Abstract
Despite recent improvements to current therapies and the emergence of novel agents to manage advanced non-small cell lung cancer (NSCLC), the patients' overall survival remains poor. Re-challenging with first-line chemotherapy upon relapse is common in the management of small cell lung cancer but is not well reported for advanced NSCLC. NSCLC relapse has been attributed to acquired drug resistance, but the repopulation of sensitive clones may also play a role, in which case re-challenge may be appropriate. Here, we report the results of re-challenge with gemcitabine plus carboplatin in 22 patients from a single institution who had previously received gemcitabine plus platinum in the first-line setting and had either partial response or a progression-free interval of longer than 6 months. In this retrospective study, the charts of patients who underwent second-line chemotherapy for NSCLC in our cancer center between January 2005 and April 2010 were reviewed. All the patients who received a combination of gemcitabine and carboplatin for re-challenge were included in the study. These patients were offered second-line treatment on confirmation of clear radiological disease progression. The overall response rate was 15% and disease control rate was 75%. The median survival time was 10.4 months, with 46% of patients alive at 1 year. These results suggest that re-challenge chemotherapy should be considered in selected patients with radiological partial response or a progression-free survival of longer than 6 months to the initial therapy.
Keywords: Non-small cell lung cancer, gemcitabine, carboplatin, systemic anti-cancer therapy, gefitinib, tyrosine kinase inhibitor
Lung cancer is the most common cancer and is the leading cause of cancer-related mortality worldwide. An estimated 40,000 new cases are diagnosed annually in the United Kingdom, and the 5-year overall survival (OS) rate from the time of diagnosis is 10% to 15% in the United States and Europe[1]. Non-small cell lung cancer (NSCLC) accounts for 75% to 80% of all lung cancer cases, of which adenocarcinoma (35% to 40%) and squamous cell carcinoma (30% to 35%) are the two most common subtypes[2]. Approximately 65% of patients present with locally advanced or metastatic disease and are not suitable for curative treatment[3].
Systemic anti-cancer therapy has been used in NSCLC to improve quality of life, disease control, and OS for lung cancer patients[4]. Platinum-based doublet chemotherapy is the mainstay first-line chemotherapy for advanced, non-adenocarcinoma NSCLC without an activating epidermal growth factor receptor (EGFR) mutation. In 2008, a meta-analysis incorporating 16 randomized trials and 2,714 cases confirmed that chemotherapy was associated with improved OS independent of factors such as age, performance status, and tumor histological subtype[5].
The majority of patients with advanced NSCLC treated with first-line, platinum-based systemic anti-cancer therapy relapse or become refractory to the treatment. Only 4% to 6% of patients survive for more than 2 years[6]–[8]. The prognosis of patients with relapsed disease remains extremely poor, with limited treatment options. However, in patients with good performance status, systemic anti-cancer therapy can be considered. In this setting, the standard options include single agent cytotoxic therapy, small-molecule EGFR tyrosine kinase inhibitors (TKI), or treatment within the context of clinical trials, where appropriate and available. Docetaxel has been shown to have an overall response rate of 7%, with a disease control rate of 50%. An improvement in progression-free survival (PFS) (11 weeks vs. 7 weeks, P < 0.001) and OS (7 months vs. 5 months, P = 0.047) was noted in patients treated with docetaxel plus best supportive care compared with best supportive care alone[9]. Patients receiving 75 mg/m2 of docetaxel had a 1-year survival rate of 37%[9]. Another phase III study compared docetaxel with pemetrexed in patients with recurrent NSCLC. A total of 571 patients were recruited in that study, and comparable clinical efficacy and 1-year survival rates of 30% were observed in both arms[10]. The study, however, demonstrated significantly increased toxicities in the docetaxel arm, with higher rates of neutropenia and neutropenic sepsis. Further subset analysis showed better activity of pemetrexed in non-squamous tumors compared to docetaxel. Following the BR21 trial (a study coordinated by the National Cancer Institute of Canada Clinical Trials Group)[11] that compared erlotinib with best supportive care, erlotinib was used for second-line treatment of NSCLC. Despite the modest overall response rate of 9% (disease control rate = 47%), patients receiving erlotinib demonstrated significantly improved OS [7 months vs. 5 months, hazard ratio (HR) = 0.70, P < 0.001], PFS (2.2 months vs. 1.8 months, P < 0.001), and quality of life compared with best supportive care[11]. Subsequently, the ISEL (Iressa Survival Evaluation in Lung Cancer) trial compared gefitinib, another TKI, with placebo and demonstrated a prolonged time-to-progression (TTP) in gefitinib arm (3.0 months vs. 2.6 months, P < 0.001); however, no significant difference in OS was observed in patients with relapsed NSCLC[12]. The overall outcome for patients treated with second-line systemic anti-cancer therapy, as assessed by OS and overall response rate, remains poor, and there is a clear need for new approaches to systemic anti-cancer therapy in this setting.
