Abstract
Type I insulin-like growth factor receptor (IGF-1R) has long been recognized for its role in tumorigenesis and growth, but only recently have the tools for targeting the IGF pathway become available. More than 10 IGF/IGF-1R inhibitors have entered clinical trials, and these belong to three main classes: (1) monoclonal antibodies against IGF-1R, (2) monoclonal antibodies against IGF-1R ligands (IGF-1 and IGF-2), and (3) IGF-1R tyrosine kinase inhibitors. These IGF-1R–targeting agents share common effects on IGF-1R signaling but differ in mechanisms of action, spectrum of target inhibition, and pharmacological features. Clinical activity of IGF-1R inhibitors has been demonstrated with sustained responses in a small number of patients with select tumor types, such as Ewing sarcoma and thymoma. However, many large clinical trials involving patients with adult tumors, including non–small cell lung cancer, breast cancer, and pancreatic cancer, failed to show clinical benefit in the overall patient population. Possible reasons for failure include the complexity of the IGF-1R/insulin receptor system and parallel growth and survival pathways, as well as a lack of patient selection markers. While IGF-1R remains a valid target for selected tumor types, identification of predictive markers and rational combinations will be critical to success in future development.
Keywords: IGF-1R, insulin receptor
Type I insulin-like growth factor receptor (IGF-1R) has been recognized for decades for its role in tumorigenesis and growth[1]. Not until recently had advances in medicinal chemistry and biotechnology provided the tools for targeting the insulin-like growth factor (IGF) pathway in patients. To date, more than 10 IGF/IGF-1R inhibitors have entered clinical trials. Clinical validation of this target has been demonstrated in select tumor types. However, several large clinical trials failed to show clinical benefit in the overall patient population. In this review we focus on the two main classes of IGF-1R inhibitors in clinical development—monoclonal antibodies (mAbs) and small molecule tyrosine kinase inhibitors (TKIs)—and discuss the background, clinical experience, and lessons learned.
IGF-1R
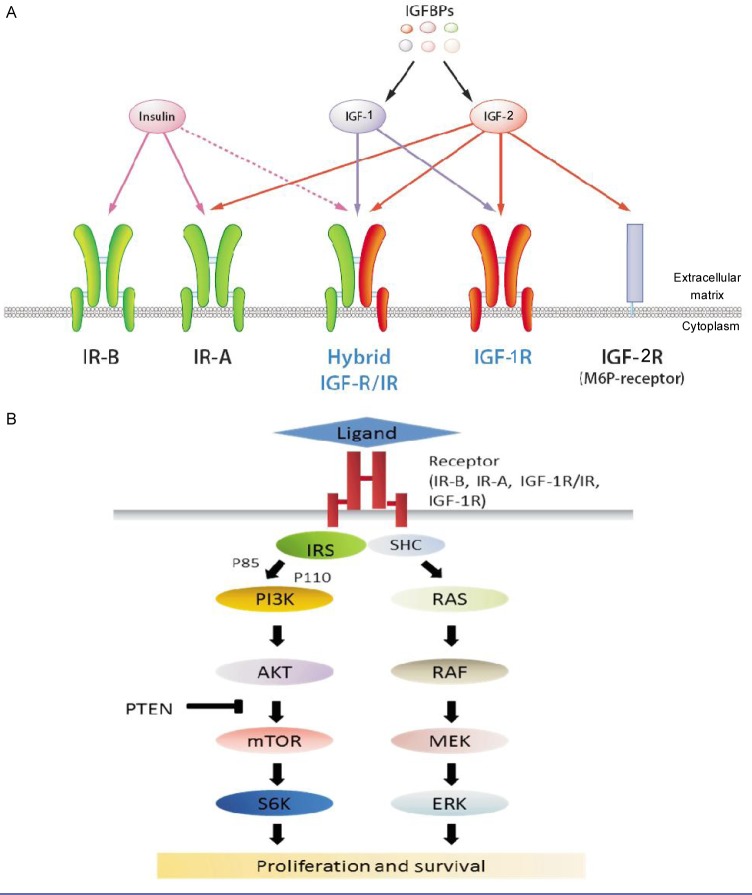
IGF-1R belongs to the insulin receptor (IR) family that includes the IR (a homodimer), IGF-1R (a homodimer), IGF-1R/IR (hybrid, heterodimeric receptors), and the mannose 6-phosphate receptor (also known as IGF-2R)[1] (Figure 1). IGF-1R can be activated by the ligands insulin-like growth factor-1 (IGF-1) or insulin-like growth factor-2 (IGF-2). IGF-1R/IR hybrids preferentially signal with the IGF ligands. IR exists in two isoforms: IR-B, traditional insulin receptors, and IR-A, a fetal form that is re-expressed in selected tumors and preferentially binds IGF-2 [2]. IGF-2R is a non-signaling receptor that acts as a “sink” for IGF-2 [3]. These receptors may coexist in a given cell, with relative abundance and activation status varying by cell type, tissue type, and physiologic or pathologic conditions.
Figure 1. Insulin and insulin-like growth factor (IGF) receptors and downstream signaling.
A, the insulin receptor family includes the insulin receptor (IR) in two isoforms (IR-A and IR-B), the type 1 insulin-like growth factor receptor (IGF-1R), and the mannose 6-phosphate (M6P) receptor (IGF-2R). IR and IGF-1R are expressed as preformed dimers, either homodimers or heterodimers. IGF-2R is a non-signaling receptor that acts as a “sink” for IGF-2. Insulin binds primarily to IR-A or IR-B, but also has weak affinity for IGF-1R. IGF-1 and IGF-2 are ligands for the IGF-1R and IGF-1R/IR hybrid receptor along with IR-A. Insulin-like growth factor binding proteins (IGFBPs) bind to and prevent IGF-1 and IGF-2 from activating receptor signaling cascades. B, signaling of the IGF-1R/IR system is mediated by the insulin receptor substrate (IRS) and Shc. PI3K-AKT activation is the predominant downstream event of IGF-1R/IR, but the MAPK pathway can also be activated.
The ligands of IGF-1R, IGF-1 and IGF-2 are abundant in the serum of adults[4] and have complex interactions with IGF-1R/IR receptors. IGF-1 is secreted primarily by the liver upon stimulation by human growth hormone (HGH), whereas IGF-2 is non-HGH dependent and is expressed in a variety of tissues. There are at least 6 well-characterized IGF-binding proteins (IGFBP-1 through -6) that bind IGFs and prevent their action on the receptors. In serum, only approximately 2% of IGF ligands exist in the unbound form. At the tissue level, bioavailability of IGF-1 and IGF-2 is modulated by IGFBP protease and the presence of the non-signaling, IGF-2-binding IGF-2R. Intracellular signaling of IGF-1R is mediated through IR substrates (IRS-1 through -4) and Src-homology collagen protein (Shc)[5], which in turn leads to activation of the mitogen-activated protein kinase (MAPK) pathway and the PI3K-AKT pathway (Figure 1B)[3]. PI3K-AKT is considered the predominant downstream signaling pathway for the IR family.
