Abstract
In recent years, it has become increasingly apparent that noncoding RNAs (ncRNA) are of crucial importance for human cancer. The functional relevance of ncRNAs is particularly evident for microRNAs (miRNAs) and long noncoding RNAs (lncRNAs). miRNAs are endogenously expressed small RNA sequences that act as post-transcriptional regulators of gene expression and have been extensively studied for their roles in cancers, whereas lncRNAs are emerging as important players in the cancer paradigm in recent years. These noncoding genes are often aberrantly expressed in a variety of human cancers. However, the biological functions of most ncRNAs remain largely unknown. Recently, evidence has begun to accumulate describing how ncRNAs are dysregulated in cancer and cancer stem cells, a subset of cancer cells harboring self-renewal and differentiation capacities. These studies provide insight into the functional roles that ncRNAs play in tumor initiation, progression, and resistance to therapies, and they suggest ncRNAs as attractive therapeutic targets and potentially useful diagnostic tools.
Keywords: ncRNA, miRNA, lncRNA, cancer cell, cancer stem cell
It is estimated that up to 90% of the genome is actively transcribed into RNAs. However, only 1.5%–2.0% of the human genome is estimated to consist of protein-coding genes[1]. In recent years, it has become apparent that the non–protein-coding portion of the genome is not spurious transcriptional noise, as originally believed. Increasing evidence suggests that the proverbial “dark matter” of the genome may play a major biological role in health and disease, particularly in cancer[2]. The oncogenic and tumor suppressive functions of noncoding transcripts are particularly evident for the most widely studied class of noncoding RNAs (ncRNAs) called microRNAs (miRNAs)[3],[4], which are implicated in a variety of cancer processes[5]–[7]. However, miRNAs may just represent the tip of the iceberg of known and newly discovered ncRNA species. Other ncRNAs include small interfering RNA (siRNA), PIWI-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), transcribed ultraconserved regions (t-UCRs), large intergenic noncoding RNAs (lincRNAs), and other species[2].
ncRNAs can be subdivided into two major classes based on their transcript size: small ncRNAs and long ncRNAs (lncRNAs), as shown in Table 1[8],[9]. snoRNAs are intermediate in size, 60 to 300 bp. Small ncRNAs, typically less than 200 nucleotides in length, include the well-documented miRNAs, siRNAs, piRNAs, and the recently described transcription initiation RNAs (tiRNAs)[10],[11]. Mammalian genomes also transcribe numerous lncRNAs, which are longer than 200 nucleotides and lack translational activity[12]–[15]. lncRNAs consist of a very heterogeneous group of RNA molecules that may be involved in a broad spectrum of cellular processes that utilize different modes of action. Accumulating studies have also described the potential functions of lncRNAs in cancer for their involvement in oncogene and tumor-suppressor pathways[16],[17].
Table 1. Types of ncRNAs.
| Name | Size | Functions | Illustrative examples | Reference(s) |
| Small ncRNA | ||||
| miRNAs | 19-24 bp | Translation inhibition, mRNA degradation | miR-15/16, miR-21 | [61],[66] |
| piRNAs | 26-31bp | Epigenetic modification | piRNAs targeting RASGRF1 and LINE1 | [144],[145] |
| tiRNAs | 17-18bp | RNAPII backtracking, nucleosome marking, and gene regulation | Associated with the CAP1 gene | [11] |
| TSSa-RNAs | 20-90 bp | Transcription maintenance | Associated with RNF12 and CCDC52 genes | [146] |
| PROMPTs | <200 bp | Transcription activation | Associated with EXT1 and RBM39 genes | [147] |
| snacRNA | 89-135 bp | Potentially maintaining stemness and differentiation | Snora61 | [120] |
| Intermediate ncRNA | ||||
| snoRNA | 60-300 bp | Synthesis and processing of cytoplasmic ribosomal RNAs; post-transcriptional modification of rRNA and snRNA by 2′-O-methylation and pseudouridylation | snoRNA U50 | [125],[126] |
| Long ncRNA | ||||
| lincRNAs | >200 bp | Epigenetic modification | HOTAIR, DD3 | [102],[134] |
| T-UCRs | >200 bp | Regulation of miRNA and mRNA levels? | uc.283+, uc.338, uc160+ | [148] |
| Other lncRNAs | >200 bp | Chromosome inactivation, telomere regulation, imprinting | XIST, TSIX, TERRAs, p15AS, H19, HYMAI | [149] |
ncRNA, noncoding RNA; miRNAs, microRNAs; piRNAs, PIWI-interacting RNAs; tiRNAs, transcription initiation RNAs; TSSa-RNAs, TSS-associated RNAs; PROMPTs, promoter upstream transcripts; snacRNA, small non-polyadenylated (NPA) conserved RNA; snoRNA, small nucleolar RNAs; lincRNA, large intergenic noncoding RNAs; T-UCRs, transcribed ultraconserved regions; RASGRF1, RAS-protein–specific guanine nucleotide-releasing factor 1; LINE1, long interspersed element-1; CAP1, adenylatecyclase-associated protein 1; RNF12, ring finger protein 12; CCDC52, coiled-coil domain containing 52; EXT1, exostosin 1; RBM39, RNA-binding motif protein 39; HOTAIR, homeobox (HOX) transcript antisense RNA; XIST, X-inactivation specific transcript; TSIX, antisense transcript of XIST; TERRAs, telomeric repeat-containing RNAs; HYMAI, hydatidiform mole associated and imprinted.
Virtually all cancers consist of phenotypically and genetically heterogeneous cells. Among these heterogenous cancer cells are a subpopulation of cells with stem cell–like properties, namely self-renewal and multipotency. These cancer stem cells (CSCs), or tumor-initiating cells, are capable of driving tumor growth and differentiating into multiple cell types to produce tumor heterogeneity[18].
In this short review, we focus on the roles of miRNAs and lncRNAs in the context of cancer and CSCs, briefly describe recent studies of other ncRNAs in cancer, and discuss their potential therapeutic applications.
