari M, et al. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecological Endocrinology. 2004;18(4):212–218. doi: 10.1080/09513590410001692492. [DOI] [PubMed] [Google Scholar]
- 141.Maccarrone M, Fride E, Bisogno T, et al. Up-regulation of the endocannabinoid system in the uterus of leptin knockout (ob/ob) mice and implications for fertility. Molecular Human Reproduction. 2005;11(1):21–28. doi: 10.1093/molehr/gah130. [DOI] [PubMed] [Google Scholar]
- 142.Wang H, Xie H, Sun X, et al. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins and Other Lipid Mediators. 2007;83(1-2):62–74. doi: 10.1016/j.prostaglandins.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Acone G, Trabucco E, Colacurci N, et al. Low type I cannabinoid receptor levels characterize placental villous in labouring delivery. Placenta. 2009;30(2):203–205. doi: 10.1016/j.placenta.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 144.Trabucco E, Acone G, Marenna A, et al. Endocannabinoid system in first trimester placenta: low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta. 2009;30(6):516–522. doi: 10.1016/j.placenta.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 145.Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chemical Neuroscience. 2012;3(5):349–355. doi: 10.1021/cn300014e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pertwee RG, Joe-Adigwe G, Hawksworth GM. Further evidence for the presence of cannabinoid CB1 receptors in mouse vas deferens. European Journal of Pharmacology. 1996;296(2):169–172. doi: 10.1016/0014-2999(95)00790-3. [DOI] [PubMed] [Google Scholar]
- 147.Grimaldi P, Orlando P, Di Siena S, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11131–11136. doi: 10.1073/pnas.0812789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Human Reproduction Update. 2011;17(3):347–361. doi: 10.1093/humupd/dmq058.dmq058 [DOI] [PubMed] [Google Scholar]
- 149.Gye MC, Kang HH, Kang HJ. Expression of cannabinoid receptor 1 in mouse testes. Archives of Andrology. 2005;51(3):247–255. doi: 10.1080/014850190898845. [DOI] [PubMed] [Google Scholar]
- 150.Rossato M, Popa FI, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. Journal of Clinical Endocrinology and Metabolism. 2005;90(2):984–991. doi: 10.1210/jc.2004-1287. [DOI] [PubMed] [Google Scholar]
- 151.Barbonetti A, Vassallo MRC, Fortunato D, Francavilla S, Maccarrone M, Francavilla F. Energetic metabolism and human sperm motility: impact of CB1 receptor activation. Endocrinology. 2010;151(12):5882–5892. doi: 10.1210/en.2010-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barboni B, Bernabò N, Palestini P, et al. Type-1 Cannabinoid receptors reduce membrane fluidity of capacitated boar sperm by impairing their activation by bicarbonate. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023038.e23038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Catanzaro G, Battista N, Rossi G, et al. Effect of capacitation on the endocannabinoid system of mouse sperm. Molecular and Cellular Endocrinology. 2011;343(1-2):88–92. doi: 10.1016/j.mce.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 154.Bernabò N, Palestini P, Chiarini M, Maccarrone M, Mattioli M, Barboni B. Endocannabinoid-binding CB1 and TRPV1 receptors as modulators of sperm capacitation. Communicative and Integrative Biology. 2012;5(1):68–70. doi: 10.4161/cib.18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pierantoni R, Cobellis G, Meccariello R, et al. CB1 activity in male reproduction: mammalian and nonmammalian animal models. Vitamins and Hormones. 2009;81:367–387. doi: 10.1016/S0083-6729(09)81014-5. [DOI] [PubMed] [Google Scholar]
- 156.Fasano S, Meccariello R, Cobellis G, et al. The endocannabinoid system: an ancient signaling involved in the control of male fertility. Annals of the New York Academy of Sciences. 2009;1163(1):112–124. doi: 10.1111/j.1749-6632.2009.04437.x. [DOI] [PubMed] [Google Scholar]
- 157.Chianese R, Ciaramella V, Scarpa D, Fasano S, Pierantoni R, Meccariello R. Anandamide regulates the expression of GnRH1, GnRH2, and GnRH-Rs in frog testis. The American Journa Physioly Endocrinology and Metabolism. 2012;303(4):475–487. doi: 10.1152/ajpendo.00086.2012. [DOI] [PubMed] [Google Scholar]
- 158.Scorticati C, Fernández-Solari J, De Laurentiis A, et al. The inhibitory effect of anandamide on luteinizing hormonereleasing hormone secretion is reversed by estrogen. Proceedings of the National Academy of Sciences. 2004;101(32):11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grimaldi P, Pucci M, Di Siena S, et al. The faah gene is the first direct target of E2 in the testis: role of histone demethylase LSD1. Cellullar Molecular Life Science. 2012;69(24):4177–4190. doi: 10.1007/s00018-012-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meeker JD, Singh NP, Hauser R. Serum concentrations of estradiol and free T4 are inversely correlated with sperm DNA damage in men from an infertility clinic. Journal of Andrology. 2008;29(4):379–388. doi: 10.2164/jandrol.107.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hamden K, Silandre D, Delalande C, Elfeki A, Carreau S. Protective effects of estrogens and caloric restriction during aging on various rat testis parameters. Asian Journal of Andrology. 2008;10(6):837–845. doi: 10.1111/j.1745-7262.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- 162.Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. Journal of Andrology. 2002;23(1):1–8. doi: 10.1002/j.1939-4640.2002.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 163.Oliveira JB, Massaro FC, Baruffi RL, et al. Correlation between semen analysis by motile sperm organelle morphology examination and sperm DNA damage. Fertility and Sterility. 2010;94(5):1937–1940. doi: 10.1016/j.fertnstert.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 164.Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. Journal of Endocrinology. 2010;205(2):117–131. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- 165.Jones R. Sperm survival versus degradation in the mammalian epididymis: an hypothesis. Biology of Reproduction. 2004;71(5):1405–1411. doi: 10.1095/biolreprod.104.031252. [DOI] [PubMed] [Google Scholar]


