Abstract
This review documents chemical structures and antifungal activities of 68 compounds isolated from 22 Piper species of the plant family Piperaceae. These compounds include amides, flavonoids, prenylated benzoic acid derivatives, lignans, phenylpropanoids, butenolides, and cyclopentendiones. Some of them may serve as leads for potential pharmaceutical or agricultural fungicide development.
Keywords: Antifungal, Piper, Piperaceae, amide, flavonoid, prenylated benzoic acid, lignan, phenylpropanoid, butenolide, cyclopentendione
INTRODUCTION
Piper is a big genus of the plant family Piperaceae, with more than 700 species widely distributed in the tropical and subtropical regions of the world. Some species are used in folk medicine as analgesics, antiseptics, insecticides, and antimicrobials or for the treatment of toothache, haemorrhoids, dysmenorrhea, and wound [1–4]. Extensive chemical studies on Piper species have resulted in the isolation of a large number of structurally diverse compounds. A previous review by Parmar V et al. has covered about 600 compounds isolated from this genus for the period of 1907 to 1996 [1]. In recent years, a number of compounds from Piper species have been reported to possess significant antifungal activities. Thus, this review attempts to document chemical structures and antifungal activities of these compounds, with the aim of providing useful information for potential pharmaceutical or agricultural fungicide development. The antifungal compounds are classified into amides, flavonoids, prenylated benzoic acid derivatives, lignans, phenylpropanoids, butenolides, and cyclopentendiones. The trivial names of the compounds that are available in the literature have been provided.
AMIDES
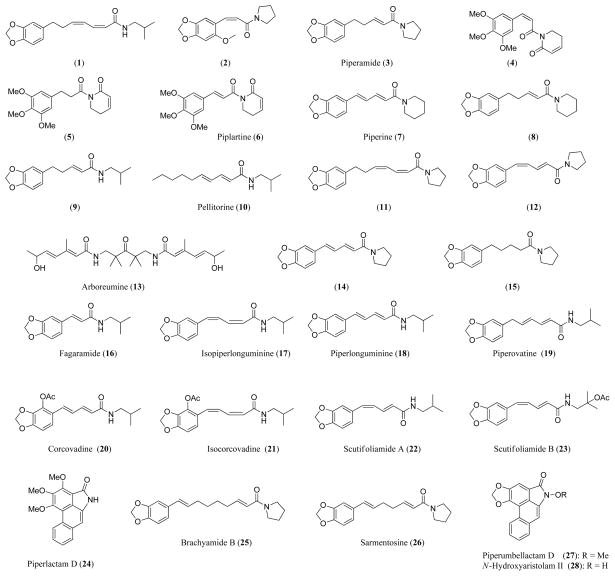
Amides 1–3 and 4–10 were isolated from the stems of P. hispidum and the seeds of P. tuberculatum, respectively, and tested for antifungal activity against Cladosporium sphaerospermum by the TLC bioautography method. The minimum amount required (MAR) for inhibition of compounds 1–10 are 5.0, 5.0, 5.0, 5.0, 0.1, 5.0, 1.0, 5.0, 5.0, and 5.0 μg, respectively [5]. Compound 5 is 5-fold more potent than the positive controls nystatin and miconazole, both of which show an MAR of 0.5 μg. The potent antifungal activity of this compound may be attributed to the α,β-unsaturated piperidinone moiety. It is also interesting to note that analogous compounds 4 and 6 (piplarine) bearing a double bond (cis or trans) between the carbonyl group and the aromatic ring show significantly reduced activity by 50-fold, which may be caused by the decreased electrophilicity of the α,β-unsaturated piperidinone moiety.
N-[7-(3′,4′-methylenedioxyphenyl)-2(Z),4(Z)-heptadienoyl]pyrrolidine (11) was isolated from a methylene chloride extract of the leaves of P. hispidum by antifungal activity-guided fractionation [6]. The structural difference between compounds 11 and 1 lies in the amide moiety, with the latter possessing an isobutyl group. Compound 11 showed an antifungal activity against C. sphaerospermum that is one eighth of miconazole, similar to compound 1 that is one fifth of miconazole [5].
