Abstract
The application of simultaneous integrated boost-intensity modulated radiotherapy (SIB-IMRT) in pediatric and adolescent nasopharyngeal carcinoma (NPC) is underevaluated. This study aimed to evaluate long-term outcome and late toxicities in pediatric and adolescent NPC after SIB-IMRT combined with chemotherapy. Thirty-four patients (aged 8–20 years) with histologically proven, non-disseminated NPC treated with SIB-IMRT were enrolled in this retrospective study. The disease stage distribution was as follows: stage I, 1 (2.9%); stage III, 14 (41.2%); and stage IV, 19 (55.9%). All patients underwent SIB-IMRT and 30 patients also underwent cisplatin-based chemotherapy. The prescribed dose of IMRT was 64–68 Gy in 29–31 fractions to the nasopharyngeal gross target volume. Within the median follow-up of 52 months (range, 9–111 months), 1 patient (2.9%) experienced local recurrence and 4 (11.8%) developed distant metastasis (to the lung in 3 cases and to multiple organs in 1 case). Four patients (11.8%) died due to recurrence or metastasis. The 5-year locoregional relapse–free survival, distant metastasis–free survival, disease-free survival, and overall survival rates were 97.1%, 88.2%, 85.3%, and 88.2%, respectively. The most common acute toxicities were grades 3–4 hematologic toxicities and stomatitis. Of the 24 patients who survived for more than 2 years, 16 (66.7%) and 15 (62.5%) developed grades 1–2 xerostomia and ototoxicity, respectively. Two patients (8.3%) developed grade 3 ototoxicity; no grade 4 toxicities were observed. SIB-IMRT combined with chemotherapy achieves excellent long-term locoregional control in pediatric and adolescent NPC, with mild incidence of late toxicities. Distant metastasis is the predominant mode of failure.
Keywords: Pediatric and adolescent, nasopharyngeal carcinoma, SIB-IMRT, outcome, late toxicity
Pediatric and adolescent nasopharyngeal carcinoma (NPC) is rare, accounting for approximately 1% of all cases of NPC in the endemic areas of southern China[1]. Undifferentiated carcinoma is the most common histologic type of pediatric and adolescent NPC and is associated with advanced locoregional disease at diagnosis and a high incidence of distant metastasis[2]. Cisplatin-based chemotherapy combined with radiotherapy currently serves as the standard treatment modality for advanced pediatric and adolescent NPC.
Historically, two-dimensional radiotherapy mainly used a large lateral opposing faciocervical field and a lower anterior cervical field, which made it difficult to sufficiently protect adjacent normal tissues. For patients with locally advanced disease, such as skull base infiltration or intracranial extension, target volume coverage and normal tissue sparing could not be well balanced. In patients with pediatric and adolescent NPC treated with two-dimensional radiotherapy, the locoregional relapse–free survival rate was appro-ximately 80%, with an incidence of late sequelae of 65%–85%[3]–[6].
Intensity-modulated radiotherapy (IMRT) is currently the mainstay radiotherapy modality for NPC; it offers excellent target volume coverage while protecting the normal tissues adjacent to the target. Superior locoregional control rates and reduced late toxicities have been reported for this modality during the treatment of adults with NPC[7]. Furthermore, IMRT in combination with the simultaneous integrated boost (SIB) technique has been reported to increase the biologically effective dose delivered to the tumor and yield an excellent local control rate with reduced late toxicities in adult patients with NPC[8]–[10].
To the best of our knowledge, only one study has examined the use of IMRT in pediatric and adolescent NPC, and this study reported a high incidence of acute and late toxicities associated with platinum-based chemotherapy plus IMRT in 5 patients[11]. Compared with the effects of IMRT in adults, these poor outcomes make it essential to improve the application of IMRT in pediatric and adolescent NPC. On this basis, the aim of this study was to analyze the long-term survival outcome and late toxicities in patients with pediatric and adolescent NPC after SIB-IMRT combined with chemotherapy.