The response rate to systemic anti-cancer therapy in small cell lung cancer is high, and the drugs used in the first-line setting are often considered on relapse. Similarly, re-challenging with the same chemotherapy is a valid treatment strategy used in several advanced malignancies, after failure on first-line systemic anti-cancer therapy[13]–[16].
In this study, we report outcomes in patients with NSCLC who had a progression-free survival of longer than 6 months with first-line gemcitabine plus platinum chemotherapy and who were re-challenged with the same treatment regimen, i.e., gemcitabine-platinum (rGC). All the patients in re-challenge setting received gemcitabine with carboplatin. We hypothesized that disease relapse is dominated by the re-growth of sensitive clones and that reintroduction of the first-line regimen may yield further response. Although re-challenge has been reported for NSCLC in an Asian population with good performance status with encouraging results[17], to our knowledge, we report the first series of patients re-challenged with a single regimen.
Patients and Methods
An existing data source in the Belfast Trust was used after approval by the hospital's audit committee. The treatment offered was a part of the hospital guidelines and did not require ethics approval. All patients who participated in the study signed the generic consent form for treatment.
Patients
All patients who received rGC as second-line therapy for NSCLC between January 2005 and April 2010, following radiological evidence of disease progression, were included in the study if they had 1) a complete or partial response to first-line gemcitabine plus platinum-based treatment, 2) a progression-free interval longer than 6 months, 3) an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and 4) at least one measurable lesion to evaluate response to treatment.
Treatment and response
Patients were treated with gemcitabine (1,250 mg/m2) on days 1 and 8 along with carboplatin area under the curve (AUC)5 on day 1 in a 3-week cycle. No more than 6 cycles were offered to each patient. Overall response rate was determined according to modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria (version 1.1)[18]. Response was evaluated in all patients with computed tomography (CT) scan. Complete response (CR) was defined as complete disappearance of the tumor. Partial response (PR) was defined as less than a 30% decrease in the maximum diameter of measurable disease, in the absence of progression in non-target lesions or new disease. Progressive disease (PD) was defined as more than a 20% increase in the maximum diameter of measurable disease, or as evidence of new disease or progression in non-target lesions. Stable disease (SD) was defined as the disease that did not fit the category of PR or PD[18]. Disease control was defined as CR, PR, or SD. Treatment was stopped in patients with radiological evidence of disease progression according to the above-mentioned criteria.
Follow-up
While patients were treated, history taking, physical examination, and hematologic and biochemical tests were performed every 3 weeks, and radiological investigations were performed at the end of the second and sixth cycles of treatment. All patients were followed up until death. Patients with PR or SD were followed up every 3 months with clinical examination and repeat chest X-ray radiograph. If patients reported any symptomatic progression or chest X-ray radiograph demonstrated any evidence of progressive disease, CT scan of the chest, abdomen, and pelvis was performed to confirm evidence of progression.
Statistical analyses
OS was calculated from the day second-line treatment was commenced until the date of death, and PFS was calculated from the day treatment was initiated to radiological or clinical disease progression, both using MedCalc software (version 12.7.0, Ostend, Belgium). Survival curves were constructed using the Kaplan-Meier method. Univariate and multivariate analyses were performed to evaluate factors that were predictive of response using SPSS software (version 19, Chicago, IL, USA).
Results
Patient characteristics
The patients' pre-treatment characteristics were balanced and similar to those of patients enrolled in clinical studies and treated in second-line setting in the context of NSCLC[9],[10] (Table 1), with the exception of the predefined selection criteria. Twenty-two patients were recruited in the study based on the above-defined selection criteria, with a median age of 63 (range, 40-80 years); of those, 18 (82%) received gemcitabine with carboplatin, and the remaining 4 (18%) received gemcitabine with cisplatin in the first-line setting (median, 4 cycles; range, 3-6 cycles). Thirteen (59%) patients had a response to first-line chemotherapy, with 3 (14%) achieving CR and 10 (45%) achieving PR. The median progression-free interval was 12.7 months. Twelve (54%) patients relapsed with new metastatic disease, and 6 of them had sites of metastatic disease larger than 3 cm. Of the remaining 10 patients, 5 had at least a 50% increase in the size of known metastatic disease, and 5 had locoregional relapse only.