Amides 12–15 isolated from the Brazilian plant P. arboretum showed antifungal activity against C. sphaerospermum with MARs of 10, 5.0, 0.1, and 5.0 μg, respectively, while amide 16 from P. tuberculatum exhibited an MAR of 10 μg against C. cladosporioides [7]. The potent antifungal activity of compound 14 indicates that 5-(3′,4′-methylenedioxyphenyl)-2(E),4(E)-pentadienoyl moiety is important. However, amide 7 that has the same acyl moiety but with a piperidinyl moiety is 10-fold less active (MAR, 1.0 μg), indicating that the pyrrolidinyl moiety in compound 14 also plays a key role for antifungal activity.
Amides 17–24 isolated from the leaves of P. scutifolium showed varying degrees of antifungal activities against C. sphaerospermum and C. cladosporioides [8], providing further information on the structure-activity relationship (SAR) of this class of compounds. Among the eight compounds, isopiperlonguminine (17) is the most potent, with an MAR of 0.25 μg. Piperlonguminine (18), piperovatine (19), and corcovadine (20) are equipotent with the positive controls nystatin and miconazole (MARs, 1.0 μg). It appears that the geometric configurations of the double bonds in these compounds are associated with antifungal activity. The aromatic lactam piperolactum (24) is the least active.
Several amides were isolated from the roots of P. sarmentosum and tested for antifungal activity against Candida albicans using a method modified from the soluble formazan assay. Only brachyamide B (25) and sarmentosine (26) showed marginal antifungal activity with 50% growth inhibitory concentration (IC50) of 41.82 and 32.82 μg/ml, respectively [2]. The positive control amphotericin B gave an IC50 of 0.01μg/ml.
The aristolactams piperumbellactam D (27) and N-hydroxyaristolam II (28) isolated from the branches of P. rumbellatum showed antifungal activity against Aspergillus flavus, Trichophyton longifusus, Microsporum canis, Fusarium solani, C. albicans, and C. glabrata using the agar tube dilution method. They exhibited higher antifungal activity than amphotericin B against A. flavus and T. longifusus in terms of inhibition rates at fixed sample concentrations [3].
FLAVONOIDS
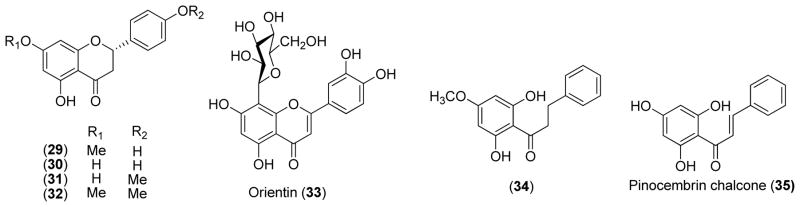
In a bioassay-guided isolation study, the flavanones sakuranetin (29) and naringenin (30) were isolated from the leaves of P. crassinervium and tested for antifungal activity using the TLC bioautography method. Compound 29 gave an MAR of 1.0 μg against both C. sphaerospermum and C. cladosporioides, the same MAR generated by the positive control nystantin, while Compound 30 afforded MAR of 1.0 and 5.0 μg, respectively [9]. Compound 29 was also isolated as an antifungal constituent from P. lhotzkyanum [10] and P. marginatum [11]. The analog 5,7-dihydroxy-4′-methoxyflavanone (31) isolated from P. marginatum [11] also demonstrated the same antifungal activity as sakuranetin against the aforementioned two fungal pathogens. When both 7,4′-dihydoxy groups in compound 30 are methylated, the resultant compound (7,4′-dimethoxy-5-hydroxy-flavanone, 32) showed decreased antifungal activity by 25-fold [11].