Patients and Materials
Patient selection
Between December 2003 and October 2010, a total of 34 young patients with newly diagnosed, untreated, and non-disseminated NPC presented to the Sun Yat-sen University Cancer Center (Guangzhou, China), and all were selected for this study. There were 24 males and 10 females, with a median age of 16 years (range, 8–20 years). Histologically 97.1% (33 of 34) of the patients were diagnosed with type III disease based on World Health Organization (WHO) criteria, and 2.9% (1 of 34) had WHO type II disease.
All patients underwent a pre-treatment evaluation that included a complete patient history, physical examination, hematology and biochemistry profiles, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal sonography, and single-photon emission computed tomography (SPECT) for whole body bone scans. Positron emission tomography (PET)/computed tomography (CT) was performed on 13 patients (38.2%). All were restaged according to the 7th edition of the UICC/AJCC staging system. The stage distribution for the patients was as follows: stage I, 1 (2.9%); stage III, 14 (41.2%); stage IVA, 14 (41.2%); and stage IVB, 5 (14.7%). Table 1 lists the characteristics of the patient cohort.
Table 1. Characteristics of the 34 patients with pediatric and adolescent nasopharyngeal carcinoma (NPC).
| Characteristic | No. of patients (%) |
| Age (years) | |
| Median | 16 |
| Range | 8-20 |
| Sex | |
| Male | 24 (70.6) |
| Female | 10 (29.4) |
| Pathology | |
| WHO II | 1 (2.9) |
| WHO III | 33 (97.1) |
| T categorya | |
| T1 | 2 (5.9) |
| T2 | 1 (2.9) |
| T3 | 16 (47.1) |
| T4 | 15 (44.1) |
| N categorya | |
| N0 | 3 (8.8) |
| N1 | 18 (52.9) |
| N2 | 8 (23.5) |
| N3 | 5 (14.7) |
| Clinical stagea | |
| I | 1 (2.9) |
| II | 0 |
| III | 14 (41.2) |
| IVA | 14 (41.2) |
| IVB | 5 (14.7) |
| Chemotherapy | |
| Yes | 30 (88.2) |
| No | 4 (11.8) |
T, tumor; N, node; WHO, World Health Organization. a As defined by the criteria of the seventh edition of the UICC/AJCC staging system for NPC.
Radiotherapy protocol
Radical radiotherapy was implemented. The patients were immobilized in a supine position using a thermoplastic mask. After administration of intravenous contrast material, 3-mm CT slices depicting the area of the head until 2 cm below the sterno-clavicular joint were acquired. The primary tumor and upper-neck area above the caudal edge of the cricoid cartilage were treated with IMRT with the SIB technique. Target volumes were delineated according to our previously described institutional treatment protocol[12], which is in agreement with International Commission on Radiation Units and Measurements Reports 50 and 62. Clinical target volumes (CTV) were individually delineated on the basis of the tumor invasion pattern[13]. Simultaneously, all normal tissues including the brainstem, spinal cord, temporal lobe, optic nerves and chiasm, parotid glands, temporomandibular joint, and mandible were carefully outlined. The contoured image was transferred to Corvus version 3.0, an inverse IMRT planning system (Peacock; Nomos Corp., Deer Park, IL). The planning target volumes (PTV) for the gross tumor volumes (GTVs) and CTVs were generated automatically after delineation of the tumor targets according to immobilization and localization uncertainties. The prescribed radiation dose was defined as follows: a total dose of 64–68 Gy in 29–31 fractions to the PTVs of the nasopharynx gross tumor volume (GTVnx), 60–64 Gy to the PTV of the nodal gross tumor volume (GTVnd), 60 Gy to the PTV of CTV-1 (i.e., high-risk regions of NPC), and 52–54 Gy to the PTV of CTV-2 (i.e., low-risk regions of NPC) and CTV-nd (i.e., neck nodal regions). The maximum dose tolerances for the critical normal structures were as follows: brainstem, 54 Gy; spinal cord, 45 Gy; optic nerve and chiasm, 54 Gy; temporal lobes, 60 Gy; and pituitary gland, 60 Gy. Radiotherapy was delivered using a dynamic, multileaf, intensity-modulating collimator (MIMiC; Nomos Corp., Sewickly, PA). A separate anterior low-neck field with spinal cord shielding was used for the lower neck and supraclavicular fossa. The prescribed dose delivered to the lower neck and supraclavicular fossa was 50 Gy over 25 fractions for prophylactic intent, and 60–66 Gy over 30–33 fractions for therapeutic intent. Treatment was delivered once daily, over 5 fractions per week. All patients completed the prescribed dose of IMRT without interruption.