Biogenesis and Function of miRNA and lncRNA
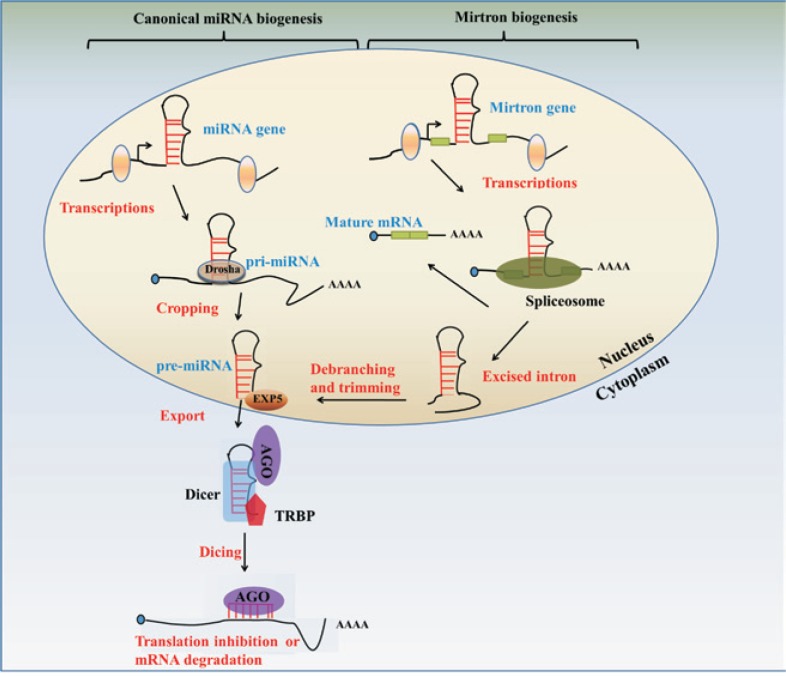
Sequence analysis of miRNA positions in the human genome reveals the majority of miRNAs are located in intergenic regions[19],[20], although a few located within exonic or intronic regions have been described[21]. The canonical pathway for miRNA biogenesis begins with transcription of primary miRNA (pri-miRNA) by RNA polymerase II (Figure 1). The pri-miRNA is processed by the RNase Drosha in the nucleus into ∼70 nucleotide precursor miRNA (pre-miRNA) products, which locally fold into stable secondary hairpin loops with ∼2 nucleotide 3′-overhangs[22]. The pre-miRNAs are then exported to the cytoplasm by the Ran-GTP–dependent transporter Exportin 5[23] for further cleavage by the RNase Dicer, which is assisted by its partner TAR RNA-binding protein (TRBP) or protein activator of the interferon-induced protein kinase, also known as protein kinase interferon-inducible double-stranded RNA-dependent activator (PRKRA), to recognize and bind pre-miRNAs[24]. The resulting imperfect miRNA:miRNA* duplex is then unwound and one strand is selected as the mature miRNA, based on sequence thermodynamic properties[25], whereas the complementary passenger strand is usually subjected to degradation. Then, TRBP recruits Argonaute 2 to form the RNA-induced silencing complex (RISC)[26],[27], where the mature miRNA recognizes its mRNA target by pairing the miRNA seed region (positions 2–8) to complementary sequences located primarily in the target 3′-untranslated regions (3′-UTRs) and sometimes in the coding regions[28]. In addition, a special type of miRNA known as mirtrons is regulated through a non-canonical pathway (Figure 1). Mirtrons are produced from spliced introns that mimic the structural features of precursor miRNAs and enter the miRNA-processing pathway independent of Drosha-mediated cleavage[29],[30]. In mammalian cells, miRNAs regulate the expression of target genes by inducing mRNA degradation or translational repression, depending on the sequence complementarity between the small RNA and the target mRNA[31]. miRNAs control a wide range of pathologic and physiologic processes, including development, differentiation, cellular proliferation, programmed cell death, oncogenesis, and metastasis[32]. Therefore, dysregulation of miRNA expression can lead to pathogenesis, including many types of cancer[33].
Figure 1. Canonical and non-canonical miRNA biogenesis pathways.
In the canonical pathway (left), long primary miRNAs (pri-miRNAs) are transcribed by RNA polymerase II and are then capped and polyadenylated, forming RNA with a hairpin secondary structure. Cropping is the first step in the maturation mediated by the RNase III enzyme Drosha and produces a ∼65 nucleotide hairpin RNA with a 2-3 nucleotide overhang termed precursor—miRNA (pre-miRNA). pre-miRNA is recognized and exported into cytoplasm by the Exportin-5 (EXP5)-Ran-GTP complex for further processing by Dicer with its partner TAR RNA-binding protein (TRBP) and Arogonaute proteins 1-4 (AGO). Dicer processing generates the miRNA:miRNA* duplex. One strand of the miRNA duplex is selected to form the RNA-induced silencing complex (RISC), which mediates translation inhibition or mRNA degradation. In the non-canonical pathway (right), some miRNAs called mirtrons are embedded in short introns and bypass the Drosha procession. After the splicing and production of the mature mRNA, the excised intron is debranched and trimmed to generate the pre-miRNA that can be EXP5 and exported by EXP5-Ran-GTP complex, and then subsequently enter the canonical pathway for miRNAs biogenesis.
Similar to miRNA primary transcripts, lncRNAs are frequently transcribed by RNA polymerase II and polyadenylated. In the genome, lncRNAs are located in sense or antisense orientations relative to the protein-coding genes, either within the introns or intergenic regions. lncRNAs are involved in diverse cellular functions[34],[35]. For example, lncRNAs can serve as the precursors of small ncRNAs to produce miRNA and endo-siRNA, or serves as a “miRNA sponge” to inhibit miRNA activity[36],[37]. lncRNAs can also act as scaffolds during the formation of cellular substructures or protein complexes[38]. The best-described function of lncRNAs is the regulation of gene expression. The interaction of lncRNAs with proteins can influence protein activity and localization. For example, nuclear factor of activated T cells (NFAT) is a cellular transcription factor; the lncRNA noncoding repressor of NFAT (NRON) interacts with NFAT and regulates its nuclear-cytoplasmic trafficking, resulting in repression of NFAT target gene expression[39]. Furthermore, lncRNAs regulate gene transcription by recruiting transcription factors to bind the promoters of their target genes and activate or repress gene expression[40]. A very recent study revealed that numerous lncRNAs were induced by Toll-like receptors, which recognized microbial products and induced antimicrobial defense signaling and adaptive immune response[40]–[42]. The lncRNA lincRNA-Cox2 was found to play a key role in activation and repression of distinct classes of immune genes. Transcriptional repression of target genes was dependent on interactions of lincRNA-Cox2 with heterogeneous nuclear ribonucleoprotein (hnRNPs) A/B and A2/B1[41]. This finding indicates that lncRNAs can serve as repressors and activators of genes through their physical interactions with hnRNPs. On the other hand, lncRNAs can also occupy the binding sites of general transcription factors and block transcription[41]. Intriguingly, a number of studies demonstrate that lncRNAs are also key components of epigenetic networks, where they can interact with chromatin remodeling complexes and induce local or global changes in chromatin packaging[43],[44]. For example, lncRNA Xist, 17 kilobases in length, was transcribed from the X chromosome. Xist recruits the polycomb repressive complex 2 (PRC2) to switch off gene expression from one X chromosome in each female cell[45]. However, exactly how Xist establishes binding pattern during the initiation of X chromosome inactivation remains unknown. Recently, Engreitz et al.[46] used a high-throughput “chromosome conformation capture” technology to identify DNA sequences in close proximity to each other within the nucleus, and found that the early binding sites for Xist correspond to loci spatially nearby the Xist transcription site, rather than those that are close along the linear sequence. They demonstrated that Xist recruited PRC2 to spread across and silence active genes using a targeting mechanism based on three-dimensional chromosome conformation, which was exploited to extrude Xist onto its early binding site targets where it then helped to modify and reorganize the X chromosome architecture[46]. Overall, lncRNAs are also emerging as important regulatory molecules in gene expression at a transcriptional, post-transcriptional, and epigenetic level.