From the Brazilian plant P. solmsianu, orientin (33), a favone C-glucoside, was isolated and showed good antifungal activity against M. canis, M. gypseum, T. mentagrophytes, T. rubrum, and Epidermophyton flocosum in an agar dilution assay with minimal inhibitory concentration (MICs) of 7, 9, 8, 8, and 9 μg/ml, comparable to the positive control ketoconazole with MICs of 8, 6, 8, 3, and >15 μg/ml [12], respectively.
Bioassay-guided fractionation of P. mollicomum led to the isolation of dihydrochalcone (34) that showed moderate antifungal activity against C. sphaerospermum and C. cladosporioides with MARs of 5 amd 10 μg, respectively [10]. Pinocembrin chalcone (35) gave an MIC of 100 μg/ml against C. albicans using a broth dilution method [13]
PRENYLATED BENZOIC ACID DERIVATIVES
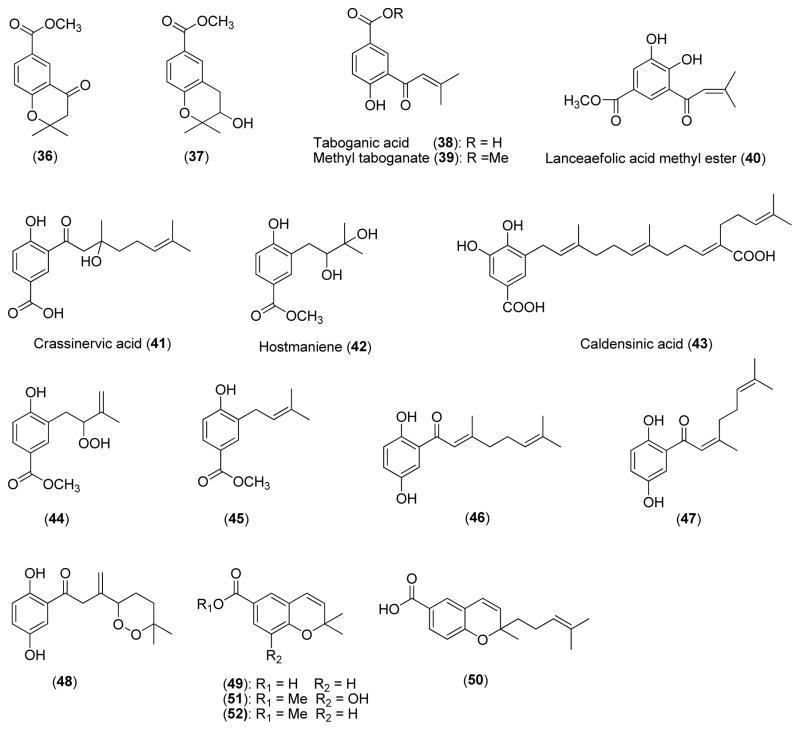
This class of compounds is derived from benzoic acid (from shikimate pathways) or decarboxylated phenol coupled with an isoprenyl, geranyl, or geranylfarnesyl unit (from mevalonate pathways). Four prenylated benzoic acid derivatives (36–39) were isolated from P. dilatatum collected in Panama and showed antifungal activity against the plant pathogenic fungus Cladosporium cucumerinum with MARs of 1.0, 3.0, 5.0, and 5.0 μg, respectively, by the TLC bioautography method [14]. The positive controls miconazole and propiconazole gave MARs of 1.0 and 0.1 μg, respectively. When tested using a dilution method, the MICs of 36 and 39 (methyl taboganate) were 40 and 60 μg/ml, respectively. Compound 38 (taboganic acid) was not active in the dilution assay. The MICs obtained for miconazole and propiconazole were 10 and 1 μg/ml, respectively. This study provides comparative data between the two assay methods.
In another bioassay-guided fractionation of P. lanceaefolium, the prenylated benzoic acid derivative 40 (lanceaefolic acid methyl ester) showed an MIC of 100 μg/ml against C. albicans using a broth dilution method [13].