Treatment plans were approved upon meeting the following criteria[8]: (1) 95% of any PTV was at or above the prescribed dose; (2) 99% of any PTV was at or above 93% of the PTV dose; (3) the hot spot was located in the gross nasopharyngeal target volume, not the nasopharyngeal mucosa; and (4) the doses to organs at risk were restricted below their tolerance doses, and a balance was reached between target volume coverage and the doses to the organs at risk.
Chemotherapy protocol
Indications for chemotherapy were based on disease staging, tumor burden, and oncologists' opinions. Overall, 4 of 34 patients (11.8%) were treated with radiotherapy only, and 30 of 34 patients (88.2%) received chemotherapy. Neoadjuvant chemotherapy was given to 21 patients using a combination of cisplatin and 5-fluorouracil for two or three cycles at a 3-week interval. Concurrent chemotherapy was delivered to 25 patients using cisplatin given weekly or on weeks 1, 4, and 7 of radiotherapy. Two patients received adjuvant chemotherapy using cisplatin and 5-fluorouracil. Among these two patients, one also received four courses of cytokine-activated killer (CIK) cell infusion.
Follow-up
The duration of patient follow-up was calculated from the first day of treatment to either the day of death or the day of last examination. Patients were examined at least every 3 months during the first 2 years and, thereafter, every 6 months until death. During every follow-up, a complete physical examination, nasopharyngoscopy, blood and biochemistry profiles, chest radiography, abdominal ultrasonography, and CT/MRI scans of the nasopharynx and cervical region were used to assess disease status. Acute and late toxicities were scored according to the Radiation Therapy Oncology Group radiation morbidity scoring criteria[14].
Statistical analysis
Statistical Package for Social Sciences, version 16.0 (SPSS, Chicago, IL, USA), was used for statistical analysis. All events were measured from the start of treatment. The following end points (time to the first defined event) were assessed: overall survival, disease-free survival, locoregional relapse–free survival, and distant metastasis–free survival. Actuarial rates were calculated using the Kaplan-Meier method[15]. The criterion for statistical significance was set at α = 0.05, and P values were determined from two-sided tests.
Results
Patterns of treatment failure and survival
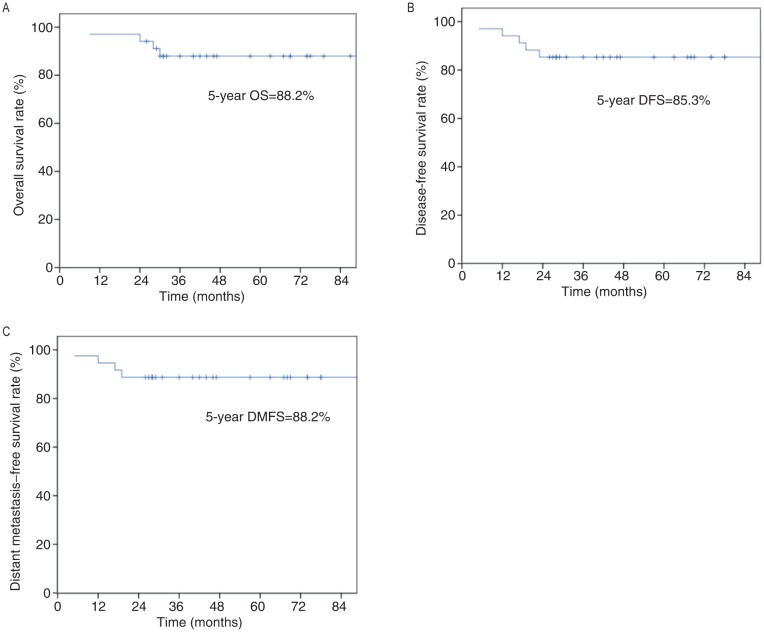
The medium follow-up time was 52 months (range, 9–111 months). Thirty of the 34 patients (88.2%) were still alive without clinical signs of disease at last follow-up. One patient (2.9%) developed local recurrence 18 months after treatment. Four patients (11.8%) developed distant metastasis: to the lung in three cases and to multiple organs in one case. The median time to the development of distant metastasis was 14.5 months (range, 5.0–19.0 months). Four patients (11.8%) died of pediatric and adolescent NPC due to recurrence or metastasis. The 5-year survival rates for the entire cohort were as follows: locoregional recurrence–free survival, 97.1%; distant metastasis–free survival, 88.2%; disease-free survival, 85.3%; and overall survival, 88.2% (Figure 1).