IGF-1R is ubiquitously expressed in normal tissues and plays an important role in growth and various physiological functions, including those involving the cardiac and neurological systems, as well as glucose homeostasis. The impact on glucose probably occurs through feedback down-regulation of HGH by circulating IGF-1 and the local effect of IGF-1 on IGF-1R in the muscles or kidneys to promote glucose uptake[6],[7].
Extensive in vitro and in vivo studies have implicated IGF-1R, IGF-1, and IGF-2 signaling in cancer development, maintenance, and progression. IGF-1R expression is critical for anchorage-independent growth, a well recognized property of malignant cells. IGF-1 and IGF-2 are strong mitogens in a wide variety of cancer cell lines, including prostate cancer[8], breast cancer[9]–[12], colon cancer[13],[14], and myeloma[15]. High circulating levels of IGF-1 have been associated with increased risk of breast, prostate, and colon cancers[1]. The IGF/IGF-1R pathway has also been shown to have extensive cross-talk with the estrogen receptor (ER), epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor 2 (HER-2) signaling pathways and to play an important role in the resistance mechanisms of cytotoxic drugs and EGFR/HER-2–targeted agents[16]. More recent work also suggests a potential role for IGF-1R in the resistance to mTOR inhibitors[17] and RAF-MEK inhibitors[18].
IGF-1R can be detected in most solid tumors and hematological malignancies examined to date, and IGF-2 overexpression, IGFBP modulations, and IGF-2R downregulation have also been seen in cancer cells [5],[19],[20]. However, unlike other growth factor receptors such as EGFR and HER-2, activating mutations of the IGF1R gene have not been reported, and gene amplification is extremely rare in the tumors that have been tested [21]. On the other hand, several genetic abnormalities can lead indirectly to IGF/IGF-1R overexpression and signaling. For example, in Ewing sarcoma (EWS), the EWS/friend leukemia integration-1 (FLI-1) translocation product can interact with the IGFBP3 promoter and repress its expression, and IGF-1R is required for transformation by the fusion protein. Some tumor types, including hepatocellular carcinoma and breast cancer, have been associated with loss of heterozygosity of the IGF2R gene[22]. Loss of imprinting of IGF-2 (loss of methylation resulting in biallelic expression), first described in Wilms tumor, has since been identified in adult tumors and is associated with an increased risk of colon cancer[23],[24]. These genetic changes may increase IGF-2 production or its bioavailability for IGF-1R signaling.
IGF-1R Inhibitors in Clinical Development
Several approaches to inhibit IGF-1R signaling have been investigated. Agents in current clinical development belong to three main classes (Tables 1 and 2): monoclonal antibodies (mAbs) against IGF-1R, mAbs against IGF-1R ligand (IGF-1 and IGF-2), and IGF-1R tyrosine kinase inhibitors (TKIs). At least eight human or humanized anti–IGF-1R mAbs entered clinical trials (Table 1), though several clinical development programs have since been discontinued. These antibodies are highly specific to IGF-1R and do not bind IR. Common mechanisms of action include blockade of the receptor from ligand binding and internalization/degradation of IGF-1R[25]. In addition, anti–IGF-1R mAbs also down-regulate the IGF-1R/IR hybrid receptor[26].
Table 1. Monoclonal antibodies that target the type I insulin-like growth factor receptor (IGF-1R) pathway.
| Target | Agent name | Sponsor | Status | Class | Phase 2 dose |
| IGF-1R | Cixutumumab (IMC-A12)[49],[84] | ImClone | Phase 2 | IgG1 | 6 mg/kg qw, 10 mg/kg q2w |
| IGF-1R | Figitumumab (CP-751,871) [35],[36],[59] | Pfizer | Discontinued after Phase 3 | IgG2 | 20 mg/kg q3w |
| IGF-1R | Dalotuzumab (MK-0646; h7C10)[85],[86] | Pierre Fabre and Merck | Phase 3 | IgG1 | 10 mg/kg q2w |
| IGF-1R | Ganitumab (AMG 479)[33] | Amgen | Phase 3 | IgG1 | 18 mg/kg q3w |
| IGF-1R | R1507[41] | Roche | Phase 2 | IgG1 | 9 mg/kg qw |
| IGF-1R | SCH 717454 (19D12)[37] | Schering Plough | Discontinued after Phase 1 | IgG1 | NA |
| IGF-1R | AVE1642 (EM164)[38] | ImmunoGen/Sanofi | Discontinued | IgG1 | 8 mg/kg q4w, 12 mg/kg q3w |
| IGF-1R | BIIB022[87],[88] | Biogen-IDEC | Discontinued after Phase 1 | IgG4 | NA |
| IGF-1 and IGF-2 | MEDI-573[27],[89] | Medlmmune | Phase 1 | IgG2 | NA |
qw, every week; q2w, every 2 weeks; q3w, every 3 weeks; q4w, every 4 weeks; NA, not available.
Table 2. Small molecule tyrosine kinase inhibitors (TKI) against IGF-1R.
| Agent | Sponsor | Class (route) | IC50 (µmol/L) against |
Sponsor | ||
| IGF-1R | IR | Othersa | ||||
| Linsitinib (OSI-906)[28],[29] | OSI | TKI (oral) ATP-competitive | 0.018 | 0.054 | None | Phase 3 |
| BMS-754807[90] | BMS | TKI (oral) ATP-competitive | <2 nmol/L | <2 nmol/L | 11 other kinases < 100 nmol/L | Phase 2 |
| BVP 51004[91] | Biovitrum | Small molecule (oral) Not ATP-competitive |
0.038 µmol/L | No effect | None | Phase 1 |
| XL228[92] | Exelixis | TKI (IV) ATP-competitive |
1.6 nmol/L (cellular) | NA | • Bcr-Abl: 5 nmol/L • Bcr-Abl T315I: 1.4 nmol/L • Src: 6.1 nmol/L • Aurora A: 3.1 nmol/L • LYN: 2 nmol/L (all cellular) |
Phase 1 |
| INSM-18 (NDGA)[93],[94] | Insmed | Phenolic compound isolated from creosote bush Larrea divaricata | 31 µmol/L (cellular) | NA | HER-2: 15 µmol/L (cellular) | Phase 1 |
a Targets for which IC50 is <50 fold of the IC50 for IGF-1R. IGF-1R, type I insulin-like growth factor receptor; IR, insulin receptor; TKI, tyrosine kinase inhibitor; IC50, half maximal inhibitory concentration; IV, intravenous; NDGA, nordihydroguaiaretic acid; OSI, OSI Pharmaceutical; BMS, Brystol-Meyers Scribb; HER-2, human epidermal growth factor receptor 2.
