Abstract
Systemic chemotherapy is the basic palliative treatment for metastatic nasopharyngeal carcinoma (NPC); however, it is not known whether locoregional radiotherapy targeting the primary tumor and regional lymph nodes affects the survival of patients with metastatic NPC. Therefore, we aimed to retrospectively evaluate the benefits of locoregional radiotherapy. A total of 408 patients with metastatic NPC were included in this study. The mortality risks of the patients undergoing supportive treatment and those undergoing chemotherapy were compared with that of patients undergoing locoregional radiotherapy delivered alone or in combination with chemotherapy. Univariate and multivariate analyses were conducted. The contributions of independent factors were assessed after adjustment for covariates with significant prognostic associations (P < 0.05). Both locoregional radiotherapy and systemic chemotherapy were identified as significant independent prognostic factors of overall survival (OS). The mortality risk was similar in the group undergoing locoregional radiotherapy alone and the group undergoing systemic chemotherapy alone [multi-adjusted hazard ratio (HR) = 0.9, P = 0.529]; this risk was 60% lower than that of the group undergoing supportive treatment (HR = 0.4, P = 0.004) and 130% higher than that of the group undergoing both systemic chemotherapy and locoregional radiotherapy (HR = 2.3, P < 0.001). In conclusion, locoregional radiotherapy, particularly when combined with systemic chemotherapy, is associated with improved survival of patients with metastatic NPC.
Keywords: Nasopharyngeal carcinoma, distant metastases, overall survival, radiotherapy, systemic chemotherapy
Nasopharyngeal carcinoma (NPC) is an endemic Epstein-Barr virus (EBV)-related neoplasm that occurs commonly in southern China, southeastern Asia, and northern Africa[1]–[5]. NPC is highly sensitive to radiotherapy and moderately sensitive to chemotherapy. The 5-year overall survival (OS) rate is around 90% for patients with early-stage NPC when treated with intensity-modulated radiotherapy (IMRT)[6] and is 68% to 74.5% for patients with non-metastatic, locoregionally advanced NPC when treated with chemoradiotherapy[7],[8]. However, among head and neck cancers, NPC is the most predisposed to distant metastasis, with an incidence of 4.4% to 6% at initial diagnosis (stage IVc)[9]–[11]. Metastatic NPC is considered incurable and devastating, with a median OS of 10–15 months when treated with palliative chemotherapy[12].
Aggressive local treatment of the primary tumor is considered futile in patients with metastatic NPC, and its use has been generally limited to local symptomatic control[11],[13]. However, an increasing amount of clinical evidence suggests that aggressive treatment of the primary tumor, including surgery or radiotherapy, could improve the OS rate of patients with metastatic renal[14],[15], breast[16],[17], and prostate cancers[18]. Recently, one case report article showed that two patients had long-term disease-free survival (29 and 91 months)[19], and two retrospective papers with case series showed that patients had prolonged median survival (25 and 36 months) after combined treatment of chemotherapy and definitive radiotherapy targeting both the primary tumor and its regional lymph nodes (locore-gional radiotherapy)[20],[21]. However, the survival of patients treated with locoregional radiotherapy has not been compared with that of patients undergoing chemotherapy alone or supportive treatment.
In the present study, we evaluated the therapeutic effect of locoregional radiotherapy alone, systemic chemotherapy alone, systemic chemotherapy in combination with locoregional radiotherapy, and supportive treatment to identify the most promising treatment strategy for patients with metastatic NPC.
Patients and Methods
Clinicopathologic characteristics
Patients with metastatic NPC were identified from a database of inpatients at Sun Yat-sen University Cancer Center (SYSUCC) in Guangzhou, China. The database listed 10,464 patients with pathologically diagnosed NPC who were admitted to SYSUCC between January 2001 and December 2009. Informed consent was obtained from each patient, and chart reviews were performed after the study protocol was approved by the local Ethics Committee. The following exclusion criteria were used: more than 3 months from the diagnosis of metastasis to pathologic proof of NPC, and missing clinical/survival data (for survival analysis). Based on the recorded clinical and radiological data, all patients were retrospectively classified into T1-4, N0-3, and M1, following the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) TNM classification system (6th edition, 2002).
Treatments
According to our institutional guideline for the palliative treatment of metastatic NPC, cisplatin-based systemic chemotherapy was first recommended to all patients as the basic treatment. Locoregional radiotherapy was administered to some patients as local symptomatic treatment or as part of a multidisciplinary approach using two-dimensional conventional radiotherapy (2D-CRT) or IMRT as previously described[7],[22]. Briefly, the nasopharynx and upper neck were irradiated with 6 or 8 MV photon and electron beams through bilateral, opposing faciocervical portals using a shrinking-field technique to limit the radiation exposure of the spinal cord. IMRT with 6 MV photons was delivered using a dynamic multileaf intensity-modulating collimator (NOMOS Corporation, Sewickley, PA) with a slice-by-slice arc rotation approach. In some patients, an additional anterior cervical field with a laryngeal block was used to treat the lower neck. The timing and combination of chemotherapy (induction, concurrent, and/or adjuvant) in relation to radiotherapy was at the discretion of the attending radiation oncologists. Local treatment of the metastatic disease, including radiotherapy, surgical resection, or ablation, or other treatments were provided to some patients to eliminate metastases in the bone, liver, lungs, or other organs.