Figure 1. The Kaplan-Meier estimated survival curves of the 34 patients with pediatric and adolescent nasopharyngeal carcinoma.
A, overall survival (OS); B, disease-free survival (DFS); C, distant metastasis-free survival (DMFS).
Acute and late toxicity
Most of the patients developed grades 1–2 acute toxicities. The incidence of grades 3–4 acute toxicities were as follows: dermatitis, 5.9%; mucositis, 29.4%; leucopenia, 11.8%; and neutropenia, 5.9%. Neurotoxicity was not observed in any patient (Table 2).
Table 2. The frequency of acute and late toxicities in patients with pediatric and adolescent NPC.
| Toxicity | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Acute toxicities | |||||
| Leucopenia | 9 (26.5) | 10 (29.4) | 11 (32.3) | 4 (11.8) | 0 |
| Neutropenia | 21 (61.8) | 3 (8.8) | 8 (23.5) | 2 (5.9) | 0 |
| Thrombocytopenia | 29 (85.3) | 3 (8.8) | 2 (5.9) | 0 | 0 |
| Anaemia | 27 (79.4) | 6 (17.6) | 1 (2.9) | 0 | 0 |
| Dermatitis | 0 | 25 (73.5) | 7 (20.6) | 2 (5.9) | 0 |
| Stomatitis (mucositis) | 0 | 10 (29.4) | 14 (41.2) | 9 (26.5) | 1 (2.9) |
| Esophagitis, dysphagia, or odynophagia | 11 (32.4) | 18 (52.9) | 5 (14.7) | 0 | 0 |
| Xerostomia | 0 | 23 (67.6) | 11 (32.4) | 0 | 0 |
| Ototoxicity | 28 (82.4) | 6 (17.6) | 0 | 0 | 0 |
| Neurotoxicity | 34 (100) | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 10 (29.4) | 18 (52.9) | 6 (17.6) | 0 | 0 |
| Late toxicitiesa | |||||
| Skin dystrophy | 19 (79.2) | 5 (20.8) | 0 | 0 | 0 |
| Subcutaneous fibrosis | 19 (79.2) | 5 (20.8) | 0 | 0 | 0 |
| Ototoxocity | 7 (29.2) | 12 (50) | 3 ( 12.5) | 2 (8.3) | 0 |
| Xerostomia | 8 (33.3) | 13 (54.2) | 3 (12.5) | 0 | 0 |
| Trismus | 23 (95.8) | 1 (4.2) | 0 | 0 | 0 |
| Cataract | 24 (100) | 0 | 0 | 0 | 0 |
| Neuropathy | 24 (100) | 0 | 0 | 0 | 0 |
| Temporal lobe necrosis | 24 (100) | 0 | 0 | 0 | 0 |
| Mandible necrosis | 24 (100) | 0 | 0 | 0 | 0 |
| Pituitary amenorrhea | 23 (95.8) | 1 (4.2) | 0 | 0 | 0 |
All values are presented as numbers of patients, with percentage in the parentheses. a The 24 patients who survived for at least 2 years were included in the analysis of late toxicities.