Table 1. Baseline characteristics of 22 patients with non-small cell lung cancer (NSCLC).
| variable | No. of patients (%) |
| Sex | |
| Male | 14 (64) |
| Female | 8 (36) |
| Smoking status | |
| Non-smoker | 2 (9) |
| Smoker | 20 (91) |
| PS score (ECOG) | |
| 0 | 3 (14) |
| 1 | 12 (55) |
| 2 | 4 (18) |
| 3 | 1 (5) |
| Undocumented | 2 (9) |
| Clinical stage | |
| IIIB | 5 (23) |
| IV | 17 (77) |
| Histological subtype | |
| Adenocarcinoma | 6 (27) |
| Squamous | 9 (41) |
| Adeno-squamous | 3 (14) |
| Unknown | 4 (18) |
PS, performance status; ECOG, Eastern Cooperative Oncology Group.
Response and survival
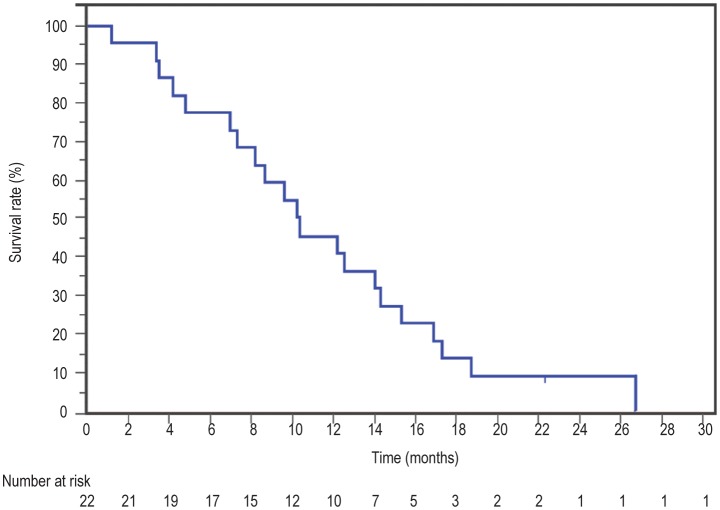
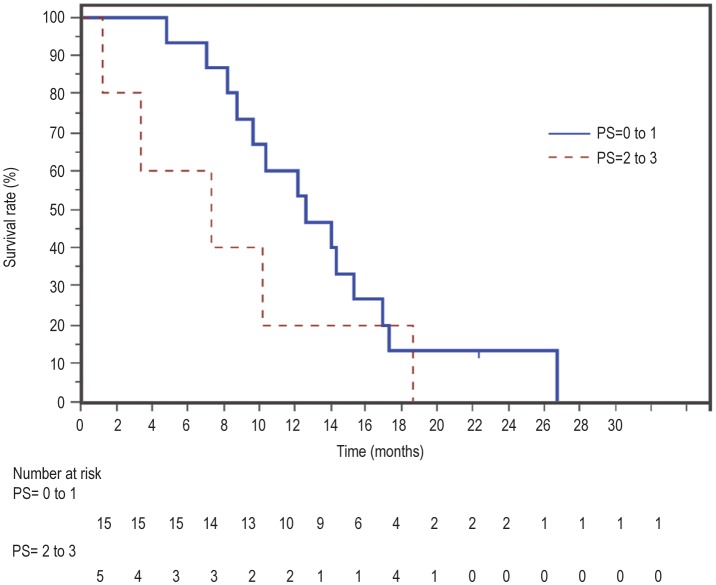
The median follow-up time was 22 months. Nineteen patients died, all from disease progression. The response rate for rGC was 15% [95% confidence interval (CI) = 0% to 16%], whereas the disease control rate was 75% (95% CI = 56% to 94%). The median OS was 10.4 months, and the 1-year OS rate was 46% (Figure 1). Patients with a performance status of 0-1 did better with rGC, with a median OS of 14 months and a 1-year OS rate of 60% (Figure 2). Factors predicting better OS with rGC included length of time from first-line treatment to the time of progression (HR = 0.9, 95% CI = 0.9 to 1.0, P = 0.018) and ECOG performance status at the start of re-challenge (HR = 2.7, 95% CI = 1.3 to 5.7, P = 0.006), both in univariate and multivariate analyses (Tables 2 and 3). Interestingly, the response rate to first-line treatment was not predictive of OS or response to rGC. The median PFS was 5.6 months.