Currently, MEDI-573 is the only monoclonal antibody in clinical development that targets the ligands IGF-1 and IGF-21[27]. MEDI-573 inhibits IGF-induced IGF-1R and IR-A activation, but does not affect insulin signaling. Several small molecule TKIs against IGF-1R are under clinical investigation. Among them, OSI-906 and BMS-754807 are the most specific, whereas others also inhibit receptor tyrosine kinases beyond the IGF-1R and IR family (Table 2). Because of the high degree of homology between IGF-1R and IR, even the most specific IGF-1R TKIs have some degree of inhibitory effect on the IR. For example, OSI-906 has a half maximal inhibitory concentration (IC50) of 0.018 µmol/L against IGF-1R and 0.054 µmol/L against IR[28],[29]. At clinically relevant doses, this agent is expected to inhibit IGF-1R and IR simultaneously. Co-inhibition of IR and IGF-1R may confer a better anti-tumor effect because IR signaling induced by insulin or IGF-2 has been implicated in a number of preclinical tumor models[30], including breast cancer[31]. Furthermore, when IGF-1R signaling is disrupted, cells may respond with an increase in IR activity[32]. However, IR inhibition is expected to impact insulin signaling and may increase the risk of toxicities, particularly hyperglycemia.
In summary, the three classes of IGF-1R–targeting agents share common effects on IGF-1R signaling, but differ in mechanisms of action, spectrum of target inhibition, and pharmacological features (Table 3). For example, anti–IGF-1R mAbs only block signaling through IGF-1R and IGF-1R/IR hybrids, whereas IGF-1/2– neutralizing mAb prevents IGF signaling through both homodimers and heterodimers of IGF-1R and IR-A, but spares insulin signaling. IGF-1R TKIs can potentially block all receptors responsible for IGF/insulin signaling. The differing spectrum of target blockade may potentially translate into different toxicity and/or activity profiles.
Table 3. Main features of monoclonal antibodies and small molecule tyrosine kinase inhibitors against the IGF/IGF-1R pathway.
| Features of interest | mAb against IGF-1R | mAb against IGF-1 and -2 | Small molecule TKI |
| Mechanism of action | • Block IGF-1R from ligand binding • Receptor degradation of IGF-1R homodimer and IGF-1R/IR hybrids • Possible ADCC (if IgG1) |
• Neutralizing ligand from binding to IGF-1Rand IR-A | • Kinase inhibition intracellular ▴ (also inhibit ligand-independent activation, if relevant) |
| Signaling affected | • Specific • Inhibit signaling of: ▴IGF-1R ▴IGF-1R/IR-A hybrid • No effect on IR-A or IR-B |
• Specific • Inhibit IGF-1 or IGF-2 signaling through: ▴ IGF-1R ▴ IGF-1R/IR-A ▴IR-A • No effect on insulin signaling |
• Less specific • Inhibit signaling of RTKs (by any ligand): ▴IGF-1R ▴IGF-1R/IR ▴IR (to a lesser degree than for IGF-1R) • May inhibit targets beyond IGF-1R and IR (XL228; INSM-18) |
| Pharmacokinetics | • Long t1/2 (days to weeks) • PK interaction less likely in combination regimens • Poor CNS uptake |
• Long t1/2 (days to weeks) • PK interaction less likely in combination regimens • Poor CNS uptake |
• Short t1/2 (hours) |
IGF-1R, type I insulin-like growth factor receptor; IR, insulin receptor; mAbs, monoclonal antibodies; TKIs, tyrosine kinase inhibitors; ADCC, antibody-dependent cell-mediated cytotoxicity; RTKs, receptor tyrosine kinases; PK, pharmacokinetics; t1/2, half life; CNS, central nervous system.
Clinical Experience with IGF-1R Inhibitors
IGF-1R–targeting mAbs
The maximum tolerated dose for monotherapy was not reached at the conclusion of the phase 1 trials for any of the anti–IGF-1R mAbs. Selection of the phase 2 doses was largely based on feasibility and the target steady-state drug levels extrapolated from preclinical in vivo tumor models. Table 1 lists the recommended phase 2 doses for monotherapy with different IGF-1R mAbs. Anti–IGF-1R mAbs are generally well tolerated as monotherapy. Common treatment-emergent adverse events include hyperglycemia, the classic side effect of all anti–IGF-1R mAbs. Hyperglycemia, which occurs in about 20% patients, is mostly grades 1–2 and can be controlled with oral diabetic medications with continued mAb treatment.
Pharmacodynamic changes tested in early clinical trials with anti–IGF-1R mAbs have shown evidence of target modulation, including down-regulation of IGF-1R in granulocytes and circulating tumor cells[33],[34], a significant increase in HGH and IGF-1, and a variable increase in the insulin level[33],[35]–[38]. Decrease in the standardized uptake values of (18)F-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) has also been observed in anecdotal cases[33].
The most notable activity of anti–IGF-1R mAbs was demonstrated in EWS, with reports of complete responses (CRs) or partial responses (PRs) and prolonged stable disease (SD) in phase 1 trials[33],[39]–[41]. These promising results led to a series of phase 2 evaluations in the indication (Table 4). The largest EWS-specific phase 2 trial used R1507[42]. In this trial, 115 patients with recurrent or refractory EWS older than 2 years of age were treated at either 9 mg/kg once a week (n = 109) or 27 mg/kg every 3 weeks (n = 6). The overall CR/PR rate was 10% (1 CR, 10 PRs), with a median response duration of 29 weeks (range, 12 to 94 weeks) and a median overall survival of 7.6 months (95% confidence interval, 6 to 9.7 months). Ganitumab (AMG 479) was also tested in patients with refractory EWS (n = 19) or desmoplastic round cell tumors (n = 16)[43], and the results were reported together. PR was noted in one patient with EWS (censored at 47 weeks) and one patient with desmoplastic round cell tumor. Overall, five additional patients had SD for more than 24 weeks. Recently, Malempati et al.[44] reported the results of a Children's Oncology Group (COG) trial involving 47 pediatric patients treated with cixutumumab (IMC-A12) at 6 mg/kg or 9 mg/kg weekly on 28 day cycles. Of the 35 patients in the EWS expansion cohorts with heavily pretreated disease, 3 patients had confirmed PRs. As with other mAbs, the median progression-free survival (PFS) in the overall patient population was short (44 days in 9 mg/kg dose cohort)[44]. The overall experience in EWS suggests that IGF-1R inhibition with mAb has major activity in this indication, but the benefit was restricted to a small subset of patients. Further studies are warranted to explore markers predictive of response, mechanisms of resistance, and combination strategies.