Cancer and CSCs
A tumor mass contains heterogeneous subsets of cells with diverse states of differentiation. CSCs are a small subpopulation identified in many types of human cancers[47]. CSCs can undergo a theoretically unlimited number of mitotic cycles, and through asymmetric cell division, form progeny that are either stem-like or more differentiated cell types, depending on intrinsic or microenvironmental factors[48]. CSCs are capable of initiating tumor formation, increasing tumor cell proliferation and expansion, and becoming differentiated tumor cells[14],[48]. CSCs can be isolated based on their growth properties or by sorting using cell surface antigens, metabolic markers such as CD44, CD24 and CD133, and activity of aldehyde dehydrogenase 1 (ALDH1)[49]. Through the common sorting approach, CSCs have been isolated from hematologic malignancies[50], breast tumors[51], brain tumors[52], colon cancer[53], and other solid tumors[54].
Current cancer therapeutics for most malignant tumors can reduce tumor size or inhibit further progression, but have a limited curative effect. CSCs, which are intrinsically resistant to conventional chemotherapy and radiation treatment, are hypothesized to lead to tumor recurrence. Thus current treatments are unlikely to result in long-term remission unless the CSCs are also targeted[47]. Treatment resistance results from multiple factors. Resistance to chemotherapy is partially attributed to the overexpression of transmembrane efflux pump proteins, which are regulated by reactive oxygen species within cells[55]. However, Zielske et al.[56] revealed a controversial result from two patients on treatment for therapy-resistant CSCs. The CD44+CD24−/low lineage and ALDH+ breast CSCs from one patients were rapidly depleted 2 weeks after treatment with radiation, resulting in a significant decrease in tumor sphere frequency and tumorigenic capacity. In contrast, CSCs from the other patient showed enrichment after radiation and resistance to therapy, suggesting that CSC variance may exist in individual patients[56]. Therefore, therapeutics that target different CSC subtypes is likely required.
The mechanisms that regulate CSC growth, in conjunction with factors that facilitate resistance to radiation and chemotherapy, are of particular clinical importance given the role CSCs have in tumorigenesis and recurrence[18],[47],[48],[54],[57]–[60]. Stem cells undergo symmetric or asymmetric cell division, producing identical stem cell progeny or cells that are more differentiated. Moreover, the cycling of stem cells varies considerably, facilitating so-called kinetic resistance, whereby slow cycling or quiescent stem cells are unaffected by DNA damaging agents or radiation compare to the way more rapidly dividing cells are. CSCs also show great drug resistance through other mechanisms, including drug effluxing. Resistance to radiation can occur through the repair of damaged DNA, redistribution of cycling cells, re-oxygenation of hypoxic tumor regions, and repopulation of the tissue, commonly considered the “four Rs.” The role of ncRNAs in regulating these mechanisms of resistance, in addition to intrinsic resistance that CSCs may have, is an exciting area of research.
miRNAs in Cancer and CSCs
Since the first characterized miRNAs, miR-15 and miR-16, were found to be deleted in patients with chronic lymphocytic leukemia (CLL) in 2002[61], a large number of miRNAs have been found to be dysregulated in virtually all cancers[62],[63]. miRNAs regulate multiple cancer processes, such as transformation, tumor cell proliferation, epithelial-mesenchymal transition (EMT), invasion, and metastasis, mainly by inhibiting the expression of critical genes in pathways that regulate cell processes including cell cycle, apoptosis, and migration[62],[63].
miRNAs can function as either oncogenes or tumor suppressors. miR-21 is a well characterized example of an oncogenic miRNA. miR-21 is overexpressed in most types of malignancies, including breast cancer, glioblastoma, colorectal cancer, lung cancer, pancreatic cancer, and leukemia[64]–[66]. In glioblastoma, miR-21 was revealed to target several important components of the epidermal growth factor receptor (EGFR) and phosphatase and tensin homolog (PTEN) signaling pathway in glioma cell lines. Inhibition of miR-21 by specific antisense oligonucleotides in U251MG cells decreased the expression of EGFR and activated AKT, CYCLIN D, and BCL2[67],[68]. Down-regulation of PTEN, followed by AKT activation, was also reported as a result of miR-26a overexpression in both NIH-3T3 fibroblast and LN-18 human glioblastoma cells[69]. In contrast, miR-34 family members (miR-34s) are known as tumor suppressive miRNAs. The tumor suppressor TP53 transcriptionally induces the miR-34 family in response to DNA damage[70]. miR-34a is encoded by a sequence on chromosome 1, whereas both miR-34b and miR-34c are processed from one primary transcript from chromosome 11[71],[72]. miR-34a deletion was associated with metastasis and recurrence of prostate cancer[73]. Restoration of miR-34a expression in pancreatic cancer cells substantially repressed cell proliferation and invasion, and sensitized cells to chemotherapy and radiation[74]. miR-34 could also be repressed by ZEB1, a transcriptional repressor of E-cadherin that is involved in promoting metastasis by remodeling cytoskeletal actin, which is required for tumor cell invasion[74]–[76].
Compelling evidence suggests critical roles that miRNAs play in regulating stem cells and in CSC biology. The regulation of stem cells by miRNA has been partially revealed through their regulation of pluripotency and differentiation. For example, the balance between stemness and differentiation is maintained by the reciprocal regulation miR-145 and the embryonic stem cell genes NANOG, OCT4, SOX2 and KLF4[77]–[81]. In fact, the use of feedback mechanisms that drives stemness while inhibiting differentiation, or vice versa, is a common occurrence in stem cell signaling. In embryonic stem cells, signaling from transcription factors OCT4, NANOG, SOX2, and MYC are regulated with LIN28 to maintain embryonic stem cells in their pluripotent state[82]–[84]. Alternatively, let-7 is activated upon differentiation and inhibits LIN28 (by binding to the gene's 3′-UTR) and MYC, creating a bi-stable switch between stemness and differentiation[85],[86]. These interlinked pathways have been exploited to create induced-pluripotent stem cells by different combinations of transcription factors or miRNAs[81]–[83].