Evaluation of treatment efficacy
The tumor response to treatment was assessed according to the WHO criteria by reviewing the results of a series of physical examinations and radiological investigations. A complete response (CR) to systemic chemotherapy was defined as the disappearance of all evidence of locoregional and distant disease. To evaluate the efficacy of locoregional radiotherapy, an independent criterion of CR (the CRLR) was defined as the disappearance of locoregional disease.
Statistical analysis
OS was defined as the survival time from the first diagnosis of metastatic NPC to the time of death or to the most recent follow-up. OS was analyzed and compared between different subgroups. The actuarial rates and the estimated median survival were calculated using the Kaplan-Meier method, and the differences were compared using the log-rank test. To evaluate the independent contribution of each variable to mortality, the covariates that were identified by univariate analyses as significantly associated (P < 0.05) with prognosis were included in the multivariate analyses. Additionally, survival curves were plotted using the Cox multivariate model. All analyses were performed with SPSS software (version 16.0, SPSS Inc., Chicago, IL), and a two-tailed P value of < 0.05 was considered significant.
Results
Survival outcomes of patients with metastatic NPC after anticancer treatments
Among the 10,464 patients in the database, 590 patients (5.64%) had distant metastases at diagnosis. A total of 408 patients, with a median age of 47 years (range, 13-90 years), were included in the analysis (Table 1).
Table 1. Clinical characteristics of 408 patients with metastatic nasopharyngeal carcinoma (NPC).
| Characteristic | Number of patients | Percentage (%) |
| Sex | ||
| Male | 342 | 83.8 |
| Female | 66 | 16.2 |
| Pathologic type | ||
| WHO I/II | 17 | 4.2 |
| WHO III | 391 | 95.8 |
| T classificationa | ||
| T1-2 | 157 | 38.5 |
| T3-4 | 251 | 61.5 |
| N classificationa | ||
| N0-1 | 144 | 35.3 |
| N2-3 | 264 | 64.7 |
| Chemotherapy (n=345) | ||
| Cisplatin-based | 293 | 84.9 |
| Non-Cisplatin | 52 | 15.1 |
| Radiotherapy (n=214) | ||
| 2D-CRT | 194 | 90.7 |
| IMRT | 20 | 9.3 |
WHO, World Health Organization; 2D-CRT, two-dimensional conventional radiotherapy; IMRT, intensity-modulated radiotherapy. aT and N classifications were performed according to the American Joint Committee on Cancer (AJCC) staging system (6th edition).
At the cutoff of January 2012, the median follow-up time was 19.2 months (range, 0.7–134.1 months). Among the 408 patients, 383 (93.9%) were offered anticancer treatments, including systemic chemotherapy, locoregional radiotherapy, and local treatment of metastatic disease alone or in combination; 25 (6.1%) declined any anticancer treatment and preferred supportive treatment, analgesic therapy, or watching and waiting (Figure 1). The cisplatin-based regimen was the most frequently used first-line chemotherapy (293/345, 84.9%), whereas gemcitabine and paclitaxol were used in the remaining patients (15.1%). The patients underwent 1-29 cycles of chemotherapy, with a median of 6 cycles. In the patients who underwent locoregional radiotherapy, most of them (194/214, 90.7%) underwent 2D-CRT with a median dose of 70 Gy (range, 40-84 Gy) at the primary site, whereas the rest (9.3%) underwent IMRT with a median dose of 72 Gy (range, 70-74 Gy) at the primary site (Table 1). After these palliative therapies, 254 (62.3%) patients died, and the estimated median survival time (MST) was 24.7 months. At 1, 2, 3, and 5 years, the OS rates were 79.2%, 51.7%, 33.7%, and 24.3%, respectively.
Figure 1. The distribution of the different anticancer treatments for 408 patients with metastatic nasopharyngeal carcinoma (NPC).
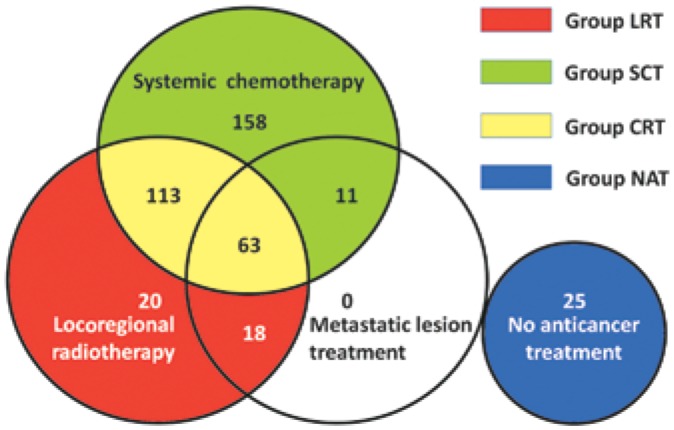
Systemic chemotherapy, locoregional radiotherapy, or local treatment of metastatic disease were offered as treatment options to 383 patients, whereas 25 patients declined any anticancer treatment. LRT, locoregional radiotherapy alone; SCT, systemic chemotherapy alone; CRT, locoregional radiotherapy plus systemic chemotherapy; NAT, no anticancer treatment.