Figure 1. Overall survival curves for 22 patients with non-small cell lung cancer (NSCLC) from the start of re-challenge chemotherapy with gemcitabine plus carboplatin (rGC).
Figure 2. Overall survival from the start of re-challenge chemotherapy according to the performance status (PS) of the patients.
Table 2. Univariate analysis to determine factors associated overall survival (OS) of patients who underwent re-challenge chemotherapy with gemcitabine plus carboplatin (rGC).
| Variate | HR | 95% CI | P |
| Sex | 1.5 | 0.6 to 3.8 | 0.412 |
| Smoking history | 0.4 | 0.1 to 2.0 | 0.253 |
| Pathologic subtype | 0.8 | 0.4 to 1.7 | 0.586 |
| RR for first-line chemotherapy | 0.8 | 0.4 to 1.5 | 0.454 |
| Interval between first-line and second-line chemotherapy | 0.9 | 0.9 to 1.0 | 0.018 |
| >6 months | 0.6 | 0.2 to 1.9 | 0.441 |
| >12 months | 0.5 | 0.2 to 1.3 | 0.052 |
| >15 months | 0.1 | 0.0 to 0.6 | 0.007 |
| PS at rGC | 2.7 | 1.3 to 5.7 | 0.006 |
| Stage at rGC | 0.8 | 0.1 to 7.7 | 0.881 |
| Third-line chemotherapy | 0.8 | 0.3 to 2.0 | 0.591 |
| RR for rGC chemotherapy | 1.9 | 0.2 to 5.2 | 0.199 |
RR, response rate; PS, performance status; HR, hazard ratio; CI, confidence interval.
Table 3. Multivariate analysis to determine factors associated with OS of patients who underwent re-challenge chemotherapy.
| Variate | HR | 95% CI | P |
| Time from first-line treatment to rGC | 0.9 | 0.8 to 1.0 | 0.016 |
| PS at rGC | 3.6 | 1.4 to 9.1 | 0.008 |
| Time from first-line treatment to rGC >15 months | 0.1 | 0.0 to 0.5 | 0.009 |
Abbreviations as in Table 2.
Toxicity
Sixteen (74%) patients undergoing rGC chemotherapy required a dose reduction. Four (18%) patients had to stop because of toxicity (predominately fatigue). One patient died within 30 days of finishing treatment; however, this death was due to disease progression.
Further treatments after second-line therapy
Following progression on rGC, 7 (32%) patients went on to have third-line treatment (3 were treated with docetaxel and 4 with erlotinib), with a median of 2 cycles (range, 1 to 3 cycles). The median survival of these 7 patients was 4.6 months from the start of third-line treatment.
Discussion
The present study demonstrates a valuable treatment strategy, albeit for a selected group of NSCLC patients. To our knowledge, only one study has been reported thus far adapting re-challenge as a treatment strategy in NSCLC. In that study, Nagano et al.[17] reported outcomes in a cohort of 28 patients treated with re-challenge chemotherapy for NSCLC and compared their outcomes with 38 patients receiving docetaxel in second-line setting. All the 28 patients treated with re-challenge chemotherapy had a performance status of 0 or 1 and had shown previous response to systemic anti-cancer therapy. In Nagano study, PFS was not used as a selection criterion in the re-challenge group; some patients relapsed within 2 months of first-line treatment. A variety of treatment regimens were used in the first-line setting and, hence, on re-challenge. Of the 28 eligible patients, 10 (36%) were treated with vinca-alkaloid and cisplatin, 3 (9%) with cisplatin and docetaxel, 2 (7%) with gemcitabine and vinorelbine, 1 (4%) with cisplatin and camptothecin, 1 (4%) with carboplatin and paclitaxel, 1 (4%) with gemcitabine and cisplatin, and details of remaining first-line therapies were not provided. Our study is therefore the first describing the approach of standardized rGC in relapsed advanced NSCLC. Nagano et al.[17] reported that 9 (32%) patients underwent third-line chemotherapy and 4 (14%) underwent fourth-line chemotherapy. The reported median survival was 17 months and the 1-year survival rate was 60%. The response rate was 29%, with a disease control rate of 75%. Median survival time was long in those with a disease-free interval longer than 6 months. These outcomes are comparable with the good performance status subgroup (0-1) in our study.