Six patients were lost to follow-up; the 24 patients who survived for at least 2 years were included in the analysis of late toxicities. The incidences of grade 1 and grade 2 late toxicities were 54.2% and 12.5% for xerostomia, and 50% and 12.5% for ototoxicity, respectively. Additionally, two patients (8.3%) developed grade 3 ototoxicity and presented moderate to severe hearing loss; one of them required hearing aids to communicate with others. One female patient (4.2%) developed pituitary amenorrhea and required hormone replacement therapy. It was noteworthy that temporal lobe necrosis, neuropathy, cataracts, mandible necrosis, secondary malignancies, and skeletal growth retardation were not observed in any patient. The acute and late toxicities are summarized in Table 2.
Dose-volume analysis
The mean radiation dose and mean fraction dose for nasopharynx gross target volume, CTV1, CTV2, left neck lymph nodes, and right neck lymph nodes were 73.17 Gy and 2.43 Gy, 68.69 Gy and 2.28 Gy, 62.62 Gy and 2.07Gy, 67.10 Gy and 2.22 Gy, and 66.91 Gy and 2.22 Gy, respectively (Table 3). For normal tissues, the brainstem, optic nerves, and temporal lobes were well spared in patients with locally advanced disease (Table 4). The mean dose to the whole parotid gland was around 35 Gy, which resulted in a relatively mild xerostomia.
Table 3. Dose-volume statistics for the target volumes.
| Volumn | Dmean (Gy) | Dmax (Gy) | Dmin (Gy) | Fraction (Gy) | D95 (Gy) | V95 (%) | Volume (cm3) |
| GTVnx | 73.17 (68.03-78.40) | 80.26 (71.45-86.00) | 60.05 (50.58-70.94) | 2.43 (2.13-2.61) | 69.68 (64.91-72.34) | 99.69 (99.41-100) | 56.11 (9.10-164.59) |
| CTV-1 | 68.69 (61.57-73.38) | 79.04 (71.45-85.01) | 51.27 (29.64-61.38) | 2.28 (2.05-2.45) | 62.90 (59.87-66.89) | 98.50 (92.24-100) | NA |
| CTV-2 | 62.62 (58.53-66.28) | 77.37 (71.45-82.10) | 37.33 (11.89-63.41) | 2.07 (1.89-2.21) | 55.38 (50.66-59.09) | 98.62 (96.60-100) | NA |
| GTVnd-L | 67.10 (61.61-72.75) | 72.33 (65.49-80.85) | 62.00 (54.70-68.84) | 2.22 (1.99-2.43) | 64.89 (57.83-69.38) | 99.96 (99.54-100) | 8.11 (0.80-31.27) |
| GTVnd-R | 66.91 (61.71-72.81) | 72.37 (64.37-82.51) | 60.79 (46.92-66.30) | 2.22 (1.99-2.43) | 64.46 (57.78-70.63) | 99.78 (99.60-100) | 10.80 (0.44-67.43) |
All values are presented as mean value of dose-volume parameter, with range in the parentheses. Dmean, mean dose; Dmax, maximum dose; Dmin, minimum dose; Gy, grays; D95 is the dose to 95% of the target volume; V95, the percentage of the target volume covered by 95% of the prescribed dose; GTVnx, gross target volume of the nasopharynx; CTV-1, clinical target volume of the high-risk regions of NPC; CTV-2, clinical target volume of the low-risk regions of NPC; GTVnd-L, gross target volume of the left neck nodes; GTVnd-R, gross target volume of the right neck nodes; NA, not available.
Table 4. Dose-volume statistics for the normal tissues.