Survival benefits attributed to locoregional radiotherapy
The association of the clinicopathologic characteristics with mortality was determined by univariate analysis (Table 2). In general, the MSTs were significantly longer in the patients who underwent anticancer treatment than in these who underwent no anticancer treatment (all P < 0.05). Furthermore, the MSTs were significantly longer in the patients with CR to treatment than in the patients without CR (P = 0.001). Longer survival was also associated with the 2005–2009 period of diagnosis, a Karnofsky Performance Scale (KPS) score ≥90, N0-1 disease, single metastatic organ/lesion involvement, and the absence of liver metastasis; however, survival was not associated with sex, age, pathologic type, T classification, or metastases in the bone, lungs, or distant lymph nodes.
Table 2. Univariate analysis of patient characteristics for overall survival (n = 408).
| Parameter | n | Overall survival (months) |
HR (95% CI) | P | |
| Median | 95% CI | ||||
| Sex | 0.826 | ||||
| Female | 66 | 23.3 | 16.4-30.2 | ||
| Male | 342 | 25.1 | 22.1-28.0 | 1.0 (0.7-1.5) | |
| Age (years) | 0.459 | ||||
| < 47 | 197 | 26.0 | 20.8-31.1 | ||
| ≥ 47 | 211 | 23.3 | 19.1-27.6 | 1.1 (0.9-1.4) | |
| Pathologic type | 0.900 | ||||
| WHO I/II | 17 | 31.8 | 14.7-48.8 | ||
| WHO III | 391 | 24.7 | 22.0-27.4 | 1.0 (0.5-1.9) | |
| Period of diagnosis | 0.011 | ||||
| 2000 to 2005 | 201 | 23.3 | 18.8-27.9 | ||
| 2006 to 2009 | 207 | 25.1 | 19.8-30.3 | 0.7 (0.5-0.9) | |
| KPS | 0.025 | ||||
| < 90 | 104 | 18.9 | 12.8-25.0 | ||
| ≥ 90 | 304 | 26.9 | 23.4-30.4 | 0.7 (0.5-0.9) | |
| T classification | 0.454 | ||||
| T1-2 | 157 | 25.3 | 21.9-28.8 | ||
| T3-4 | 251 | 24.6 | 20.3-28.5 | 0.9 (0.7-1.2) | |
| N classification | 0.003 | ||||
| N0-1 | 144 | 30.5 | 23.2-37.7 | ||
| N2-3 | 264 | 21.9 | 19.0-24.9 | 1.5 (1.1-2.0) | |
| Bone metastasis | 0.725 | ||||
| Absent | 145 | 24.6 | 21.1-28.0 | ||
| Present | 263 | 27.0 | 22.7-31.3 | 1.0 (0.7-1.2) | |
| Liver metastasis | 0.008 | ||||
| Absent | 261 | 28.7 | 25.7-31.7 | ||
| Present | 147 | 20.7 | 17.7-23.7 | 1.4 (1.1-1.8) | |
| Lung metastasis | 0.598 | ||||
| Absent | 317 | 24.4 | 21.8-28.0 | ||
| Present | 91 | 26.8 | 22.5-31.0 | 1.1 (0.8-1.5) | |
| Distant lymph node metastasis | 0.063 | ||||
| Absent | 346 | 26.8 | 23.4-30.1 | ||
| Present | 62 | 22.7 | 21.3-24.1 | 1.4 (1.0-2.0) | |
| No. of metastatic organs | 0.001 | ||||
| Single | 286 | 28.0 | 24.0-32.1 | ||
| Multiple | 122 | 20.4 | 16.5-24.3 | 1.6 (1.2-2.1) | |
| No. of metastatic lesions | 0.005 | ||||
| Single | 70 | 35.3 | 23.6-47.0 | ||
| Multiple | 338 | 23.3 | 20.2-26.5 | 1.7 (1.2-2.4) | |
| Secondary metastasis | 0.966 | ||||
| No | 357 | 24.4 | 21.4-27.4 | ||
| Yes | 51 | 26.9 | 22.3-31.5 | 1.0 (0.7-1.5) | |
| Anticancer treatment | <0.001 | ||||
| No | 25 | 11.4 | 10.7-12.0 | ||
| Yes | 383 | 26.9 | 23.8-30.0 | 0.4 (0.2-0.6) | |
| Systemic chemotherapy | <0.001 | ||||
| No | 63 | 15.0 | 9.8-20.2 | ||
| Yes | 345 | 26.9 | 23.4-30.4 | 0.5 (0.4-0.7) | |
| Locoregional radiotherapy | <0.001 | ||||
| No | 194 | 17.7 | 15.5-19.9 | ||
| Yes | 214 | 34.0 | 26.8-41.2 | 0.4 (0.3-0.5) | |
| Local treatment of metastatic disease | 0.017 | ||||
| No | 316 | 23.0 | 19.8-26.2 | ||
| Yes | 92 | 33.3 | 28.8-37.7 | 0.7 (0.5-0.9) | |
| CR | 0.001 | ||||
| No | 375 | 23.1 | 19.9-26.3 | ||
| Yes | 33 | 79.0 | 28.6-129.4 | 0.4 (0.2-0.7) | |
KPS, Karnofsky Performance Scale; CR, complete response; CI, confidence interval; HR, hazard ratio.