Interlinked ncRNA and protein signaling pathways are aberrantly perturbed and result in transformation and oncogenic activity. For example, let-7 represses cell proliferation and is often down-regulated in many tumors[87],[88]. The role of let-7 and LIN28 in inhibiting or enhancing oncogenic signaling via RAS, NF-κB, and high mobility group AT-hook 2 (HMGA2) make these pathways attractive therapeutic targets[88]–[90]. Studies that demonstrate the important feedback inhibition of enhanced LIN28 activity or repressed let-7 in regulating stem cell activity[86],[91] highlight how these miRNAs are important to oncogenesis. LIN28 inhibits the tumor suppressor activity in breast cancer and regulates the ALDH1, a marker for breast and ovarian CSCs[92]. Recently, a comparative analysis of miRNA expression profiles between embryonic stem cells and breast CSCs showed an overlap between the two groups, and 37 miRNAs were found to be differentially expressed in CD44+CD24−/low breast CSCs. In breast CSCs, three clusters of miR-200 family miRNAs, miR-200c-141, miR-200b-200a-429, and miR-183-96-182, were significantly down-regulated[76]. Recently, miR-22 was found to suppress the expression of miR-200 family members in breast CSCs via direct targeting of chromatin remodeling enzymes such as ten-eleven translocation (TET) family members. Inhibition of TETs leads to hypermethylation of the miR-200 promoter and induction of EMT and stemness in breast CSCs[93]. In CD133+ colon CSCs, a distinct miRNA signature of 11 overexpressed and 8 underexpressed miRNAs was shown to be involved in self-renewal and differentiation[94],[95]. In glioma CSCs, miR-128 could specifically block the self-renewal capacity of CSCs through down-regulation of BMI1, whereas miR-125 promoted cell proliferation by regulating CDK6 and CDC25[96],[97]. In EpCAM-positive hepatocellular CSC, miR-181 expression was shown to be involved in the regulation of differentiation by targeting transcription factors CDX2 and GATA6[98],[99]. In pancreatic carcinoma, CSCs showed a signature of 210 miRNAs involved in self-renewal and differentiation, including miR-99a, miR-100, miR-125b, miR-192, and miR-429[100]. miR-34 is down-regulated in pancreatic CSCs, and restoring miR-34 in these CSCs repressed self-renewal by blocking the expression of the BCL2 and NOTCH signaling pathways[101]. Taken together, these findings demonstrate that miRNAs play a critical role in regulating CSC self-renewal, differentiation, and tumorigenesis.
lncRNAs in Cancer Cells
In addition to well-characterized miRNAs, lncRNAs have also emerged as important regulators in oncogenic and tumor suppressor pathways (Table 2)[102]. Accumulating evidence provides mechanistic insight demonstrating how lncRNAs regulate important cellular signaling pathways in cancer cells at transcriptional, post-transcriptional, and epigenetic levels[103]. Among the better characterized oncogenic lncRNAs is lincRNA-HOTAIR (HOX antisense intergenic RNA), which is located in the mammalian homeobox C (HOXC) locus on chromosome 12q13.13[104]. HOTAIR was highly up-regulated in primary and metastatic breast tumors. Depletion of HOTAIR resulted in an altered pattern of H3K27 methylation and decreased invasiveness, whereas restoration of HOTAIR had the opposite effect[105]. Furthermore, the expression level of HOTAIR in primary breast tumors was a powerful predictor of patient outcomes and correlated with metastasis and low survival rates[106]. Expression of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), located at chromosome 11q13.1, associates with metastasis and poor prognosis in patients with non–small cell lung cancer (NSCLC)[106]. Studies in cervical and lung cancers implicate MALAT1 in regulating the invasive potential of cancer cells[107],[108]. Most recently, Yang et al.[109] reported that two lncRNAs, PRNCR1 and PCGEM1, were highly expressed in a large number of aggressive prostate cancers and drive androgen receptor (AR)–associated transcriptional programs to promote prostate cancer growth. In this study, PRNCR1 was found to directly interact with the acetylated carboxyl terminus of AR and recruit the enzyme DOT1L, resulting in methylation of the amino terminus of AR and subsequent interaction with PCGEM1. PCGEM1 then induced PYGO2, an important component of the Wnt signaling transcriptional complex, to activate the expression of AR-targeted genes by binding to H3K4me3 chromatin marks in the genes' promoter sequences[109],[110].
Table 2. Examples of lncRNAs in cancer.
| lncRNA | Cancer types | Functions | Molecular interactors | Reference(s) |
| Oncogene | ||||
| ANRIL/p15AS | Prostate, leukemia | Suppression of senescence via INK4A | PRC1, PRC2 | [139],[150] |
| HOTAIR | Breast, hepatocellular | Promotes metastasis | PRC2, LSD1 | [104],[105] |
| MALAT1/NEAT2 | Lung, prostate, breast, colon | Promotes metastasis | Unknown | [106]–[109] |
| PCGEM1 | Prostate | Inhibits apoptosis; promotes cell proliferation | Unknown | [151] |
| TUC338 | Hepatocellular | Promotes cell proliferation | Unknown | [152] |
| H19 | Breast, hepatocellular | Promotes cell proliferation | Unknown | [139]–[141] |
| Tumor suppressor | ||||
| GAS5 | Breast | Induces apoptosis and growth arrest | GR | [153],[154] |
| linc-p21 | Mouse model, lung cancer cells, sarcoma, lymphoma | Induces apoptosis | hnRNP-K | [115] |
| MEG3 | Meningioma, hepatocellular | Inhibits cell proliferation | Unknown | [10],[111]–[114] |
| PTENP1 | Prostate, colon | Binds PTEN-suppressing miRNAs | Unknown | [155] |
ANRIL, antisense noncoding RNA in the INK4 locus; HOTAIR, HOX antisense intergenic RNA; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; NEAT2, nuclear enriched abundant transcript 2; PCGEM1, prostate-specific transcript 1; TUC338, transcribed ultra-conserved region 338; GAS5, growth arrest-specific 5; MEG3, maternally expressed 3; PTENP1, phosphatase and tensin homolog pseudogene 1; PRC, polycomb complex; LSD1, lysine-specific demethylase 1; GR, glucocorticoid receptor.
Several lncRNAs are involved in the regulation of p53 tumor suppressor signaling. Maternally expressed gene 3 (MEG3), an imprinted lncRNA on chromosome 14q32.3 in humans, was shown to activate p53 and facilitate p53 signaling, including enhancing p53 interactions with the promoters of its target genes[111]. Frequent hypermethylation within the MEG3 promoter is widely observed in human cancers, including pituitary tumors[10], meningioma[112], and leukemia[113]. Overexpression of MEG3 suppresses cell proliferation in meningioma and hepatocellular carcinoma cell lines[112],[114], suggesting that MEG3 functions as a putative tumor suppressor. In addition, some lncRNAs are under the regulation of p53. For example, linc-p21, a murine lncRNA located near the p21 gene, has also emerged as an important gene in p53 pathways. In murine lung cancer, sarcoma, and lymphoma, linc-p21 expression was induced by p53 stimulation and repressed p53 target genes via interaction with hnRNP-K, a protein that binds the promoters of p53 downstream genes[115]. However, it is still unknown whether the human homolog of linc-p21 has a similar mechanism, although lincRNA-p21 seems to be conserved and was also up-regulated in human fibroblasts after induction of DNA damage[115].