The extracts of the leaves of P. crassinervium and P. hostmannianum from Brazil showed high growth inhibitory activity against C. sphaerospermum and C. cladosporioides. Under bioassay-guided fractionation, two potent antifungal benzoic acid derivatives crassinervic acid (41) and hostmaniene (42) were isolated from P. crassinervium and P. hostmannianum, respectively. Compounds 41 and 42 are equipotent, giving the same MAR of 0.5 μg against C. cladosporioides and C. sphaerospermum [15].
Caldensinic acid (43) was isolated from the leaves of P. caldense by bioassay-guided fractionation. Using a direct TLC bioautography assay to evaluate antifungal activity, it gave MARs of 5.0 μg and 25.0 μg against C. sphaerospermum and C. cladosporioides, respectively [16].
Bioassay-guided fractionation of the extract from the leaves of Brazilian P. hostmannianum and P. aduncum led to the isolation of two antifungal prenylated methyl benzoates (44 and 45). Compounds 44 and 45 demonstrated equipotent antifungal activity in a bioautography assay, giving the same MAR of 1.0 μg against C. cladosporioides and C. sphaerospermum [4].
Compounds 46–48 were isolated from the leaves of P. crassinervium by bioassay-guided fractionation. Compound 46 with C-2′ trans-double bond in the monoterpenoid side chain showed antifungal activity equipotent to nystatin and miconazole against C. sphaerospermum and C. cladosporioides (MARs, all 1.0 μg), while compound 47 with C-2′ cis-double bond in the side chain showed decreased antifungal activity by 5 to 10-fold. Compound 48 with a peroxy group in the side chain is equipotent to 47 [17].
Compounds 49 and 50 isolated from the leaves of P. lhotzkyanum showed equipotent antifungal activity with a MAR of 5.0 μg against both C. sphaerospermum and C. cladosporioides. Two analogs 51 and 52 with methylation on the carboxyl group showed decreased antifungal activity (by 2-fold and 5-fold, respectively) [10].
LIGNANS
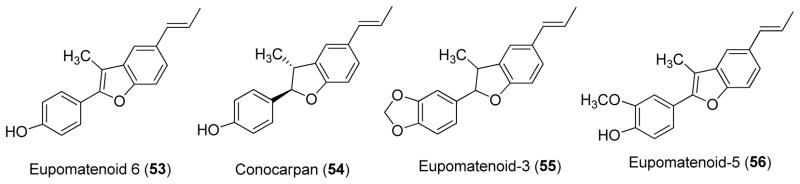
Bioassay-guided fractionation of the methylene chloride extract from the leaves of P. fulvescens using an agar overlay bioautography method afforded two neolignans, eupomatenoid-6 (53) and conocarpan (54). The MICs of 53 and 54 were determined by a broth dilution method. Compound 54 demonstrated antifungal activity against all the species tested, namely C. albicans, Cryptococcus neoformans, M. gypseum, Saccharomyces cerevisiae, and T. mentagrophytes with MICs of 8, 16, 16, 4, and 8 μg/ml, respectively, compared against the positive control amphotericin B with MICs of 0.0625, 0.125, 0.25, 0.0625, and 0.125 μg/ml, respectively. Compound 53 was only active against M. gypseum, S. cerevisiae, T. mentagrophytes with MICs of 0.5, 16, and 1 μg/ml, respectively [18]. Compounds 53 and 54 were also isolated as antifungal constituents from P. abutiloides [19].
The methanol extract of the leaves of P. regnellii showed strong activity against the dermatophyte fungi T. mentagrophytes, T. rubrum, M. canis and M. gypseum. Bioassay-guided fractionation led to the isolation of two antifungal neolignans, eupomatenoid-3 (55) and eupomatenoid-5 (56). Compounds 55 and 56 exhibited antifungal activity against T. rubrum with MICs of 50.0 and 6.2 μg/ml, respectively [20].