Table 4. Activity of IGF-1R monoclonal antibodies.
| Tumor type | Agent | Trial and regimen | Phase of trial | Activity | Reference |
| EWS | Figitumumab | Monotherapy | Phase 1 (n=14) | 1 CR, 1 PR, 8 SD | [95] |
| EWS | Cixutumumab (IMC-A12) | Monotherapy | Phase 1/2 (n=35 on EWS expansion) | 3 PRs | [44] |
| EWS | R1507 | Monotherapy | Phase 2 (n=115) | 1 CR, 10 PR | [42] |
| EWS and DRCT | Ganitumab (AMG 479) | Monotherapy | Phase 2 (n=16) | 1 PR in EWS, 1 PR in DRCT, 5 SD | [43] |
| HCC | Cixutumumab | Monotherapy | Phase 2 (n=24) | No PR/CR, 7 SD for > 4 months | [47] |
| ACC | Figitumumab | Monotherapy | Phase 2 (n=14) | No PR, 8 SD | [48] |
| Metastatic castration-resistant prostate cancer | Citxutumumab | Monotherapy | Phase 2 (n=31 in q2w dose cohort and n=10 in q3w dose cohort) | 9 patients had SD > 6 months in q2w cohort; 3 patients had SD |
[50] |
| Thymoma | Citxutumumab | Monotherapy | Phase 2 (n=30) | 4 patients had PRs, 23 had SD | [46] |
| NSCLC (squamous cell) | Figitumumab | Chemotherapy ± figitumumab | Phase 3 | Experimental vs. control; OS: 8.5 vs. 10.3 months |
[60] |
| NSCLC | Erlotinib ± figitumumab | Phase 3 | Early termination for futility | [71] | |
| Erlotinib ± R1507 | Phase 2 | No difference in 12-week PFS | [70] | ||
| Pancreatic cancer | Ganitumab | Gemcitabine ± ganitumab | Phase 2 | Increase in PFS and OS | [55] |
| Cixutumumab | Gemcitabine + erlotinib ± cixutumumab | Phase 2 | No difference in PFS and OS | [56] | |
| Colorectal cancer | Dalotuzumab | Irinotecan-cetuximab ± dalotuzumab | Phase 2/3 | No difference in PFS | [72] |
| Breast cancer | Ganitumab | Exemustane or fulvestrant ± ganitumab | Phase 2 | No difference in ORR or PFS | [65] |
IGF-1R, type I insulin-like growth factor receptor; EWS, Ewing sarcoma; DRCT, desmoplastic round cell tumor; HCC, hepatocellular carcinoma; ACC, adrenocortical carcinoma; NSCLC, non–small cell lung cancer; CR, complete response; PR, partial response; SD, stable disease; PFS, progression-free surivial; OS, overall surivial ORR, overall response rate.
Phase 1 trials of IGF-1R–targeting mAbs have also shown objective responses in patients with neuroendocrine tumors[45] and prolonged SD in patients with hepatocellular carcinoma, thymoma, and prostate cancer. A phase 2 trial of cixutumumab was initiated in patients with thymoma and thymic carcinoma[46]. For the 30 evaluable patients with thymoma, 4 patients had PRs, 23 had SDs, and only 3 had progressive disease. No response was observed in the thymic carcinoma cohort. Of note, 8 of 33 patients with thymoma developed autoimmune symptoms, 4 of which were new onset, while on study, including immune thrombocytopenic purpura, myositis, myocarditis, colitis, pure red cell aplasia, and a food allergy. The underlying mechanism of the autoimmune phenomena is unclear.
IGF-1R–targeting mAbs have shown less single agent activity in other tumors. Abou-Alfa et al. [47] reported the results of a phase 2 trial of cixutumumab in hepatocellular carcinoma. In that trial with 22 evaluable patients, no objective responses were observed; 7 (29%) had SD for at least four months. The median overall survival (OS) was 8 months. Similarly, 14 patients with adrenal cortical cancer were treated with figitumumab in a phase 2 trial, in which eight patients had stable disease, but none had objective responses[48]. A phase 2 trial of cixutumumab in 31 patients with metastatic castration-resistant prostate cancer showed no objective responses, although the result was considered promising as some patients achieved stabilization of their initially progressing tumors[49],[50]. The median time to progression was 3.8 months, with 9 patients showing SD for more than 6 months. Combination regimens in castration-sensitive prostate cancer are currently being explored.
IGF-1R TKIs
Phase 1 trials of the IGF-1R TKI OSI-906 explored two main schedules: continuous oral dosing (once or twice daily without interruption) and intermittent oral dosing (days 1–3 every 14 days)[51]–[53]. DLTs included: grade 3 hyperglycemia, grade 3 QT prolongation, and grade 4 ALT/AST elevation. Daily dosing up to 300 mg indicated a linear PK, with median terminal t1/2 of 2–4 h. The recommended phase 2 doses were determined to be 150 mg, twice per day, 500 mg daily, or 600 mg daily on Day 1 to Day 3 in 14-day cycles. The plasma concentrations in the phase 1 trials exceeded the “efficacious” concentration (IC50) in the in vitro models (1 µmol/L).
OSI-906's target effect was reflected by a dose-dependent increase in the insulin levels. SD more than 12 weeks in duration was seen in patients with thymic, adrenocortical, and colorectal cancer. Interestingly, in the phase 1 trial for the intermittent schedule[51], 1 of the 3 patients with adrenocortical carcinoma had a confirmed PR in the primary tumor and multiple lung metastases, whereas another patient had prolonged SD of 32 weeks.
Currently, OSI-906 is being tested in combination with erlotinib in patients with NSCLC with EGFR activating mutation, in pancreatic cancer for combination with standard of care, and in head and neck cancer for combination with cetuximab. A phase 3 study comparing OSI-906 versus placebo in patients with advanced adrenocortical cancer is ongoing.
IGF-Neutralizing mAb
The only IGF-1–neutralizing mAb in clinical trials, MEDI-573, is still in the early stage of development. In a phase 1 trial of MEDI-573, the drug was well tolerated in the 25 patients treated, and the maximum tolerated dose was not reached. Hyperglycemia occurred in 2 patients[54]. No objective responses were seen in the phase 1 trial. Phase 2 trials are ongoing in breast cancer for combination with endocrine therapy.