| Critical organ | Average maximum dose (Gy) | Average mean dose (Gy) |
| Brain stem | 56.81 (42.40-73.80) | 26.85 (17.27-40.93) |
| Spinal cord | 33.03 (22.12-42.73) | 20.00 (11.69-32.88) |
| Pituitary gland | 49.94 (24.50-63.42) | 39.83 (20.64-61.05) |
| Optic nerve | ||
| Left | 43.83 (23.40-65.10) | 23.56 (9.34-46.14) |
| Right | 45.77 (22.21-62.86) | 27.29 (10.58-76.98) |
| Optic chiasm | 42.39 (4.80-65.86) | 27.58 (4.21-59.15) |
| Temporal lobe | ||
| Left | 64.87 (48.00-75.20) | 18.26 (8.07-29.86) |
| Right | 64.72 (47.82-75.26) | 18.89 (9.90-40.43) |
| Parotid gland | ||
| Left | 65.00 (60.15-70.49) | 35.33 (30.68-39.42) |
| Right | 64.99 (57.80-68.62) | 35.82 (27.31-41.61) |
| Temporomandibular joint | ||
| Left | 48.41 (20.99-67.17) | 33.76 (20.07-56.25) |
| Right | 55.32 (43.95-70.19) | 38.40 (26.63-55.88) |
Discussion
In this retrospective study, we showed superior clinical efficacy with mild incidence of late toxicities for patients with pediatric and adolescent NPC receiving SIB-IMRT and chemotherapy. These results may provide a benchmark for further exploration of the benefits of IMRT in the treatment of pediatric and adolescent NPC.
Improved locoregional control
After conventional two-dimensional radiotherapy and combined chemotherapy, the locoregional control rate for patients with pediatric and adolescent NPC was less than satisfactory[3]–[6]. Laskar et al.[5] compared conventional radiotherapy with IMRT in 34 cases of pediatric and adolescent NPC and observed improved locoregional control in the IMRT arm (84.21% vs. 68.28%), though this difference did not reach statistical significance, probably because of the relatively small sample size. The survival benefits of IMRT have been explicitly demonstrated in adult NPC. Peng et al.[7] verified that IMRT improved local recurrence–free survival, especially in late-stage NPC, in a phase III randomized trial comparing IMRT with conventional radiotherapy. In the present study, the locoregional recurrence–free survival rate was 97.1%. This encouraging result suggests that IMRT offers excellent locoregional control in pediatric and adolescent NPC—even in locally advanced disease, which is the main form of pediatric and adolescent NPC.
The recent, rapid developments in radiology have also brought exciting improvements. The uses of MRI and PET/CT enable more accurate staging, help to better guide individualized treatment and improve the accuracy of gross tumor delineation with reference to MRI scans.
It is worth mentioning that chemotherapy also plays a role in survival. Previous retrospective studies have reported the survival benefit offered by radiotherapy combined with chemotherapy in pediatric and adolescent NPC[3],[16],[17]. In this study, 30 patients (88.2%) received chemotherapy and the 5-year overall survival for the entire cohort was 88.2%.
Acute toxicities
A moderate incidence of acute toxicities was observed in this study. All patients demonstrated good compliance and successfully completed radiation on the basis of intensive supportive treatment. Laskar et al.[5] showed that IMRT significantly reduced and delayed the onset of acute toxicities in patients with pediatric and adolescent NPC. Our results are consistent with those of Laskar et al.[5] and indicate that the incidence of acute toxicities in pediatric and adolescent NPC after SIB-IMRT and chemotherapy was acceptable, even after administration of high-dose radiation.
Late toxicities
Previously, two-dimensional conventional radiotherapy was widely used; however, survival was achieved at the expense of severe late toxicities. Cheuk et al.[6] reported that the 15-year cumulative incidence of any morbidity in patients with pediatric and adolescent NPC was (83.7 ± 5.4)%. In that study, the three most commonly observed complications were the following: sensorineural hearing loss, (52.9 ± 6.7)%; primary hypothyroidism, (42.7 ± 6.6)%; and trismus, (32.3 ± 6.2)%.
In recent years, IMRT has gained popularity in the treatment of head and neck cancer. Although the superiority of IMRT has been confirmed in adult patients with NPC, the insufficient evidence in childhood and adolescent patients deserves to be taken seriously. In the present study, grades 1–2 xerostomia and ototoxicity were the most common late toxicities. Only two patients (8.3%) developed grade 3 ototoxicity, and no patients developed grade 4 toxicities. Louis et al.[11] provided the first assessment of late toxicities in five cases of pediatric and adolescent NPC treated with IMRT. Compared to our results, a lower radiation dose and higher incidence of late toxicity were evident in the previous study by Louis et al.[11]; the authors speculated that the large CTV may possibly have explained the numerous late toxicities.