PHENYLPROPANOIDS
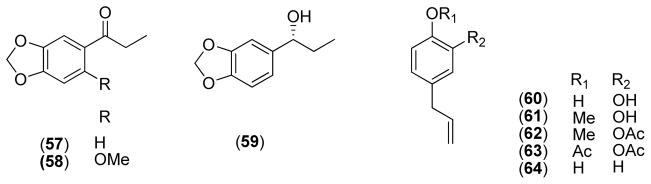
Three simple phenyl propanoids (57–59) were isolated from the leaves of P. marginatum by means of bioassay-guided fractionation and exhibited moderate antifungal activity against C. sphaerospermum with MARs of 5.0, 5.0, and 10.0 μg, respectively, and C. cladosporioides with MARs of 5.0, 5.0, 10.0 μg, respectively [11].
Five phenylpropenes (60–64) isolated the leaves of P. betle showed varying degrees of antifungal activities against C. cucumerinum with MARs of 1, 3, 3, 10, and 30 μg, respectively. The relative potencies of these compounds indicate that substitution patterns play a significant role for antifungal activity [21].
BUTENOLIDES
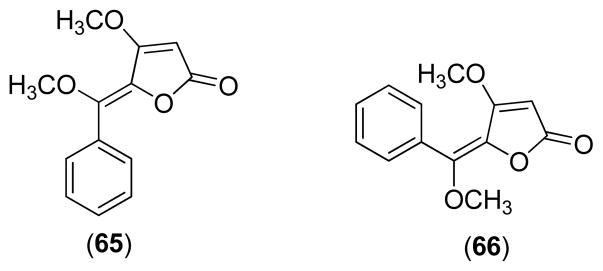
Bioassay-guided fractionation of the methylene chloride extract from the leaves of P. malacophyllum led to the identification of butenolides 65 and 66. Antifungal testing indicated that the trans-geometric isomer 66 had equipotent antifungal activity with miconazole (MAR, 1.0 μg) against C. cladosporioides and C. sphaerospermum, while the cis-isomer 66 was 5- to 10-fold less active than 65 [22].
CYCLOPENTENDIONES
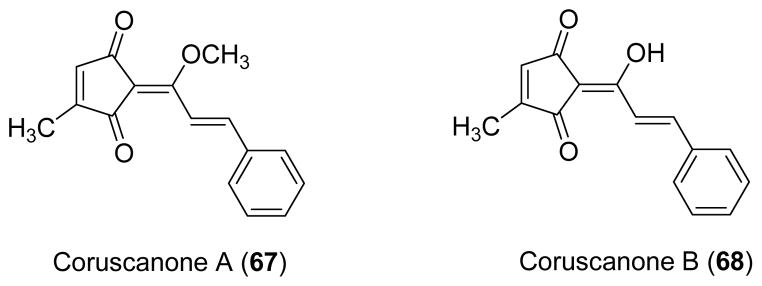
Two cyclopentenedione derivatives, coruscanone A (67) and B (68) were isolated from the ethanol extract of the Peruvian plant P. coruscans. Compound 67 showed potent activity against C. albicans (MIC 0.78 μg/ml) and C. neoformans (MIC 6.25 μg/ml), while compound 68 exhibited marginal antifungal activity against C. albicans (MIC 50.0 μg/ml) [23]. Compound 67 is probably the most potent antifungal compound isolated from plants against C. albicans. Synthetic analogs have been prepared by modification of the cyclopentenedione ring, the enolic methoxy functionality, and the styrene moiety to afford SAR information for this class of compounds [24].
CONCLUSION
The survey of chemical and biological investigations of Piper species has revealed structurally diverse antifungal compounds. A total of 68 compounds were isolated from 22 Piper species (P. hispidum, P. tuberculatum, P. arboretum, P. scutifolium, P. sarmentosum, P. rumbellatum, P. crassinervium, P. lhotzkyanum, P. marginatum, P. solmsianu, P. mollicomum, P. dilatatum, P. lanceaefolium, P. hostmannianum, P. caldense, P. aduncum, P. fulvescens, P. abutiloides, P. regnellii, P. betle, P. malacophyllum, and P. coruscans). While the majority of these antifungal compounds (especially amides) were only evaluated by the simple bioautography method against agricultural fungal species, it will be worthwhile to test these compounds against clinically important human fungal pathogens. In addition, a medicinal chemistry approach to synthesizing analogs may be employed to study the SAR of these antifungal compounds in order to discover better leads for the development of antifungal drugs or agricultural fungicides.