Other ncRNA Species in Cancer Cells and CSCs
In addition to miRNAs and lncRNAs, other ncRNA species such as piRNAs and snoRNAs are gaining a greater appreciation for their role in carcinogenesis[33]. piRNAs are small ncRNAs of 24–30 nucleotides that are found in the germ line of flies and vertebrates[116]. piRNAs are generated independent of Dicer processing and interact with the PIWI subfamily of the argonaute protein family that are implicated in maintaining germ line genome stability[116]. Recently, piRNA-651 was found to be up-regulated in several cancer cell lines including gastric, lung, mesothelial, breast, liver, and cervical cancer cell lines[117]. The inhibition of piRNA-651 by antisense oligonucleotides led to the repression of proliferation in gastric cancer cells and cell cycle arrest at the G2/M phase[117]. In contrast, piRNA-823 was significantly decreased in gastric cancer tissues when compared with non-cancerous tissues. Restoration of piR-823 in gastric cancer cells inhibited cell proliferation and tumor growth in a xenograft nude mouse model[118]. Both piRNAs and PIWI proteins can play an important role in tumor growth. It was recently shown that ectopic expression of germ line PIWI genes can drive brain tumor development in Drosophila[119]. This evidence indicates that the piRNA pathway is not limited to germ line cells, but also plays roles in tumorigenesis, although the specific functions of piRNAs are largely unknown.
Recent next-generation sequencing studies have highlighted differentially expressed ncRNA between stem cells and differentiated cells. For example, small non-polyadenylated (NPA)–conserved RNA (snacRNA) differs significantly between embryonic stem cells and differentiated cells[120]. It is unknown whether snacRNAs have functional activity in cancer cells or CSCs.
snoRNAs are a group of intermediate-sized ncRNAs (60–300 bp) characterized in eukaryotes. snoRNAs are enriched in the nucleolus, where they provide the cellular locale for the synthesis and processing of cytoplasmic ribosomal RNAs (rRNAs)[121]. In vertebrates, most snoRNAs are transcribed by RNA polymerase II from introns of protein-coding genes[122]. In addition, they can also be processed from introns of longer ncRNA precursors[123]. snoRNAs interact with specific proteins to form snoRNPs, which are responsible for post-transcriptional modification of rRNA and snRNA by 2′-O-methylation and pseudouridylation[121]. Recently, it was reported that human snoRNAs also have an important role in tumorigenesis. Various snoRNAs were shown to be differentially expressed in NSCLC when compared to the corresponding matched tissue[124]. A germ line homozygous 2-bp (TT) deletion of the snoRNA U50 was found in prostate tumors and loss of U50 is associated with tumorigenesis. Furthermore, frequent deletion of snoRNA U50 was also observed in breast cancer[125],[126]. Given that translation is often perturbed in cancer cells, it is possible that snoRNAs contribute to tumorigenesis by regulating protein translation[127]. Taken together, emerging evidence suggests that dysfunctions of other ncRNA species, such as piRNAs and snoRNAs, are also involved in the development and progression of cancer. However, the functional roles of these ncRNAs in the biology and tumorigenesis of cancer and CSCs are still in question. Therefore, further understanding the molecular mechanisms of how these aberrant ncRNAs contribute to the development and progression of cancer is warranted.
Noncoding RNAs as Potential Therapeutics for Cancer
miRNAs have now been recognized as promising therapeutic targets for anticancer treatments[128]. This is because modulating the level of a single miRNA can eventually affect its multiple target genes, simultaneously altering oncogenic and tumor suppressor pathways. Oncogenic miRNAs (Figure 2), which are usually overexpressed in tumors when compared with healthy tissues, can be altered by several means. Based on the knowledge that miRNAs control their targets through base pair complementarity, antisense oligonucleotides (ASOs) have been developed to inhibit miRNA function. In order to increase ASOs' stability and efficacy, different chemical modifications, such as locked nucleic acids (LNAs), anti-miRNA oligonucleotides (AMOs), and antagomirs, are incorporated in the skeleton of ASOs[129]–[131]. For example, a specific antagomir was used to knockdown the oncogene miR-21 in breast cancer MCF-7 cells, resulting insignificant inhibition of MCF-7 tumor growth in vitro and in tumor xenografts through inhibiting cell proliferation and inducing apoptosis[66]. Alternatively, “miRNA sponges” have been developed to inhibit miRNA activity in cancer cells and mouse models by incorporating the complementary binding sites of target miRNA into RNA transcripts expressed from strong promoters[132]. For instance, inhibition of miR-22 by the specific sponge in LM2 cells, a highly metastatic breast cancer cell line derived from the MDA-MB-231 cell line, resulted in a reduction of breast cancer metastasis to the lung using xenograft models[93].
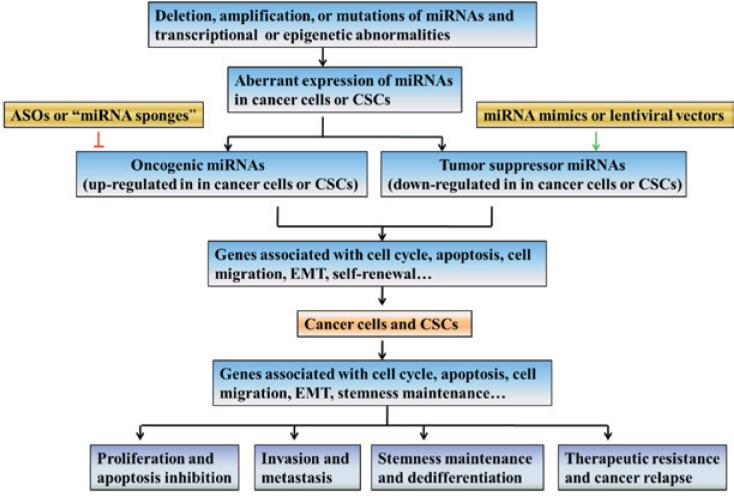
Figure 2. miRNA in cancer and cancer stem cells (CSCs).
Aberrant expression of miRNAs, either oncogenic or tumor suppressive, may be due to deletion, amplification, or mutations of miRNA genes, and dysegulation of transcriptional and epigenetic factors that regulate the miRNA genes. Dysregulation of genes linked to cell cycle, apoptosis, cell migration, epithelial-mesenchymal transition (EMT), and self-renewal results in carcinogenesis, invasion, metastasis, and maintenance of stemness. It is proposed that miRNA inhibition can knockdown the effects of oncogenic miRNAs, and miRNA mimics or lentiviral vectors expressing target miRNAs can restore the capabilities of tumor suppressor miRNAs. Therefore, miRNAs have great therapeutic potential against cancer progression, therapy resistance, and relapse. ASOs, antisense oligonucleotides.