To test the potential bias in the patient assignment, all characteristics of the patients undergoing locoregional radiotherapy and those not undergoing locoregional radiotherapy were analyzed within each subgroup (Table 3). Patients who were younger, had earlier T classifications (T1-2), and had single organ/lesion involvement without metastasis to the liver or distant lymph nodes, or no secondary metastasis tended to undergo locoregional radiotherapy. The proportion of patients who underwent local treatment of metastatic disease was higher in the locoregional radiotherapy subgroup than in the non-locoregional radiotherapy subgroup. Nevertheless, the distribution of systemic chemotherapy was similar between these two subgroups.
Table 3. χ2-test analysis of patient distributions in the locoregional radiotherapy (LRT) and non-LRT groups.
| Parameter | LRT [cases (%)] | Non-LRT [cases (%)] | P |
| Sex | 0.481 | ||
| Female | 32 (15.0) | 34 (17.5) | |
| Male | 182 (85.0) | 160 (82.5) | |
| Age (years) | 0.012 | ||
| < 47 | 116 (54.2) | 81 (41.8) | |
| ≥ 47 | 98 (45.8) | 113 (58.2) | |
| T classification | 0.03 | ||
| T1-2 | 93 (43.5) | 64 (33.0) | |
| T3-4 | 121 (56.5) | 130 (67.0) | |
| Liver metastasis | <0.001 | ||
| Absent | 158 (73.8) | 103 (53.1) | |
| Present | 56 (26.2) | 91 (46.9) | |
| Distant lymph node metastasis | 0.001 | ||
| Absent | 193 (90.2) | 153 (78.9) | |
| Present | 21 (9.8) | 41 (21.1) | |
| No. of metastatic organs | <0.001 | ||
| Single | 177 (82.7) | 109 (56.2) | |
| Multiple | 37 (17.3) | 85 (43.8) | |
| No. of metastatic lesions | <0.001 | ||
| Single | 57 (26.6) | 13 (6.7) | |
| Multiple | 157 (73.4) | 181 (93.3) | |
| Secondary metastasis | 0.002 | ||
| No | 177 (82.7) | 180 (92.8) | |
| Yes | 37 (17.3) | 14 (7.2) | |
| Systemic chemotherapy | 0.174 | ||
| No | 38 (7.9) | 25 (12.9) | |
| Yes | 176 (92.1) | 169 (87.1) | |
| Local treatment of metastatic disease | <0.001 | ||
| No | 133 (62.1) | 183 (94.3) | |
| Yes | 81 (37.9) | 11 (5.7) |
Multivariate analysis of all the studied potential prognostic factors using an adjusted Cox model identified the N classification, systemic chemotherapy, locoregional radiotherapy, and CR to treatment as independent prognostic factors. In particular, locoregional radiotherapy was associated with a 60% reduction in the risk of death [multi-adjusted hazard ratio (HR) = 0.4, 95% confidence interval (CI) = 0.3–0.5] (Table 4).
Table 4. Multivariate analysis of prognostic factors in patients with metastatic NPC.
| Variable | Categorical variable | P | HR | 95% CI for HR |
| N classification | N1-2 vs. N2-3 | <0.001 | 1.7 | 1.3-2.2 |
| Chemotherapy | No vs. yes | <0.001 | 0.5 | 0.3-0.6 |
| LRT | No vs. yes | <0.001 | 0.4 | 0.3-0.5 |
| CR | No vs. yes | 0.001 | 0.4 | 0.2-0.6 |
| No. of metastatic organs | Single vs. multiple | 0.087 | 1.3 | 1.0-1.7 |
All potential prognostic factors were taken into account by the adjusted Cox model, including sex, age, period of diagnosis, KPS acore, N classification, liver metastasis, number of metastatic organs and lesions, anticancer treatment, systemic chemotherapy, locoregional radiotherapy alone (LRT), local treatment of metastatic disease, and CR. Only N classifications, systemic chemotherapy, LRT, and CR emerged as independent prognostic factors in patients with metastatic NPC according to the multivariate analysis.