Combination of IGF-1R Inhibitors and Chemotherapy or Targeted Agents
Combination with chemotherapy
A number of clinical trials have been initiated involving the combination of anti–IGF-1R mAbs and standard chemotherapies in multiple tumor types. In pancreatic cancer, the randomized phase 2 trial for gemcitabine with or without ganitumab revealed a statistically significant improvement in the combination arm in PFS [median of 5.1 months vs. 2.1 months, hazard ratio (HR) = 0.6, P = 0.07] and OS (median of 8.7 vs. 5.9 months, HR = 0.67, P = 0.12)[55]. On the other hand, no difference in PFS was observed when cixutumumab was added to gemcitabine and erlotinib as the backbone regimen in pancreatic cancer[56]. The reason for the differing outcomes of the two trials is unclear. Interestingly, a population pharmacokinetics (PK) analysis in the ganitumab trial in pancreatic cancer showed a positive association between OS and PFS with higher exposure of ganitumab[57]. Unfortunately, a definitive phase 3 trial in advanced pancreatic cancer for gemcitabine with or without ganitumab was recently halted because the independent data monitoring committee concluded that the addition of ganitumab is unlikely to result in a statistically significant improvement in the primary endpoint of OS. More data from that trial will be forthcoming in the final analysis[58].
Data from a randomized phase 2 trial for figitumumab in combination with carboplatin and paclitaxel in advanced, treatment-naïve non–small cell lung cancer (NSCLC) showed promising results with higher overall response rates and PFS, particularly in patients with squamous cell carcinoma[59], but this result was not confirmed in a large, international phase 3 trial. The trial was terminated early after interim analysis indicated an increase in early deaths as well as futility of the experimental arm[60]. Biomarker exploration indicated an association between IGF-1 with toxicity and efficacy. Patients with low baseline free IGF-1 had worse survival with the addition of figitumubmab (HR = 1.6, P = 0.006), whereas patients with high free IGF-1 had better outcomes with figitumumab and chemotherapy compared to chemotherapy alone (HR = 0.62, P = 0.13). Confirmation for this marker is limited at this time.
Combination with anti-estrogen therapy
A key growth and survival mechanism of estrogen-dependent tumors is the functional cross-talk and co-dependence between IGF/IGF-1R and ER[16],[61],[62]. Anti-IGF-1R agents are highly active in estrogen-dependent, tamoxifen-responsive cell lines but generally ineffective in tamoxifen-resistant cells[63]. Furthermore, addition of anti–IGF-1R antibodies to tamoxifen enhanced the anti-tumor activity in T61 and MCF-7 tamoxifen-sensitive breast cancer models[26],[63],[64].
These results support the clinical evaluation of addition of IGF-1R inhibitors to anti-estrogen therapies. However, results from clinical trials combining IGF-1R mAbs and antiestrogen agents have been disappointing. The combination of ganitumab and fulvestrant or exemestane did not delay or reverse resistance to hormonal therapy[65]. Reasons for the failure of this trial are unclear but could be related to intrinsic or induced activation of IR and/or lack of patient selection. Given the important role of IR in breast cancer, evaluation of receptor-targeting antibodies with IGF-1R TKIs or IGF-1–and IGF-2–neutralizing mAbs would be interesting.
Combination with EGFR or HER-2 inhibitors
IGF-1R signaling has been causally linked to de novo or acquired resistance to trastuzumab (Herceptin®) and EGFR-targeting agents in numerous models[16]. In vitro and in vivo tumor models have also demonstrated direct interactions between IGF-1R, EGFR/HER-2[16],[62],[66]–[68], and, in some studies, co-localization of IGF-1R and HER-2[68],[69]. Treatment of resistant cells with IGF-1R inhibitors was shown to inhibit transactivation of HER-2 and restore sensitivity to trastuzumab[16],[68],[69]. Similarly, addition of anti–IGF-1R agents to EGFR TKIs or anti-EGFR mAbs has been shown to prevent, delay, or reverse resistance to EGFR inhibitors[66],[67]. The preclinical rationale supported clinical evaluations for the combination of IGF-1R and EGFR inhibitors in NSCLC, colorectal cancer, and head and neck cancers, as well as the combination of anti–IGF-1R mAb with lapatinib in HER2-positive breast cancer.
Clinical results from the combination of IGF-1R and EGFR inhibitors have been disappointing. A randomized phase 2 trial of erlotinib with or without the anti–IGF-1R mAb R1507 failed to show difference in the primary endpoint of 12-week PFS. Intriguingly, in 36 patients with KRAS mutation (27% of the patients who were evaluable for mutation status), the 12-week PFS appeared better in the R1507 group as compared with the erlotinib-alone arm (36% vs. 0%), although this result was based on retrospective analysis on a fraction of the patients[70]. A phase 3 trial of figitumumab and erlotinib versus erlotinib alone in unselected NSCLC, not including adenocarcinoma patients, was closed early after an interim analysis suggesting futility and toxicity concerns[71].
A randomized phase 2 trial in colorectal cancer with dalotuzumab (MK-0646) in combination with cetuximab and irinotecan also had a negative outcome[72]. A subsequent analysis of biomarkers suggested that patients with high tumor expression of IGF-1R may derive benefit from the addition of dalotuzumab, whereas those with IGF-2R overexpression were likely to be resistant to anti–IGF-1R mAb[73]. These intriguing results remain to be confirmed and warrant further studies.
Combination with mTOR inhibitors
Recent studies in preclinical models and tumor biopsies from patients demonstrate that treatment with mTOR inhibitors leads to up-regulation of AKT phosphorylation[74],[75]. They further suggest that the IGF/IGF-1R pathway mediated feedback activation of AKT and that combining rapamycin and IGF-1R inhibitors enhanced antitumor effects[74],[75]. The most significant synergism was observed in pediatric tumor models such as those for EWS and osteosarcoma, where the combination of an anti–IGF-1R mAb and rapamycin led to complete tumor regression, whereas single agents only induced modest growth delay[76].
This combination strategy has been pursued with several IGF-1R antibody agents[77]–[81] and TKIs[85]. A phase 1 trial with cixutumumab and temsirolimus (CCI-779, Torisel®) has shown that the combination is feasible at the full doses of both agents[80]. Mucositis was the most common toxicity. In a preliminary study of efficacy in the expansion for 20 patients with heavily pretreated EWS, 35% patients achieved either CR/PR or SD for five months. Interestingly, 1 patient who had progressed on a previous anti–IGF-1R mAb achieved a CR that lasted for more than 20 months[77]. In another phase 1 trial for the same strategy, with ganitumab and everolimus, CR was observed in 2 patients with refractory NSCLC[78]. Efficacy data were also promising for patients with ER-positive breast cancer in a phase 1 trial for dalotuzumab and ridaforolimus, warranting further studies for the combination in the indication[79]. A phase 2 evaluation of the combination is ongoing in pediatric malignancies, neuroendocrine tumors, and breast cancer.