Tumor suppressor miRNAs are usually repressed in cancer cells. miRNA mimics or lentiviral vectors can be used to restore their expression levels. miR-34 is a well-known tumor suppressor and is down-regulated in many types of cancers. In gastric cancer cells, a miR-34 mimic could arrest the cell cycle in the G1 phase and induce apoptosis by attenuating its downstream target genes, including BCL2, Notch, and HMGA2[133]. However, the delivery of the miRNA mimic into mice can only last a few days, limiting long-term efficacy. To overcome this dilemma, lentiviral vectors can be used to express sequences targeting miRNAs in cancer cells. Then these modified cancer cells with stable expression of miRNAs can be utilized for in vivo xenograft models. For example, expression of miR-34a by a lentiviral vector in pancreatic CSCs inhibited tumor sphere formation and tumor growth in vivo[101]. Therefore, miRNA mimic and lentiviral-expressed miRNAs have great potential in restoring tumor suppressor miRNAs in cancer and CSCs.
Similar to their smaller miRNA counterparts, lncRNAs represent an important untapped resource in terms of developing diagnostics and therapies. Many lncRNAs are expressed in a tissue- and cancer-type restricted manner. These lncRNAs can be exploited for the development of novel biomarkers. The highly expressed prostate lncRNA DD3 has been shown to be a prognostic marker for prostate cancer[134]. HOTAIR expression was highly increased in primary and metastatic breast tumors, and expression levels of HOTAIR were directly correlated with poor outcomes for patients with breast cancer[135]. Similarly, the high expression levels of HOTAIR were also found in hepatocellular carcinomas[136]. Therefore, HOTAIR can serve as a biomarker to predict tumor recurrence in primary breast tumor and hepatocellular carcinomas.
In comparison to well-utilized miRNA, lncRNAs are just beginning to be incorporated into therapeutic strategies against cancer. Although our knowledge of the molecular mechanisms of lncRNA function is limited, some unique characteristics of lncRNAs make these ncRNAs potential candidates for therapeutic intervention. Many lncRNAs appear to adopt some characteristic secondary structures for protein binding, thereby providing possible avenues for cancer intervention[137]. For example, inhibition of the interactions between HOTAIR and the PRC2 or LSD1 complexes restricted the metastatic potential of breast cancer cells[138]. In addition, the certain lncRNAs are expressed in specific types of cancers, which limits the off-target effects and risk to normal tissues that are associated with transgene-based therapies. For example, H19 is highly and specifically expressed in many types of human cancers[139]–[141]. As such, a construct with a diphtheria toxin gene, driven by H19 specific promoter sequences, was developed and tested in treating bladder cancer. This plasmid was administered into tumor-bearing mice through intratumoral injection either as naked DNA or as a polyplex vector consisting of the cationic polymer polyethylenimine (PEI), which increases DNA uptake efficiency. Upon uptake in the tumor, high levels of diphtheria toxin were expressed, leading to a reduction in tumor growth[142],[143]. Collectively, these studies indicate that lncRNAs provide novel strategies in cancer diagnostics and therapies.
Conclusions
Dysregulation of ncRNAs is involved in regulation of cellular signaling in cancer and CSCs. A better understanding of the molecular mechanisms and pathways regulating ncRNAs and how they control tumor phenotype and malignancy offers promise for developing more effective cancer therapies. Emerging evidence demonstrates that lncRNAs are involved in self-renewal of embryonic and pluripotent stem cells, but it is still unknown whether ncRNAs drive the transformation process or what role they play in maintaining stemness and rendering therapeutic resistance of CSCs. Therefore, studies of ncRNA biology will ultimately yield further insights into the molecular mechanisms of tumorigenesis and lead to the development of new therapeutic strategies against cancer.
Acknowledgments
This work was supported in part by grants from the US NIH grant CA130966, CA158911 to S. Y. Cheng, the Zell Scholar Award from the Zell Family Foundation and funds from Northwestern Brain Tumor Institute and Department of Neurology at Northwestern University Feinberg School of Medicine to S. Y. Cheng, and the Brain Cancer Research Award from the James S. McDonnell Foundation to B. Hu.
References
- 1.Alexander RP, Fang G, Rozowsky J, et al. Annotating non-coding regions of the genome. Nat Rev Genet. 2010;11:559–571. doi: 10.1038/nrg2814. [DOI] [PubMed] [Google Scholar]
- 2.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 3.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 4.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 5.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Hammond SM. MicroRNAs as tumor suppressors. Nat Genet. 2007;39:582–583. doi: 10.1038/ng0507-582. [DOI] [PubMed] [Google Scholar]
- 8.Brosnan CA, Voinnet O. The long and the short of noncoding RNAs. Curr Opin Cell Biol. 2009;21:416–425. doi: 10.1016/j.ceb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Mattick JS. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2001;2:986–991. doi: 10.1093/embo-reports/kve230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taft RJ, Glazov EA, Cloonan N, et al. Tiny RNAs associated with transcription start sites in animals. Nat Genet. 2009;41:572–578. doi: 10.1038/ng.312. [DOI] [PubMed] [Google Scholar]
- 12.Chen LL, Carmichael GG. Long noncoding RNAs in mammalian cells: what, where, and why? Wiley Interdiscip Rev RNA. 2010;1:2–21. doi: 10.1002/wrna.5. [DOI] [PubMed] [Google Scholar]
- 13.Dinger ME, Pang KC, Mercer TR, et al. Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Computat Biol. 2008;4:e1000176. doi: 10.1371/journal.pcbi.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipovich L, Johnson R, Lin CY. MacroRNA underdogs in a microRNA world: evolutionary, regulatory, and biomedical significance of mammalian long non-protein-coding RNA. Biochim Biophys Acta. 2010;1799:597–615. doi: 10.1016/j.bbagrm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Spizzo R, Almeida MI, Colombatti A, et al. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YK, Kim VN. Processing of intronic microRNAs. EMBO J. 2007;26:775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez A, Griffiths-Jones S, Ashurst JL, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass drosha processing. Nature. 2007;448:83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi R, Qin Y, Macara IG, et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhayani MK, Calin GA, Lai SY. Functional relevance of miRNA sequences in human disease. Mutat Res. 2012;731:14–19. doi: 10.1016/j.mrfmmm.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Okamura K, Liu N, Lai EC. Distinct mechanisms for microRNA strand selection by drosophila argonautes. Mol Cell. 2009;36:431–444. doi: 10.1016/j.molcel.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chendrimada TP, Gregory RI, Kumaraswamy E, et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haase AD, Jaskiewicz L, Zhang H, et al. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farazi TA, Spitzer JI, Morozov P, et al. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berezikov E, Chung WJ, Willis J, et al. Mammalian mirtron genes. Mol Cell. 2007;28:328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamura K, Hagen JW, Duan H, et al. The mirtron pathway generates microRNA-class regulatory RNAs in drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 32.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Ann Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 33.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 34.Guttman M, Donaghey J, Carey BW, et al. LincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annl Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Liu X, Wu H, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemson CM, Hutchinson JN, Sara SA, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33:717–726. doi: 10.1016/j.molcel.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willingham AT, Orth AP, Batalov S, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 40.Feng J, Bi C, Clark BS, et al. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter S, Aiello D, Atianand MK, et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nature Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 43.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Sun BK, Erwin JA, et al. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JT. Gracefully ageing at 50, X-chromosome inactivation becomes a paradigm for RNA and chromatin control. Nat Rev Mol Cell Biol. 2011;12:815–826. doi: 10.1038/nrm3231. [DOI] [PubMed] [Google Scholar]