Effect of locoregional radiotherapy combined with systemic chemotherapy on metastatic NPC
Regardless of their local treatment of metastatic disease, the patients were divided into four groups, namely into those undergoing locoregional radiotherapy alone (LRT), systemic chemotherapy alone (SCT), locoregional radiotherapy plus systemic chemotherapy (CRT), and supportive treatment (NAT, no anticancer treatment). Analyses using the log-rank test and a Cox model adjusting for confounders found a similar risk of death in the LRT and SCT groups (HR = 0.9, 95% CI = 0.6–1.3, P = 0.529), but a 60% reduced risk of death in the LRT group compared to the NAT group (HR = 0.4, 95% CI = 0.2–0.8, P = 0.004). Notably, the CRT group had the highest survival rates among all four groups (Figure 2).
Figure 2. The survival curves of patients underwent different anticancer treatments.
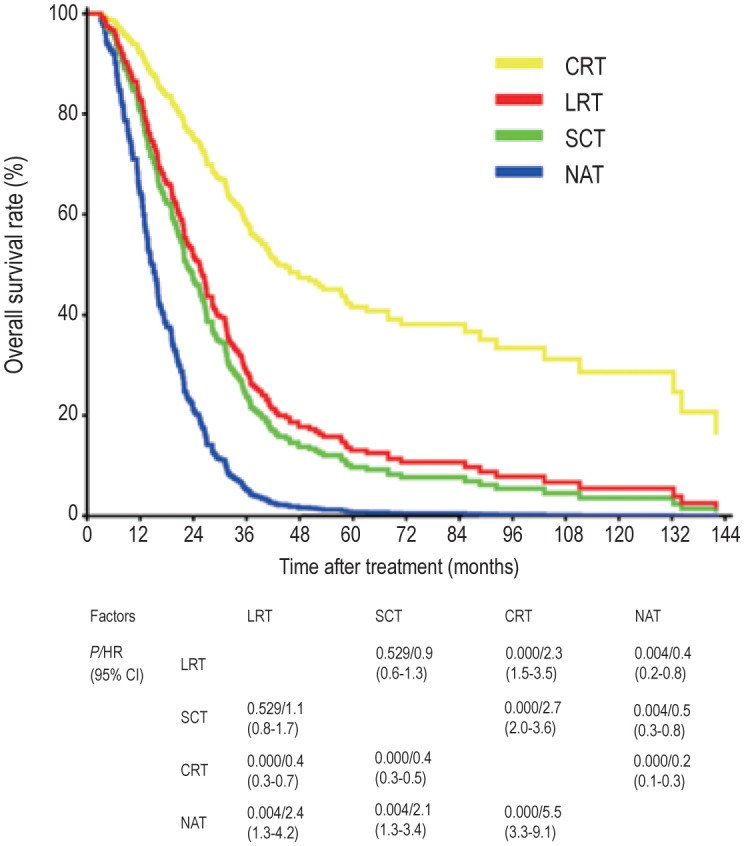
The survival curves were plotted as estimated from the Cox multivariate model with adjustments for sex, age, period of diagnosis, Karnofsky performance scale (KPS) value, N classification, liver metastasis, number of metastatic organs and lesions, and complete response (CR). The patients who underwent locoregional radiotherapy plus systemic chemotherapy (CRT group) exhibited the highest survival rate. The survival of the patients who underwent locoregional radiotherapy alone (LRT group) was similar to that of the patients who underwent systemic chemotherapy alone (SCT group), but all treatment groups had better survival than the patients who underwent no anticancer treatment (NAT group).
Effects of anticancer treatments on the survival of patients who underwent chemotherapy or radiotherapy
We further analyzed the characteristics of the subgroups undergoing different anticancer treatments (Table 5). Although the cisplatin-based regimen was the most frequently used first-line chemotherapy, it did not provide any survival benefit compared to the other regimens, including gemcitabine and paclitaxol, which were used in the remaining patients (MST: 26.9 vs. 27.3 months, P = 0.593). Furthermore, no significant survival benefit was observed from more than 6 cycles of chemotherapy compared to less than 6 cycles (MST: 27.6 vs. 23.2 months, P = 0.099). In the subgroup undergoing LRT, 79.9% (171/214) of patients underwent high-dose radiation (≥ 66 Gy) at the nasopharynx, of which 20 underwent IMRT. These patients showed better survival than the remaining 20.1% of patients who underwent low-dose radiation (< 66 Gy) at the locoregional lesion (MST: 41.0 vs. 21.3 months, P = 0.003). To determine the most promising combination of chemotherapy and radiotherapy, we further analyzed the CRT subgroup (n = 176) and found that induction chemotherapy was associated with significantly better survival (MST: 51.0 vs. 30.3 months, P = 0.007), whereas concurrent and adjuvant chemotherapy were not (P > 0.05).