Fig. 1.
Antifungal amides from Piper spp.
Fig. 2.
Antifungal flavonoids from Piper spp.
Fig. 3.
Antifungal prenylated benzoic acid derivatives from Piper spp.
Fig. 4.
Antifungal lignans from Piper spp.
Fig. 5.
Antifungal phenylpropanoids from Piper spp.
Fig. 6.
Antifungal butenolides from Piper spp.
Fig. 7.
Antifungal cyclopentenones from Piper spp.
Acknowledgments
This work was supported by the Nature Science Fund of China No. 31160075, the NIH, NIAID, Division of AIDS, Grant No. AI 27094, and the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
References
- 1.Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Ali MS, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM. Phytochemistry of the genus Piper. Phytochemistry. 1997;46:597–673. [Google Scholar]
- 2.Tuntiwachwuttiku P, Phansa P, Pootaengon Y, Taylo WC. Chemical constituents of the roots of Piper sarmentosum. Chem Pharm Bull. 2006;54:149–151. doi: 10.1248/cpb.54.149. [DOI] [PubMed] [Google Scholar]
- 3.Tabopda TK, Ngoupayo J, Liu J, Mitaine-Offer AC, Tanoli SAK, Khan SN, Ali MS, Ngadjui BT, Tsamo E, Lacaille-Dubois M-A, Luu B. Bioactive aristolactams from Piper umbellatum. Phytochemistry. 2008;69:1726–1731. doi: 10.1016/j.phytochem.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Lago JHG, Chen A, Young MCM, Guimarães EF, Oliveira A, Kato MJ. Prenylated benzoic acid derivatives from Piper aduncumL and P hostmannianum C DC (Piperaceae) Phytochem Lett. 2009;2:96–98. [Google Scholar]
- 5.Navickiene HM, Alecio AC, Kato MJ, Bolzani VS, Young MC, Cavalheiro AJ, Furlan M. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry. 2000;55:621–626. doi: 10.1016/s0031-9422(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 6.Alecio AC, Bolzani VS, Young MCM, Kato MJ, Furlan M. Antifungal amide from leaves of Piper hispidum. J Nat Prod. 1998;61:637–639. doi: 10.1021/np9703656. [DOI] [PubMed] [Google Scholar]
- 7.Silva RV, Navickiene HM, Kato MJ, Bolzani VS, Meda CI, Young MC, Furlan M. Antifungal amides from Piper arboreum and Piper tuberculatum. Phytochemistry. 2002;59:521–527. doi: 10.1016/s0031-9422(01)00431-9. [DOI] [PubMed] [Google Scholar]
- 8.Marques JV, Kitamura RS, Lago JHM, Young MC, Guimarães EF, Kato MJ. Antifungal amides from Piper scutifolium and Piper hoffmanseggianum. J Nat Prod. 2007;70:2036–2039. doi: 10.1021/np070347g. [DOI] [PubMed] [Google Scholar]
- 9.Danelutte A, Lago J, Young M, Kato M. Antifungal flavanones and prenylated hydroquinones from Piper crassinervium Kunth. Phytochemistry. 2003;64:555–559. doi: 10.1016/s0031-9422(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 10.Lago JH, Young MC, Reigada JB, Soares MG, Roesler BP, Kato MJ. Antifungal derivatives from Piper mollicomum and P. Lhotzkyanum (Piperaceae). Quim. Nova. 2007;30:1222–1224. [Google Scholar]
- 11.Reigada JB, Tcacenco CM, Andrade LH, Kato MJ, Porto ALM, Lago JHG. Chemical constituents from Piper marginatum Jacq. (Piperaceae) - antifungal activities and kinetic resolution of (R/S)-marginatumol by Candida antarctica lipase (Novozym 435) Tetrahedron: Asymmetry. 2007;18:1054–1058. [Google Scholar]