Previous clinical trials in the second-line setting have shown a median survival of 7 to 8 months, with a 1-year OS rate of 30%. Response rates of 7% to 10% and disease control rates of 45% to 55% are typically seen[9]–[11]. However, clinical trials are generally guided by strict inclusion criteria, recruiting a better group of patients with good performance status and organ function reserve. In clinical practice, the results often tend to be worse than those reported in clinical trials. Our own unpublished audit of 94 patients with relapsed NSCLC treated in the second-line setting showed a median survival of 6 months and 1-year OS rate of 15%. Overall, our outcomes are inferior to those of the Nagano study, but the outcomes are comparable when only patients of performance status 0-1 are analyzed.
The selection criteria in our study may be argued to have elicited a population with slow-biology disease, making it more likely that disease stabilization or slow progression would have been achieved. However, before entry into the study, 6 (27%) of patients had bulky (greater than 3 cm) new metastatic disease, and 8 (36%) had disease that had increased by more than 50% since their previous imaging. Only 6 (27%) had small volume (less than 3 cm) new metastatic disease, and 2 (9%) had locoregional relapse that had grown less than 50% from previous imaging.
Toxicity was difficult to define because the current study was retrospective in nature. Most patients required a dose reduction, and a significant number stopped due to toxicity, mainly fatigue. No patients died from chemotherapy-related toxicity.
There are several proposed mechanisms of resistance to systemic anti-cancer therapy. Overexpression of metallothionein (MT)-related metabolic enzymes has been implicated in enzymatic inactivation of platinum drugs in NSCLC patients previously treated with cisplatin[19]. Likewise, inherent resistance to gemcitabine is associated with low deoxycytidine kinase (dCK) levels or high multidrug-resistant protein 5 (ABCC5)[20],[21]. These studies imply that patients with low dCK levels can have resistance not only to gemcitabine but also to pemetrexed and other cytotoxic agents[22]. These results were further reproduced in a clinical study which showed that the response to previous chemotherapy might predict the efficacy of second-line treatment with pemetrexed[23]. Acquired resistance to gemcitabine has a different mechanism and is associated with increased expression of ribonucleotide reductase M1 (RRM1)[24]. In the current study, we showed that patients who once progressed on gemcitabine-platinum combination still demonstrated a response rate of 15%, suggesting that, theoretically, re-challenge can temporarily overcome the resistance mechanism.
Nevertheless, there remains a lack of clinical biomarkers to inform us about the potential outcome of patients treated with systemic anti-cancer therapy. In many studies, the expression of excision repair cross-complementation group 1 (ERCC1) predicted response rate in patients treated with cisplatin[25]. Olaussen et al.[26] demonstrated that patients with ERCC1-negative tumors who underwent 4 cycles of adjuvant cisplatin therapy had longer OS and disease-free survival compared with those with ERCC1-positive tumors. Similarly, the BRCA1 gene has also been implicated as a potential predictive marker for response to adjuvant platinum therapy in NSCLC[27]. RRM1 and RRM2 expression have been associated with reduced response rates in patients treated with gemcitabine plus docetaxel in the first-line setting in a phase III clinical study, whereas the overexpression of BRCA1 was associated with a better response to therapy[28],[29]. Our study highlights the importance of further developing these biomarkers; indeed, there may be a group of patients who benefit from re-challenge with existing treatment options, and we may be able to achieve comparable and cost-effective clinical efficacy for them to that observed with new treatment options.
There is strong body of evidence from both preclinical and clinical studies that specific biomarkers can potentially predict response to a particular type of chemotherapy, but no such biomarkers have been used so far in standard clinical practice. Although improved knowledge about the biology of NSCLC and the use of targeted agents has led to significant inroads in the management of this disease, there is a strong and unmet need to define biomarkers to inform clinicians about the choice of chemotherapy. In the absence of such robust biomarkers in standard practice, the current second-line management of NSCLC is highly dependent on clinical factors such as performance status and previous response to the treatment.
Our study is retrospective in nature and only a small number of patients have been selected based on their good performance status and previous response to the rGC regimen, which may be a small proportion of all NSCLC patients. We, however, present a valid treatment option that can be considered in a selected group of patients.