Table 5. Effect of anticancer treatments on the survival of patients who underwent chemotherapy or radiotherapy.
| Treatment | n | Overall survival (months) |
HR | 95% CI | P | |
| Median | 95% CI | |||||
| Chemotherapy | 345 | |||||
| First-line regimen | 0.593 | |||||
| Cisplatin-based | 293 | 26.9 | 22.9-30.9 | 1.0 | Reference | |
| Others | 52 | 27.3 | 20.0-34.5 | 0.9 | 0.6-1.3 | |
| Cycles | 0.099 | |||||
| < 6 | 168 | 23.2 | 18.4-28.1 | 1.0 | Reference | |
| ≥ 6 | 177 | 27.6 | 23.7-31.5 | 0.8 | 0.6-1.045 | |
| Locoregional radiotherapy | 214 | |||||
| Technique | 0.017 | |||||
| 2D-CRT | 194 | 31.8 | 27.3-36.3 | 1.0 | Reference | |
| IMRT | 20 | NA | NA | 0.2 | 0.1-0.8 | |
| Radiation dose of the nasopharynx | 0.003 | |||||
| < 66 Gy | 43 | 21.3 | 16.3-26.4 | 1.0 | Reference | |
| ≥ 66 Gy | 171 | 41.0 | 28.4-53.6 | 0.5 | 0.4-0.8 | |
| Sequential chemotherapy combined with locoregional radiotherapy | 176 | |||||
| Induction chemotherapy | 0.007 | |||||
| No | 33 | 30.3 | 20.3-40.4 | 1.0 | Reference | |
| Yes | 143 | 51.0 | 31.0-71.0 | 0.5 | 0.3-0.8 | |
| Concurrent chemotherapy | 0.234 | |||||
| No | 75 | 33.3 | 22.3-44.2 | 1.0 | Reference | |
| Yes | 101 | 51.0 | 16.4-85.7 | 0.8 | 0.5-1.2 | |
| Adjuvant chemotherapy | 0.088 | |||||
| No | 124 | 51.0 | 18.5-83.6 | 1.0 | Reference | |
| Yes | 52 | 32.8 | 23.5-42.1 | 1.5 | 0.9-2.3 | |
2D-CRT, two-dimensional conventional radiotherapy; IMRT, intensity-modulated radiotherapy; NA, not available.
Discussion
Distant metastasis, including synchronous distant metastasis at diagnosis and metachronous distant metastasis after radical radio-therapy, is a leading cause of death in patients with NPC[9]–[11]. However, to date, there is no effective treatment for either synchronous or metachronous metastases. Although chemotherapy has not been shown to advantageously improve patient survival over supportive care, systemic chemotherapy is still the recommended first-line palliative treatment and the most commonly used strategy for metastatic NPC, in the hopes of achieving long-term survival in some patients after CR[23]. The relative benefits of aggressive locoregional radiotherapy to treat the primary tumor versus other palliative treatments and supportive therapy have not yet been clearly evaluated in patients with metastatic NPC at diagnosis. In our retrospective cohort study, the OS rates of all patients were higher at 1 and 2 years than the corresponding rates previously reported in patients with metastatic NPC who underwent monotherapy (39% and 14%, respectively)[24], but were similar at 1, 2, 3, and 5 years to the corresponding rates previously reported in patients with metastatic NPC who underwent multidisciplinary treatment (67.5%, 50.3%, 37%, and 16.6%, respectively)[20]. These differences may be partially explained by that nearly half of our patients underwent locoregional radiotherapy (214/408, 52.3%) and/or combination treatment (176/408, 43.1%). Notably, locoregional radiotherapy targeting the primary tumor and regional lymph nodes, particularly when combined with systemic chemotherapy, was found to be associated with significantly improved survival.
The present study focused on synchronous distant metastasis at diagnosis, which accounted for 5.64% of our patients with NPC, and we found a 53.2% reduction in the risk of death in patients undergoing chemotherapy compared to patients undergoing supportive treatment. Chemotherapy was also verified as an independent prognostic factor for patients with metastatic NPC at diagnosis. It is difficult to achieve CR to therapy in all patients with locoregional and metastatic NPC, and CR was achieved in only 8.1% (33/408) of the patients in our study. Therefore, the optimal number of chemotherapy cycles and the tumor response after chemotherapy that optimize the efficacy of chemotherapy should be examined. A previous study reported that the OS was similar in patients undergoing no less than 4 cycles of chemotherapy and those undergoing less than 4 cycles[20]. In our study, the OS of patients undergoing no less than 6 cycles of chemotherapy was also not better than that of patients undergoing less than 6 cycles, which raises the concern of acquired chemoresistance in NPC patients after 6 cycles of chemotherapy. Cisplatin-based doublet chemotherapy is the main therapeutic regimen for metastatic NPC. However, this treatment does not exhibit a higher efficiency than other new drugs, such as gemcitabine and paclitaxel, which were reported to show promising efficacies and even lower toxicities than cisplatin[25]–[27]. Therefore, well-designed prospective trials are warranted to further validate the efficacy of these therapeutic strategies in patients with metastatic NPC.