Combination with MEK inhibitor
Preclinical work suggested activation of IGF-1R as a potential mechanism of escape from treatment with MAPK inhibitors[18]. Also, MEK and IGF-1R inhibitors had additive effects in preclinical models with RAF or RAS mutations[18]. Ahmed et al.[83] recently reported the first clinical experience with combined IGF-1R and MEK inhibition in a National Cancer Institute (NCI)–sponsored trial of cixutumumab and selumetinib (AZD6244). Dose-limiting toxicities included visual changes, requiring dose reduction of selumetinib to 50 mg twice daily in combination with full dose cixutumumab (20 mg/kg every 3 weeks) at maximum tolerated dose. Pharmacodynamic endpoints and preliminary efficacy evaluations are ongoing in the expansion cohort.
Considerations in Predictive Markers and Resistance Mechanisms
Experience with IGF-1R inhibitors indicates that the pathway is a valid target in human cancers, but the clinical benefit seems to be restricted to a small subset of patients. Identifying patient selection markers is a critical step for success in IGF-1R inhibitor development. Several factors may also confer de novo or acquired resistance, including absence or biological irrelevance of the intended target, IGF-1R; escape mechanisms within or outside the IGF-1R/IR system; or constitutively activate downstream effector molecules.
At the current time, no predictive markers for IGF-1R inhibitors are available. In view of the complexity of the IGF-1R and IR family, as well as their extensive interaction with several other signal transduction pathways, it may be difficult to identify a uniform set of predictive markers for all tumor types and molecular contexts, and for all classes of IGF/IGF-1R inhibitors. Ongoing studies focus on the following areas:
Genomic alterations within the IGF-1R axis in tumors;
Genetic alterations outside the IGF-1R axis that may affect the IGF-1R signaling (e.g., chromosomal translocations resulting in transcriptional modulations of ligands or receptors);
Absolute and relative levels as well as phosphorylation status of IGF-1R and IR; conformations of the receptors (homodimers or heterodimers)
Bioavailability of ligands (including IGF-1, IGF-2, IGFBP3, and decoy receptor IGF-2R);
Activating mutations of downstream molecules such as AKT, PI3K, RAS, and RAF;
Markers related to parallel pathways such as EGFR and vascular endothelial growth factor (VEGF); and
Markers related to epithelial-mesenchymal transition.
Preliminary data based on a limited number of patients suggested a few markers—free IGF-1 in the circulation and tumor expression of IGF-1, IGF-1R, and IR—warrant research and confirmation in prospective randomized trials is required. Additional studies on tumor biology and the molecular consequences of IGF-1R blockade are needed to provide guidance for patient selection and combination strategies.
Summary
The three classes of IGF/IGF-1R inhibitors—anti-IGF-1R mAbs, IGF-1- and IGF-2–neutralizing mAbs, and IGF-1R TKIs—have distinct mechanisms of actions and potentially different resistance/escape mechanisms. Given the complexity of the IGF-1R/IR family and the dynamic predominance of specific receptors and ligands in individual tumors, each class of anti–IGF/IGF-1R agents may have unique advantages in selected tumor settings and different toxicity profiles.
Currently available clinical data with anti–IGF-1R mAbs and TKIs have demonstrated that these targeting approaches are feasible and can induce strong anti-tumor activities in several tumor types, including rare tumors refractory to standard therapies. However, the efficacy is likely to be limited to a small patient subset.
A variety of mechanisms may confer intrinsic or acquired resistance, highlighting the need for rational combination strategies. Preliminary data support further studies for the combination of IGF-1R–specific inhibitors with insulin/IR–targeting agents, mTOR inhibitors, and MEK inhibitors. Critical tasks for future research include deeper and broader understanding of the biology of IGF-1R, exploration of predictive markers, and most importantly, integration of biomarker studies in all clinical investigations of these agents and combination regimens.
References
- 1.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 2.Frasca F, Pandini G, Sciacca L, et al. et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 3.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 4.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncol. 2002;63:317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 5.Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1–6. doi: 10.1006/excr.1999.4667. [DOI] [PubMed] [Google Scholar]
- 6.Clemmons DR. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol. 2006;6:620–625. doi: 10.1016/j.coph.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab. 2007;3:302–310. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- 8.Nickerson T, Chang F, Lorimer D, et al. et al. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61:6276–6280. [PubMed] [Google Scholar]
- 9.Cullen KJ, Yee D, Sly WS, et al. et al. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res. 1990;50:48–53. [PubMed] [Google Scholar]
- 10.Gooch JL, Van Den Berg CL, Yee D. Insulin-like growth factor (IGF)-I rescues breast cancer cells from chemotherapy-induced cell death—proliferative and anti-apoptotic effects. Breast Cancer Res Treat. 1999;56:1–10. doi: 10.1023/a:1006208721167. [DOI] [PubMed] [Google Scholar]
- 11.Lee AV, Yee D. Insulin-like growth factors and breast cancer. Biomed Pharmacother. 1995;49:415–421. doi: 10.1016/0753-3322(96)82678-3. [DOI] [PubMed] [Google Scholar]
- 12.Peyrat JP, Bonneterre J. Type 1 IGF receptor in human breast diseases. Breast Cancer Res Treat. 1992;22:59–67. doi: 10.1007/BF01833334. [DOI] [PubMed] [Google Scholar]
- 13.Hassan AB, Macaulay VM. The insulin-like growth factor system as a therapeutic target in colorectal cancer. Ann Oncol. 2002;13:349–356. doi: 10.1093/annonc/mdf096. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Yakar S, Zhao L, et al. et al. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 15.Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth. Blood. 2000;96:2856–2861. [PubMed] [Google Scholar]
- 16.Gee JM, Robertson JF, Gutteridge E, et al. et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12:S99–S111. doi: 10.1677/erc.1.01005. Suppl. 1. [DOI] [PubMed] [Google Scholar]