Furthermore, our results are comparable with studies in adults with NPC. Hearing loss was the dominant toxicity that affected quality of life in our cohort. Understandably, the middle and inner ear cannot be easily spared due to their proximity to the target volume. By delivering a proximal dose to the target volume, Xiao et al.[8] reported no grade 3 hearing loss, and Kam et al.[10] found that 13% of patients developed grade 3 hearing loss with this modality in adults with NPC. With regard to xerostomia, many studies have reported that the incidence of xerostomia appears to decrease with time after treatment, and nearly 9% of patients with NPC experienced grade 2 or higher xerostomia at 24 months after treatment[8],[18],[19]. A similar incidence of xerostomia was observed in our cohort of patients with pediatric and adolescent NPC.
It is worth mentioning that hypothyroidism was a relatively common complication in adults with NPC treated with conformal radiotherapy[20]. It is possible that inadequate monitoring of hormone levels may have contributed to an underestimation of endocrine dysfunction in this study.
Distant metastasis is the predominant mode of failure
Distant metastasis remains the major pattern of failure in pediatric and adolescent NPC. In this series, 4 of the 34 patients (11.8%) developed distant metastasis. Other studies have also revealed a higher rate of distant metastasis than locoregional failure in childhood and adolescent cancers[21]. The use of more potent systemic therapy in pediatric and adolescent NPC is warranted to improve survival.
Two prospective, multicenter studies—NPC-91-GPOH[22] and NPC-2003-GPOH/DCOG[23]—demonstrated favorable results for multimodal treatment (induction chemotherapy, and radiotherapy followed by interferon-β) in patients with pediatric and adolescent NPC. The overall survival and event-free survival rates were 95%–97% and 91%–92.4% after a median follow-up time of 48 months and 30 months, respectively. These promising outcomes are superior to all other reported results[2]–[4],[6] and indicate that interferon-β may provide a survival benefit in patients with pediatric NPC.
Varan et al.[24] first evaluated the effectiveness of neoadjuvant cisplatin and docetaxel plus radiotherapy in pediatric and adolescent NPC. The 2-year overall survival rate was 90% and the event-free survival rate was 70%. These preliminary results indicate the potential benefits of docetaxel. Furthermore, a combination of cisplatin, 5-fluorouracil, and docetaxel improved overall survival and progression-free survival in patients with unresectable squamous cell carcinoma of the head and neck, compared with the standard regimen of cisplatin and 5-fluorouracil[25]. The effectiveness of this combined regimen needs to be explored in pediatric and adolescent NPC.
As a retrospective analysis, the current study has some limitations. Prognostic factors could not be analyzed due to the limited sample size and low numbers of positive events. Increased numbers of patients need to be recruited to identify effective prognostic factors in pediatric and adolescent NPC.
Conclusions
This study revealed that SIB-IMRT combined with chemotherapy results in excellent locoregional control and low incidence of late toxicities in pediatric and adolescent NPC. However, distant metastasis remains the major cause of treatment failure; therefore, more efficient multimodal therapies that incorporate novel chemotherapy agents or immunotherapy need to be investigated to further improve survival in pediatric and adolescent NPC.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (No. 81071836) and Sun Yat-sen University 5010 Projects (No. 050243).