- 12.Campos MP, Filho VC, Silva RS, Yunes RA, Zacchino S, Juarez S, Cruz RB, Cruz AB. Evaluation of antifungal activity of Piper solmsianum C. DC. var. solmsianum (Piperaceae) Biol Pharm Bull. 2005;28:1527–1530. doi: 10.1248/bpb.28.1527. [DOI] [PubMed] [Google Scholar]
- 13.Lopez A, Ming DS, Towers HN. Antifungal activity of benzoic acid derivatives from Piper lanceaefolium. J Nat Prod. 2002;65:62–64. doi: 10.1021/np010410g. [DOI] [PubMed] [Google Scholar]
- 14.Terreaux C, Gupta MP, Hostettmann K. Antifungal benzoic acid derivatives from Piper dilatatum. Phytochemistry. 1998;49:461–464. [Google Scholar]
- 15.Lago JHG, Ramos CS, Casanova DCC, Morandim AA, Bergamo DCB, Cavalheiro AJ, Bolzani AS, Furlan M, Guimaraes EF, Young MCM, Kato MJ. Benzoic acid derivatives from Piper Species and their fungitoxic activity against Cladosporium cladosporioides and C. sphaerospermum. J Nat Prod. 2004;67:1783–1788. doi: 10.1021/np030530j. [DOI] [PubMed] [Google Scholar]
- 16.Freitas GC, Kitamura ROS, Lago JHG, Young MCM, Guimarães EF, Kato MJ. Caldensinic acid, a prenylated benzoic acid from Piper caldense. Phytochem Lett. 2009;2:119–122. [Google Scholar]
- 17.Danelutte A, Lago J, Young M, Kato M. Antifungal flavanones and prenylated hydroquinones from Piper crassinervium Kunth. Phytochemistry. 2003;64:555–559. doi: 10.1016/s0031-9422(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 18.Freixa B, Vila R, Ferro EA, Adzet T, Canigueral S. Antifungal principles from Piper fulvescens. Planta Med. 2001;67:873–875. doi: 10.1055/s-2001-18838. [DOI] [PubMed] [Google Scholar]
- 19.Johann S, Cota BB, Souza-Fagundes EM, Pizzolatti MG, Resende MA, Zani CL. Antifungal activities of compounds isolated from Piper abutiloides Kunth. Mycoses. 2009;52:499–506. doi: 10.1111/j.1439-0507.2008.01636.x. [DOI] [PubMed] [Google Scholar]
- 20.Koroishi AM, Foss SR, Cortez DAG, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. In vitro antifungal activity of extracts and neolignans from Piper regnellii against dermatophytes. J Ethnopharmcol. 2008;117:270–277. doi: 10.1016/j.jep.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Evans PH, Bowers WS, Funk EJ. Identification of Fungicidal and Nematocidal Components in the Leaves of Piper betle (Piperaceae) J Agric Food Chem. 1984;32:1254–1256. [Google Scholar]
- 22.Lago JHG, Tanizaki TM, Young MCM, Guimarães EF, Kato MJ. Antifungal piperolides from Piper malacophyllum (Prels) C. DC. J Braz Chem Soc. 2005;16:153–156. [Google Scholar]
- 23.Li XC, Ferreira D, Jocob MR, Zhang QF, Khan SI, ElSohly HN, Nagle DG, Smillie TY, Khan IA, Walker LA, Clark AM. Antifungal cyclopentenediones from Piper coruscans. J Am Chem Soc. 2004;126:6872–6873. doi: 10.1021/ja048081c. [DOI] [PubMed] [Google Scholar]
- 24.Babu KS, Li XC, Jocob MR, Zhang QF, Khan SI, Ferreira D, Clark AM. Synthesis, antifungal activity, and structure-activity relationships of coruscanone A analogues. J Med Chem. 2006;49:7877–7886. doi: 10.1021/jm061123i. [DOI] [PMC free article] [PubMed] [Google Scholar]