It is believed that removing the primary tumor is a good way to prevent further metastasis, but it has not been proven that controlling the locoregional tumors adds any survival benefit to chemotherapy alone or supportive treatment. In our study, locoregional radiotherapy alone was associated with a 65.3% reduction in the risk of death compared to supportive treatment, and its efficacy was similar to that of chemotherapy alone. In addition, both locoregional radiotherapy and systemic chemotherapy were found to be independent prognostic factors for increased survival in patients with metastatic NPC at initial diagnosis. Furthermore, Lin et al.[20] have found that survival was extended more by high-dose irradiation (>65 Gy) of the primary tumor than by low-dose radiation (<65 Gy), which was consistent with our observation. All these results suggest that controlling the primary and regional tumors help prolong the survival of NPC patients even after the tumor has spread to other organs. Moreover, we found that patients treated with locoregional radiotherapy combined with systemic chemotherapy had a significantly longer survival than patients treated with either chemotherapy alone or locoregional radiotherapy alone. These results suggest that sequential chemotherapy and locoregional radiotherapy should be considered the treatment of choice for newly diagnosed metastatic NPC. Furthermore, induction-based sequential chemotherapy was found to have a better response in patients than other sequential chemotherapy regimens. These results suggest that induction-based sequential chemotherapy plus high-dose LRT should be considered a priority for patients with newly diagnosed metastatic NPC.
Undetectable micrometastases from metastatic NPC have been considered the main obstacle to increasing patient survival after aggressive local treatments for visible metastatic lesions. However, the survival of NPC patients with metachronous metastasis after primary radiotherapy was shown to increase following local treatment of solitary liver or pulmonary metastatic lesions[28]–[31]. Notably, it was reported that a NPC patient with a synchronous solitary bone metastasis gained more than 5 years of disease-free survival after a combination of systemic chemotherapy, locoregional radiotherapy, and radiotherapy for metastatic bone disease[32]. The present study found a significantly longer median survival in patients who underwent local treatment of metastatic disease than in the patients who did not undergo this treatment. However, local treatment of metastatic disease was not identified as an independent prognostic factor of survival by the multivariate analysis, although this could be partially explained by the fact that more locoregional radiotherapy-treated patients were selected to undergo local treatment of metastatic disease than non-locoregional radiotherapy-treated patients.
Many independent prognostic factors of metachronous metastatic NPC, but not of synchronous metastatic NPC, have been identified. Systemic relapse is more frequently seen in those who present with locally advanced disease (T3-4 and/or N2-3 disease), especially N2-3 disease[9],[11]. In our study, N2-3 disease was identified as an independent negative prognostic predictor of survival in patients with metastatic NPC, indicating that there may be a positive correlation between lymphatic and hematogenous metastasis. This suggests that the status of the cervical lymph nodes may reflect the metastatic behavior of the cancer and thus predict the survival of patients to some extent. Patients with metastasis limited to the lungs were reported to survive relatively longer than those with metastases to other sites[10],[30], but we did not observe such a tendency. Consistent with previous studies[20], our study found that single-site or single-organ metastasis, when compared to widespread metastasis, was related to a relatively better prognosis. Interestingly, we also found that liver metastasis had a negative effect whereas bone metastasis had a positive effect on the OS of patients with metastatic NPC. However, neither liver nor bone metastasis was identified as an independent prognostic predictor by the multivariate analysis.
In summary, we conducted a retrospective observational study to evaluate the benefits of locoregional aggressive treatment in patients with metastatic NPC and found that locoregional radiotherapy as well as chemotherapy, N classification, and CR were independent prognostic indicators of survival for NPC patients with distant metastasis at initial diagnosis. We also found that locoregional radiotherapy, particularly when combined with systemic chemotherapy, was associated with improved survival of patients with metastatic NPC. However, well-designed prospective studies are warranted to control patient selection bias, to reevaluate the treatment paradigm of systemic chemotherapy combined with locoregional radiotherapy, and to determine the best sequence of radiochemotherapy in patients who are diagnosed with metastatic NPC.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81071890 and No. 81030043), Training Programme Foundation for the Talents by Sun Yat-sen University Cancer Center, and Program for New Century Excellent Talents in University in China, Program for New Century Excellent Talents in University (NCET-12-0562), Sun Yat-sen University Clinical Research 5010 Program (201310), Guangdong Provincial Natural Science Foundation of China (S2013020012726) (to M.Y. Chen).