References
- 1.Ayan I, Kaytan E, Ayan N. Childhood nasopharyngeal carcinoma: from biology to treatment. Lancet Oncol. 2003;4:13–21. doi: 10.1016/s1470-2045(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 2.Afqir S, Ismaili N, Alaoui K, et al. Nasopharyngeal carcinoma in adolescents: a retrospective review of 42 patients. Eur Arch Otorhinoharyngol. 2009;266:1767–1773. doi: 10.1007/s00405-009-0911-1. [DOI] [PubMed] [Google Scholar]
- 3.Wolden SL, Steinherz PG, Kraus DH, et al. Improved long-term survival with combined modality therapy for pediatric naso-pharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:859–864. doi: 10.1016/s0360-3016(99)00493-9. [DOI] [PubMed] [Google Scholar]
- 4.Ozyar E, Selek U, Laskar S, et al. Treatment results of 165 pediatric patients with non-metastatic nasopharyngeal carcinoma: a Rare Cancer Network study. Radiother Oncol. 2006;81:39–46. doi: 10.1016/j.radonc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Laskar S, Bahl G, Muckaden M, et al. Nasopharyngeal carcinoma in children: comparison of conventional and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:728–736. doi: 10.1016/j.ijrobp.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Cheuk DK, Billups CA, Martin MG, et al. Prognostic factors and long-term outcomes of childhood nasopharyngeal carcinoma. Cancer. 2011;117:197–206. doi: 10.1002/cncr.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–293. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Xiao WW, Huang SM, Han F, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;17:1874–1883. doi: 10.1002/cncr.25754. [DOI] [PubMed] [Google Scholar]
- 9.Kwong DL, Sham JS, Leung LH, et al. Preliminary results of radiation dose escalation for locally advanced nasopharyngeal carcinoma. Int J Radiat OncolBiol Phys. 2006;64:374–381. doi: 10.1016/j.ijrobp.2005.07.968. [DOI] [PubMed] [Google Scholar]
- 10.Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440–1450. doi: 10.1016/j.ijrobp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Louis CU, Paulino AC, Gottschalk S, et al. A single institution experience with pediatric nasopharyngeal carcinoma: high incidence of toxicity associated with platinum-based chemotherapy plus IMRT. J Pediatr Hematol Oncol. 2007;29:500–505. doi: 10.1097/MPH.0b013e3180959af4. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Han F, Lu LX, et al. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004;23:1532–1537. [in Chinese] [PubMed] [Google Scholar]
- 13.Liang SB, Sun Y, Liu LZ, et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: improvement of clinical target volume delineation. Int J Radiat Oncol Biol Phys. 2009;75:742–750. doi: 10.1016/j.ijrobp.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;13:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Selek U, Ozyar E, Ozyigit G, et al. Treatment results of 59 young patients with nasopharyngeal carcinoma. Int J Pediatr Otorhinolaryngol. 2005;69:201–207. doi: 10.1016/j.ijporl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Bakkal BH, Kaya B, Berberoglu S, et al. The efficiency of different chemoradiotherapy regimens in patients with paediatric nasopharynx cancer: review of 46 cases. Int J Clin Pract. 2007;61:52–61. doi: 10.1111/j.1742-1241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin S, Pan J, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys. 2009;75:1071–1078. doi: 10.1016/j.ijrobp.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Su SF, Han F, Zhao C, et al. Long-term outcomes of early-stage nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy alone. Int J Radiat Oncol Biol Phys. 2012;82:327–333. doi: 10.1016/j.ijrobp.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Boomsma MJ, Bijl HP, Christianen ME, et al. A prospective cohort study on radiation-induced hypothyroidism: development of an NTCP model. Int J Radiat Oncol Biol Phys. 2012;84:351–356. doi: 10.1016/j.ijrobp.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Laskar S, Sanghavi V, Muckaden MA, et al. Nasopharyngeal carcinoma in children: ten years' experience at the TATA memorial hospital, MUMBAI. Int J Radiat Oncol Biol Phys. 2004;58:189–195. doi: 10.1016/s0360-3016(03)00773-9. [DOI] [PubMed] [Google Scholar]
- 22.Mertens R, Granzen B, Lassay L, et al. Treatment of nasopharyngeal carcinoma in children and adolescents: definitive results of a multicenter study (NPC-91-GPOH) Cancer. 2005;104:1083–1089. doi: 10.1002/cncr.21258. [DOI] [PubMed] [Google Scholar]
- 23.Buehrlen M, Zwaan CM, Granzen B, et al. Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults: preliminary results from the prospective, multicenter study NPC-2003-GPOH/DCOG. Cancer. 2012;118:4892–4900. doi: 10.1002/cncr.27395. [DOI] [PubMed] [Google Scholar]
- 24.Varan A, Ozyar E, Corapçioğlu F, et al. Pediatric and young adult nasopharyngeal carcinoma patients treated with preradiation Cisplatin and docetaxel chemotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1116–1120. doi: 10.1016/j.ijrobp.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]