- 46.Engreitz JM, Pandya-Jones A, McDonel P, et al. The XIST lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 48.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 51.Al-Hajj M, Wicha MS, Benito-HeRNAndez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 53.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 54.Valent P, Bonnet D, De Maria R, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 55.Tothova Z, Gilliland DG. A radical bailout strategy for cancer stem cells. Cell Stem Cell. 2009;4:196–197. doi: 10.1016/j.stem.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Zielske SP, Spalding AC, Wicha MS, et al. Ablation of breast cancer stem cells with radiation. Transl Oncol. 2011;4:227–233. doi: 10.1593/tlo.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen LV, Vanner R, Dirks P, et al. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 59.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 60.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–1890; discussion 1895–1886. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 61.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of microRNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li M, Li J, Ding X, et al. MicroRNA and cancer. AAPS J. 2010;12:309–317. doi: 10.1208/s12248-010-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 64.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 65.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 67.Ren Y, Zhou X, Mei M, et al. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer. 2010;10:27. doi: 10.1186/1471-2407-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou X, Ren Y, Moore L, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of pten status. Lab Invest. 2010;90:144–155. doi: 10.1038/labinvest.2009.126. [DOI] [PubMed] [Google Scholar]
- 69.Ciafre SA, Galardi S, Mangiola A, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 70.Kong YW, Ferland-McCollough D, Jackson TJ, et al. MicroRNAs in cancer management. Lancet Oncol. 2012;13:e249–e258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 71.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corney DC, Flesken-Nikitin A, Godwin AK, et al. MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 73.Watahiki A, Wang Y, Morris J, et al. MicroRNAs associated with metastatic prostate cancer. PloS One. 2011;6:e24950. doi: 10.1371/journal.pone.0024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn YH, Gibbons DL, Chakravarti D, et al. Zeb1 drives prome-tastatic actin cytoskeletal remodeling by downregulating miR-34a expression. J Clin Invest. 2012;122:3170–3183. doi: 10.1172/JCI63608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimono Y, Zabala M, Cho RW, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adammek M, Greve B, Kassens N, et al. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil Steril. 2013;99:1346–1355. doi: 10.1016/j.fertnstert.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 78.Jain AK, Allton K, Iacovino M, et al. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012;10:e1001268. doi: 10.1371/journal.pbio.1001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jia Y, Liu H, Zhuang Q, et al. Tumorigenicity of cancer stem-like cells derived from hepatocarcinoma is regulated by microRNA-145. Oncol Rep. 2012;27:1865–1872. doi: 10.3892/or.2012.1701. [DOI] [PubMed] [Google Scholar]
- 80.Sureban SM, May R, Qu D, et al. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PloS One. 2013;8:e73940. doi: 10.1371/journal.pone.0073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu N, Papagiannakopoulos T, Pan G, et al. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 82.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 83.Judson RL, Babiarz JE, Venere M, et al. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 85.Chang TC, Zeitels LR, Hwang HW, et al. Lin-28b transactivation is necessary for myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A. 2009;106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rybak A, Fuchs H, Smirnova L, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 87.Boyerinas B, Park SM, Shomron N, et al. Identification of let-7-regulated oncofetal genes. Cancer Res. 2008;68:2587–2591. doi: 10.1158/0008-5472.CAN-08-0264. [DOI] [PubMed] [Google Scholar]
- 88.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee ST, Chu K, Oh HJ, et al. Let-7 microRNA inhibits the prolifera-tion of human glioblastoma cells. J Neurooncol. 2011;102:19–24. doi: 10.1007/s11060-010-0286-6. [DOI] [PubMed] [Google Scholar]
- 91.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang X, Lin X, Zhong X, et al. Double-negative feedback loop between reprogramming factor lin28 and microRNA let-7 regulates aldehyde dehydrogenase 1-positive cancer stem cells. Cancer Res. 2010;70:9463–9472. doi: 10.1158/0008-5472.CAN-10-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song SJ, Poliseno L, Song MS, et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154:311–324. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu XF, Zou J, Bao ZJ, et al. miR-93 suppresses proliferation and colony formation of human colon cancer stem cells. World J Gastroenterol. 2011;17:4711–4717. doi: 10.3748/wjg.v17.i42.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang H, Li W, Nan F, et al. MicroRNA expression profile of colon cancer stem-like cells in HT29 adenocarcinoma cell line. Biochem Biophys Res Commun. 2011;404:273–278. doi: 10.1016/j.bbrc.2010.11.106. [DOI] [PubMed] [Google Scholar]
- 96.Schraivogel D, Weinmann L, Beier D, et al. Camta1 is a novel tumour suppressor regulated by miR-9/9* in glioblastoma stem cells. EMBO J. 2011;30:4309–4322. doi: 10.1038/emboj.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi L, Zhang J, Pan T, et al. miR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Res. 2010;1312:120–126. doi: 10.1016/j.brainres.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 98.Ji J, Wang XW. Identification of cancer stem cell-related microRNAs in hepatocellular carcinoma. Methods Mol Biol. 2012;826:163–175. doi: 10.1007/978-1-61779-468-1_14. [DOI] [PubMed] [Google Scholar]
- 99.Meng F, Glaser SS, Francis H, et al. Functional analysis of microRNAs in human hepatocellular cancer stem cells. J Cell Mol Med. 2012;16:160–173. doi: 10.1111/j.1582-4934.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung DE, Wen J, Oh T, et al. Differentially expressed microRNAs in pancreatic cancer stem cells. Pancreas. 2011;40:1180–1187. doi: 10.1097/MPA.0b013e318221b33e. [DOI] [PubMed] [Google Scholar]
- 101.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PloS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–R161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheetham SW, Gruhl F, Mattick JS, et al. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA hotair reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 107.Rajaram V, Knezevich S, Bove KE, et al. DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation. Genes Chromosomes Cancer. 2007;46:508–513. doi: 10.1002/gcc.20437. [DOI] [PubMed] [Google Scholar]