- 17.Shin DH, Min HY, El-Naggar AK, et al. et al. Akt/mTOR counteract the antitumor activities of cixutumumab, an anti-insulin-like growth factor I receptor monoclonal antibody. Molecular Cancer Therapeutics. 2011;10:2437–2448. doi: 10.1158/1535-7163.MCT-11-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villanueva J, Vultur A, Lee JT, et al. et al. Acquired Resistance to BRAF Inhibitors Mediated by a RAF Kinase Switch in Melanoma Can Be Overcome by Cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iravani S, Zhang HQ, Yuan ZQ, et al. et al. Modification of insulin-like growth factor 1 receptor, c-Src, and Bcl-XL protein expression during the progression of Barrett's neoplasia. Hum Pathol. 2003;34:975–982. doi: 10.1053/s0046-8177(03)00354-x. [DOI] [PubMed] [Google Scholar]
- 20.LeRoith D, Baserga R, Helman L, et al. et al. Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 21.Berns EM, Klijn JG, van Staveren IL, et al. et al. Sporadic amplification of the insulin-like growth factor 1 receptor gene in human breast tumors. Cancer Res. 1992;52:1036–1039. [PubMed] [Google Scholar]
- 22.De Souza AT, Hankins GR, Washington MK, et al. et al. Frequent loss of heterozygosity on 6q at the mannose 6-phosphate/insulin-like growth factor II receptor locus in human hepatocellular tumors. Oncogene. 1995;10:1725–1729. [PubMed] [Google Scholar]
- 23.Cui H, Cruz-Correa M, Giardiello FM, et al. et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 24.Kaneda A, Feinberg AP. Loss of imprinting of IGF2: a common epigenetic modifier of intestinal tumor risk. Cancer Res. 2005;65:11236–11240. doi: 10.1158/0008-5472.CAN-05-2959. [DOI] [PubMed] [Google Scholar]
- 25.Burtrum D, Zhu Z, Lu D, et al. et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. [PubMed] [Google Scholar]
- 26.Cohen BD, Baker DA, Soderstrom C, et al. et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Chesebrough JW, Cartlidge SA, et al. et al. Dual IGF-I/II–neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71:1029–1040. doi: 10.1158/0008-5472.CAN-10-2274. [DOI] [PubMed] [Google Scholar]
- 28.Ji QS, Mulvihill M, Franklin M, et al. et al. Properties of small molecule IGF-IR kinase inhibitors in preclinical models. AACR Meeting Abstracts. 2007 Apr;:2373. [Google Scholar]
- 29.Ji QS, Mulvihill M, Rosenfeld-Franklin M, et al. et al. Preclinical characterization of OSI-906: a novel IGF-1R kinase inhibitor in clinical trials. AACR Meeting Abstracts. 2007 Oct;:C192. [Google Scholar]
- 30.Morrione A, Valentinis B, Xu SQ, et al. et al. Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci USA. 1997;94:3777–3782. doi: 10.1073/pnas.94.8.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milazzo G, Giorgino F, Damante G, et al. et al. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 1992;52:3924–3930. [PubMed] [Google Scholar]
- 32.Zhang H, Pelzer AM, Kiang DT, et al. et al. Down-regulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res. 2007;67:391–397. doi: 10.1158/0008-5472.CAN-06-1712. [DOI] [PubMed] [Google Scholar]
- 33.Tolcher AW, Sarantopoulos J, Patnaik A, et al. et al. A phase I pharmacokinetic and pharmacodynamic study of AMG 479, a fully human monoclonal antibody against insulin-like growth factor type 1 receptor. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 34.de Bono JS, Attard G, Adjei A, et al. et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin Cancer Res. 2007;13:3611–3616. doi: 10.1158/1078-0432.CCR-07-0268. [DOI] [PubMed] [Google Scholar]
- 35.Haluska P, Shaw HM, Batzel GN, et al. et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 36.Lacy MQ, Alsina M, Fonseca R, et al. et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 37.Seraj J, Tsai M, Wang Y, et al. et al. Evaluation of pharmacodynamic properties of a fully human IGF-1 receptor antibody, SCH 717454, in healthy volunteers. AACR Meeting Abstracts. 2009 Apr;:3615. [Google Scholar]
- 38.Tolcher AW, Patnaik A, Till E, et al. et al. A phase I study of AVE1642, a humanized monoclonal antibody IGF-1R (insulin like growth factor 1 receptor) antagonist, in patients (pts) with advanced solid tumor (st) J Clin Oncol. 2008;26(suppl):abstr 3582. [Google Scholar]
- 39.Patel S, Pappo A, Crowley J, et al. et al. A SARC global collaborative phase II trial of R1507, a recombinant human monoclonal antibody to the insulin-like growth factor-1 receptor (IGF1R) in patients with recurrent or refractory sarcomas. J Clin Oncol. 2009;27(suppl):abstr 10503. [Google Scholar]
- 40.Postel-Vinay S, Okuno S, Schuetze S, et al. et al. Safety, pharmacokinetics and preliminary activity of the anti–IGF-IR antibody CP-751,871 in patients with sarcoma. EJC Supplements. 2008;6:122. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodon J, Patnaik A, Stein M, et al. et al. A phase I study of q3w R1507, a human monoclonal antibody IGF-1R antagonist in patients with advanced cancer. J Clin Oncol. 2007;25(suppl):abstr 3590. [Google Scholar]
- 42.Pappo AS, Patel SR, Crowley J, et al. et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tap WD, Demetri G, Barnette P, et al. et al. Phase II study of ganitumab, a fully human anti–type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. 2012;30:1849–1856. doi: 10.1200/JCO.2011.37.2359. [DOI] [PubMed] [Google Scholar]
- 44.Malempati S, Weigel B, Ingle AM, et al. et al. Phase I/Il trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothenberg ML, Tolcher A, Sarantopoulos J, et al. et al. AMG479 monotherapy to treat patients with advanced Gl carcinoid tumors: a subset analysis from the first-in-human study. 2009 Gastrointestinal Cancers Symposium. 2009:A386. [Google Scholar]
- 46.Rajan A, Riely GJ, Carter CA, et al. et al. Phase II study of cixutumumab (IMC-A12) in thymic malignancies. J Clin Oncol. 2012;30(suppl):abstr 7033. [Google Scholar]
- 47.Abou-Alfa GK, Gansukh B, Chou JF, et al. et al. Phase II study of cixutumumab (IMC-A12, NSC742460; c) in hepatocellular carcinoma (HCC) J Clin Oncol. 2011;29(suppl):abstr 4043. doi: 10.1016/j.jhep.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haluska P, Worden F, Olmos D, et al. et al. Safety, tolerability, and pharmacokinetics of the anti–IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65:765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higano C, Alumkal J, Ryan C, et al. et al. A phase II study evaluating the efficacy and safety of single agent IMC A12, a monoclonal antibody (mAb), against the insulin-like growth factor-1 receptor (IGF-IR), as monotherapy in patients with metastatic, asymptomatic castration-resistant prostate cancer. J Clin Oncol. 2009;27(suppl):abstr 5142. [Google Scholar]