Conclusions
Re-challenging with the same cytotoxic therapy is a valuable option in many malignancies, including small cell lung cancer, breast cancer, and ovarian cancer. Based on our results, we propose that, in selected group of patients, rCG should be considered a valid treatment strategy.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 3.Novello S, Le Chevalier T. Chemotherapy for non-small-cell lung cancer. Part 1: Early-stage disease, Oncology (Williston Park) 2003;17:357–364. [PubMed] [Google Scholar]
- 4.Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004;59:828–836. doi: 10.1136/thx.2003.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group NM-AC Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–1626. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto T, Maruyama R, Shoji F, et al. Long-term survivors in stage IV non-small cell lung cancer. Lung Cancer. 2005;47:85–91. doi: 10.1016/j.lungcan.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Satoh H, Ishikawa H, Ohara G, et al. Long-term survivors after chemotherapy in advanced non-small cell lung cancer. Anticancer Res. 2007;27:4457–4460. [PubMed] [Google Scholar]
- 9.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 10.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 12.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 13.Bearz A, Talamini R, Rossoni G, et al. Re-challenge with pemetrexed in advanced mesothelioma: a multi-institutional experience. BMC Res Notes. 2012;5:482. doi: 10.1186/1756-0500-5-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okines AF, Asghar U, Cunningham D, et al. Rechallenge with platinum plus fluoropyrimidine +/- epirubicin in patients with oesophagogastric cancer. Oncology. 2010;79:150–158. doi: 10.1159/000322114. [DOI] [PubMed] [Google Scholar]
- 15.Giaccone G, Ferrati P, Donadio M, et al. Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol. 1987;23:1697–1699. doi: 10.1016/0277-5379(87)90452-4. [DOI] [PubMed] [Google Scholar]
- 16.Rosti G, Bevilacqua G, Bidoli P, et al. Small cell lung cancer. Ann Oncol. 2006;17(Suppl. 2):ii5–10. doi: 10.1093/annonc/mdj910. [DOI] [PubMed] [Google Scholar]
- 17.Nagano T, Kim YH, Goto K, et al. Re-challenge chemotherapy for relapsed non-small-cell lung cancer. Lung Cancer. 2010;69:315–318. doi: 10.1016/j.lungcan.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer. 2000;87:881–886. [PubMed] [Google Scholar]
- 19.Matsumoto Y, Oka M, Sakamoto A, et al. Enhanced expression of metallothionein in human non-small-cell lung carcinomas following chemotherapy. Anticancer Res. 1997;17:3777–3780. [PubMed] [Google Scholar]
- 20.Oguri T, Achiwa H, Sato S, et al. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: a role of ABCC5 in gemcitabine sensitivity. Mol Cancer Ther. 2006;5:1800–1806. doi: 10.1158/1535-7163.MCT-06-0025. [DOI] [PubMed] [Google Scholar]
- 21.Bergman AM, Giaccone G, van Moorsel CJ, et al. Cross-resistance in the 2′,2′-difluorodeoxycytidine (gemcitabine)-resistant human ovarian cancer cell line AG6000 to standard and investigational drugs. Eur J Cancer. 2000;36:1974–1983. doi: 10.1016/s0959-8049(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 22.Pratt S, Shepard RL, Kandasamy RA, et al. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 23.Sun JM, Oh DY, Lee SH, et al. The relationship between response to previous systemic treatment and the efficacy of subsequent pemetrexed therapy in advanced non-small cell lung cancer. Lung Cancer. 2010;68:427–432. doi: 10.1016/j.lungcan.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Bergman AM, Eijk PP, Ruiz van Haperen VW, et al. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res. 2005;65:9510–9516. doi: 10.1158/0008-5472.CAN-05-0989. [DOI] [PubMed] [Google Scholar]
- 25.Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol. 2007;18:522–528. doi: 10.1093/annonc/mdl435. [DOI] [PubMed] [Google Scholar]
- 26.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemo-therapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 27.Rosell R, Skrzypski M, Jassem E, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One. 2007;2:e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boukovinas I, Papadaki C, Mendez P, et al. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLoS One. 2008;3:e3695. doi: 10.1371/journal.pone.0003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souglakos J, Boukovinas I, Taron M, et al. Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. Br J Cancer. 2008;98:1710–1715. doi: 10.1038/sj.bjc.6604344. [DOI] [PMC free article] [PubMed] [Google Scholar]