- 108.Tano K, Mizuno R, Okada T, et al. Malat-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584:4575–4580. doi: 10.1016/j.febslet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 109.Yang L, Lin C, Jin C, et al. LncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popadiuk CM, Xiong J, Wells MG, et al. Antisense suppression of pygopus2 results in growth arrest of epithelial ovarian cancer. Clin Cancer Res. 2006;12:2216–2223. doi: 10.1158/1078-0432.CCR-05-2433. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Y, Zhong Y, Wang Y, et al. Activation of p53 by MEG3 non-coding RNA. J Biol Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, Gejman R, Mahta A, et al. Maternally expressed gene 3, an imprinted noncoding RNA gene, is associated with meningioma pathogenesis and progression. Cancer Res. 2010;70:2350–2358. doi: 10.1158/0008-5472.CAN-09-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Benetatos L, Hatzimichael E, Dasoula A, et al. CpG methylation analysis of the MEG3 and SNRPN imprinted genes in acute myeloid leukemia and myelodysplastic syndromes. Leuk Res. 2010;34:148–153. doi: 10.1016/j.leukres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 114.Braconi C, Kogure T, Valeri N, et al. MicroRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepato-cellular cancer. Oncogene. 2011;30:4750–4756. doi: 10.1038/onc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huarte M, Guttman M, Feldser D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ishizu H, Siomi H, Siomi MC. Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines. Genes Dev. 2012;26:2361–2373. doi: 10.1101/gad.203786.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng J, Guo JM, Xiao BX, et al. piRNA, the new non-coding RNA, is aberrantly expressed in human cancer cells. Clin Chim Acta. 2011;412:1621–1625. doi: 10.1016/j.cca.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 118.Cheng J, Deng H, Xiao B, et al. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Lett. 2012;315:12–17. doi: 10.1016/j.canlet.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 119.Janic A, Mendizabal L, Llamazares S, et al. Ectopic expression of germline genes drives malignant brain tumor growth in drosophila. Science. 2010;330:1824–1827. doi: 10.1126/science.1195481. [DOI] [PubMed] [Google Scholar]
- 120.Livyatan I, Harikumar A, Nissim-Rafinia M, et al. Non-polyadeny-lated transcription in embryonic stem cells reveals novel non-coding RNA related to pluripotency and differentiation. Nucleic Acids Res. 2013;41:6300–6315. doi: 10.1093/nar/gkt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kiss T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell. 2002;109:145–148. doi: 10.1016/s0092-8674(02)00718-3. [DOI] [PubMed] [Google Scholar]
- 122.Bortolin ML, Kiss T. Human U19 intron-encoded snoRNA is processed from a long primary transcript that possesses little potential for protein coding. RNA. 1998;4:445–454. [PMC free article] [PubMed] [Google Scholar]
- 123.Weinstein LB, Steitz JA. Guided tours: from precursor snoRNA to functional snoRNP. Curr Opin Cell Biol. 1999;11:378–384. doi: 10.1016/S0955-0674(99)80053-2. [DOI] [PubMed] [Google Scholar]
- 124.Liao J, Yu L, Mei Y, et al. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Mol Cancer. 2010;9:198. doi: 10.1186/1476-4598-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dong XY, Guo P, Boyd J, et al. Implication of snoRNA U50 in human breast cancer. J Genet Genomics. 2009;36:447–454. doi: 10.1016/S1673-8527(08)60134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong XY, Rodriguez C, Guo P, et al. SnoRNA U50 is a candidate tumor-suppressor gene at 6q14.3 with a mutation associated with clinically significant prostate cancer. Hum Mol Genet. 2008;17:1031–1042. doi: 10.1093/hmg/ddm375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mannoor K, Liao J, Jiang F. Small nucleolar RNAs in cancer. Biochim Biophys Acta. 2012;1826:121–128. doi: 10.1016/j.bbcan.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elmen J, Lindow M, Schutz S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 130.Esau CC. Inhibition of microRNA with antisense oligonucleotides. Methods. 2008;44:55–60. doi: 10.1016/j.ymeth.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 131.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with “antagomirs”. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 132.Cohen SM. Use of microRNA sponges to explore tissue-specific microRNA functions in vivo. Nat Methods. 2009;6:873–874. doi: 10.1038/nmeth1209-873. [DOI] [PubMed] [Google Scholar]
- 133.Ji Q, Hao X, Meng Y, et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tinzl M, Marberger M, Horvath S, et al. DD3PCA3 RNA analysis in urine—a new perspective for detecting prostate cancer. Eur Urol. 2004;46:182–186. doi: 10.1016/j.eururo.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 135.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang Z, Zhou L, Wu LM, et al. Overexpression of long non-coding RNA hotair predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 137.Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol. 2010;7:582–585. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Castle JC, Armour CD, Lower M, et al. Digital genome-wide ncRNA expression, including snoRNAs, across 11 human tissues using polya-neutral amplification. PloS One. 2010;5:e11779. doi: 10.1371/journal.pone.0011779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lustig O, Ariel I, Ilan J, et al. Expression of the imprinted gene H19 in the human fetus. Mol Reprod Dev. 1994;38:239–246. doi: 10.1002/mrd.1080380302. [DOI] [PubMed] [Google Scholar]
- 141.Poirier F, Chan CT, Timmons PM, et al. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development. 1991;113:1105–1114. doi: 10.1242/dev.113.4.1105. [DOI] [PubMed] [Google Scholar]
- 142.Amit D, Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. J Transl Med. 2010;8:134. doi: 10.1186/1479-5876-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scaiewicz V, Sorin V, Fellig Y, et al. Use of H19 gene regulatory sequences in DNA-based therapy for pancreatic cancer. J Oncol. 2010;2010:178174. doi: 10.1155/2010/178174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Di Giacomo M, Comazzetto S, Saini H, et al. Multiple epigenetic mechanisms and the piRNA pathway enforce LINE1 silencing during adult spermatogenesis. Mol Cell. 2013;50:601–608. doi: 10.1016/j.molcel.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 145.Watanabe T, Tomizawa S, Mitsuya K, et al. Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse RASGRF1 locus. Science. 2011;332:848–852. doi: 10.1126/science.1203919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seila AC, Calabrese JM, Levine SS, et al. Divergent transcription from active promoters. Science. 2008;322:1849–1851. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Preker P, Nielsen J, Kammler S, et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science. 2008;322:1851–1854. doi: 10.1126/science.1164096. [DOI] [PubMed] [Google Scholar]
- 148.Sana J, Hankeova S, Svoboda M, et al. Expression levels of transcribed ultraconserved regions UC.73 and UC.388 are altered in colorectal cancer. Oncology. 2012;82:114–118. doi: 10.1159/000336479. [DOI] [PubMed] [Google Scholar]
- 149.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kotake Y, Nakagawa T, Kitagawa K, et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Prensner JR, Iyer MK, Balbin OA, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Braconi C, Valeri N, Kogure T, et al. Expression and functional role of a transcribed noncoding RNA with an ultraconserved element in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2011;108:786–791. doi: 10.1073/pnas.1011098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kino T, Hurt DE, Ichijo T, et al. Noncoding RNA GAS5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mourtada-Maarabouni M, Pickard MR, Hedge VL, et al. GAS5, a non-protein-coding RNA, controls apoptosis and is down-regulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 155.Poliseno L, Salmena L, Zhang J, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]