References
- 1.Chang ET, Adami HO. The enigmatic epidemiology of naso-pharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 2.Titcomb CJ. High incidence of nasopharyngeal carcinoma in Asia. J Insur Med. 2001;33:235–238. [PubMed] [Google Scholar]
- 3.Wee JT, Ha TC, Loong SL, et al. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29:517–526. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 4.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30:114–119. doi: 10.5732/cjc.010.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adham M, Kurniawan AN, Muhtadi AI, et al. Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation. Chin J Cancer. 2012;31:185–196. doi: 10.5732/cjc.011.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su SF, Han F, Zhao C, et al. Long-term outcomes of early-stage nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy alone. Int J Radiat Oncol Biol Phys. 2012;82:327–333. doi: 10.1016/j.ijrobp.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Xiao WW, Huang SM, Han F, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117:1874–1883. doi: 10.1002/cncr.25754. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Tung SY, Chan AT, et al. Preliminary results of a ran-domized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:142–151. doi: 10.1016/j.ijrobp.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 9.Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261–270. doi: 10.1016/0360-3016(92)90740-9. [DOI] [PubMed] [Google Scholar]
- 10.Teo PM, Kwan WH, Lee WY, et al. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer. 1996;77:2423–2431. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2423::AID-CNCR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Sham JS, Choy D, Choi PH. Nasopharyngeal carcinoma: the significance of neck node involvement in relation to the pattern of distant failure. Br J Radiol. 1990;63:108–113. doi: 10.1259/0007-1285-63-746-108. [DOI] [PubMed] [Google Scholar]
- 12.Loong HH, Ma BB, Chan AT. Update on the management and therapeutic monitoring of advanced nasopharyngeal cancer. Hematol Oncol Clin North Am. 2008;22:1267–1278. doi: 10.1016/j.hoc.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Morgan SC, Parker CC. Local treatment of metastatic cancer—killing the seed or disturbing the soil? Nat Rev Clin Oncol. 2011;8:504–506. doi: 10.1038/nrclinonc.2011.88. [DOI] [PubMed] [Google Scholar]
- 14.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 15.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 16.Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol. 2006;24:2743–2749. doi: 10.1200/JCO.2005.04.2226. [DOI] [PubMed] [Google Scholar]
- 17.Le Scodan R, Stevens D, Brain E, et al. Breast cancer with synchronous metastases: survival impact of exclusive locoregional radiotherapy. J Clin Oncol. 2009;27:1375–1381. doi: 10.1200/JCO.2008.19.5396. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Tangen C, Basler J, et al. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002;168:1008–1012. doi: 10.1016/S0022-5347(05)64562-4. [DOI] [PubMed] [Google Scholar]
- 19.Setton J, Wolden S, Caria N, et al. Definitive treatment of metastatic nasopharyngeal carcinoma: report of 5 cases with review of literature. Head Neck. 2012;34:753–757. doi: 10.1002/hed.21608. [DOI] [PubMed] [Google Scholar]
- 20.Lin S, Tham IW, Pan J, et al. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol. 2012;35:474–479. doi: 10.1097/COC.0b013e31821a9452. [DOI] [PubMed] [Google Scholar]
- 21.Lin H, Lin H, Cai X, et al. Chemotherapy plus radiotherapy makes curability a possibility in nasopharyngeal carcinoma patients with distant metastasis at diagnosis. Head Neck Oncol. 2013;5:1. [Google Scholar]
- 22.Wang WY, Twu CW, Chen HH, et al. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res. 2010;16:1016–1024. doi: 10.1158/1078-0432.CCR-09-2796. [DOI] [PubMed] [Google Scholar]
- 23.Fandi A, Bachouchi M, Azli N, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. 2000;18:1324–1330. doi: 10.1200/JCO.2000.18.6.1324. [DOI] [PubMed] [Google Scholar]
- 24.Yeh SA, Tang Y, Lui CC, et al. Treatment outcomes of patients with AJCC stage IVC nasopharyngeal carcinoma: benefits of primary radiotherapy. Jpn J Clin Oncol. 2006;36:132–136. doi: 10.1093/jjco/hyi245. [DOI] [PubMed] [Google Scholar]
- 25.Foo KF, Tan EH, Leong SS, et al. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol. 2002;13:150–156. doi: 10.1093/annonc/mdf002. [DOI] [PubMed] [Google Scholar]
- 26.Au E, Tan EH, Ang PT. Activity of paclitaxel by three-hour infusion in Asian patients with metastatic undifferentiated nasopharyngeal cancer. Ann Oncol. 1998;9:327–329. doi: 10.1023/a:1008255220284. [DOI] [PubMed] [Google Scholar]
- 27.Ma BB, Tannock IF, Pond GR, et al. Chemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer. 2002;95:2516–2523. doi: 10.1002/cncr.10995. [DOI] [PubMed] [Google Scholar]
- 28.Cheng LC, Sham JS, Chiu CS, et al. Surgical resection of pulmonary metastases from nasopharyngeal carcinoma. Aust N Z J Surg. 1996;66:71–73. doi: 10.1111/j.1445-2197.1996.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheng LC, Chiu CS, Lee JW. Surgical resection of pulmonary metastases. J Cardiovasc Surg (Torino) 1998;39:503–507. [PubMed] [Google Scholar]
- 30.Ma J, Wen ZS, Lin P, et al. The results and prognosis of different treatment modalities for solitary metastatic lung tumor from nasopharyngeal carcinoma: a retrospective study of 105 cases. Chin J Cancer. 2010;29:787–795. doi: 10.5732/cjc.010.10098. [DOI] [PubMed] [Google Scholar]
- 31.Pan C, He N, Zhao M, et al. Subdividing the M1 stage of liver metastasis for nasopharyngeal carcinoma to better predict metastatic survival. Med Oncol. 2011;28:1349–1355. doi: 10.1007/s12032-010-9643-8. [DOI] [PubMed] [Google Scholar]
- 32.Lim A, Corry J, Lau E, et al. Prolonged remission in a patient with nasopharyngeal carcinoma with a solitary bone metastasis. J Clin Oncol. 2011;29:e135–e137. doi: 10.1200/JCO.2010.31.9053. [DOI] [PubMed] [Google Scholar]