- 50.Higano CS, Alumkal JJ, Ryan CJ, et al. et al. A phase II study of cixutumumab (IMC-A12), a monoclonal antibody (mAb) against the insulin-like growth factor 1 receptor (IGF-IR), monotherapy in metastatic castration-resistant prostate cancer (mCRPC): Feasibility of every 3-week dosing and updated results. Genitourinary Cancers Symposium. 2010. p. Abstract 189. ( http://meetinglibrary.asco.org/content/30567-30573)
- 51.Carden CP, Frentzas S, Langham M, et al. et al. Preliminary activity in adrenocortical tumor (ACC) in phase I dose escalation study of intermittent oral dosing of OSI-906, a small-molecule insulin-like growth factor-1 receptor (IGF-1R) tyrosine kinase inhibitor in patients with advanced solid tumors. J Clin Oncol. 2009;27(suppl):abstr 3544. [Google Scholar]
- 52.Lindsay CR, Chan E, Evans TR, et al. et al. Phase I OSI-906, an insulin like growth factor-1 receptor (IGF-1R) tyrosine kinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2009;27(suppl):abstr 2559. [Google Scholar]
- 53.Evans T, Lindsay CR, Chan E, et al. et al. Phase I dose-escalation study of continuous oral dosing of OSI-906, a dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR), in patients with advanced solid tumors. J Clin Oncol. 2010;28(suppl):abstr 2531. [Google Scholar]
- 54.Haluska P, Huang J, Lam B, et al. et al. MEDI-573 as a novel approach to IGF-1R and IR-A signaling inhibition by blocking IGF ligands: Phase I PK/PD, safety data, and disease linkage studies in breast cancer. J Clin Oncol. 2011;29(suppl 27):abstr 271. [Google Scholar]
- 55.Kindler HL, Richards DA, Stephenson J, et al. et al. A placebo-controlled, randomized phase ii study of conatumumab (C) or AMG 479 (A) or placebo (P) plus gemcitabine (G) in patients (pts) with metastatic pancreatic cancer (mPC) J Clin Oncol. 2010;28(suppl):abstr 4035. [Google Scholar]
- 56.Philip P, Goldman B, Ramanathan R, et al. et al. Dual blockade of epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor-1 (IGF-1R) signaling in metastatic pancreatic cancer: Phase I/randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG-0727) J Clin Oncol. 2012;30(suppl):abstr 4019. doi: 10.1002/cncr.28744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu J, Deng H, Tang R, et al. et al. Exposure-response (E-R) analysis to facilitate phase III (P3) dose selection for AMG 479 (A479) in combination with gemcitabine (G) to treat metastatic pancreatic cancer (mPC) J Clin Oncol. 2011;29(suppl 4):abstr 263. [Google Scholar]
- 58.Pancreatic cancer. amgen halts phase III trial of ganitumab plus gemcitabine. The Clinical Cancer Letter. 2012;35:3. [Google Scholar]
- 59.Karp DD, Paz-Ares LG, Novello S, et al. et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 60.Jassem J, Langer CJ, Karp DD, et al. et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non–small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(suppl):abstr 7500. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klotz DM, Hewitt SC, Ciana P, et al. et al. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1) induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 62.Nicholson RI, Hutcheson IR, Knowlden JM, et al. et al. Nonendocrine pathways and endocrine resistance: observations with antiestrogens and signal transduction inhibitors in combination. Clin Cancer Res. 2004;10:346S–354S. doi: 10.1158/1078-0432.ccr-031206. [DOI] [PubMed] [Google Scholar]
- 63.Frogne T, Jepsen JS, Larsen SS, et al. et al. Antiestrogen-resistant human breast cancer cells require activated protein kinase B/Akt for growth. Endocr Relat Cancer. 2005;12:599–614. doi: 10.1677/erc.1.00946. [DOI] [PubMed] [Google Scholar]
- 64.Ye JJ, Liang SJ, Guo N, et al. et al. Combined effects of tamoxifen and a chimeric humanized single chain antibody against the type I IGF receptor on breast tumor growth in vivo. Horm Metab Res. 2003;35:836–842. doi: 10.1055/s-2004-814145. [DOI] [PubMed] [Google Scholar]
- 65.Robertson JF, Steger GG, Neven P, et al. et al. Activity of fulvestrant in HER2-overexpressing advanced breast cancer. Ann Oncol. 2010;21:1246–1253. doi: 10.1093/annonc/mdp447. [DOI] [PubMed] [Google Scholar]
- 66.Camirand A, Zakikhani M, Young F, et al. et al. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005;7:R570–579. doi: 10.1186/bcr1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 68.Nahta R, Yuan LX, Zhang B, et al. et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 69.Jones HE, Gee JM, Taylor KM, et al. et al. Development of strategies for the use of anti-growth factor treatments. Endocr Relat Cancer. 2005;12:S173–182. doi: 10.1677/erc.1.01004. Suppl. 1. [DOI] [PubMed] [Google Scholar]
- 70.Ramalingam SS, Spigel DR, Chen D, et al. et al. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non–small-cell lung cancer. J Clin Oncol. 2011;29(suppl):abstr 7527. doi: 10.1200/JCO.2011.36.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belani CP, Goss G, Blumenschein G., Jr Recent clinical developments and rationale for combining targeted agents in non–small cell lung cancer (NSCLC) Cancer treatment reviews. 2012;38:173–184. doi: 10.1016/j.ctrv.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Watkins DJ, Tabernero J, Schmoll HJ, et al. et al. A randomized phase II/III study of the anti–IGF-1R antibody MK-0646 (dalotuzumab) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory metastatic colorectal cancer (mCRC) with wild-type (wt) KRAS status. J Clin Oncol. 2011;29(suppl):abstr 3501. [Google Scholar]
- 73.Watkins DJ, Ayers M, Cunningham D, et al. et al. Molecular analysis of the randomized phase Il/Ill study of the anti–IGF-1R antibody dalotuzumab (MK-0646) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory KRAS wild-type metastatic colorectal cancer (mCRC) J Clin Oncol. 2012;30(suppl):abstr 3531. [Google Scholar]
- 74.O'Reilly KE, Rojo F, She QB, et al. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wan X, Harkavy B, Shen N, et al. et al. Rapamycin induces feedback activation of AKT signaling through an IGF-1R–dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 76.Kurmasheva R, Boltz C, Phelps D, et al. et al. Combination of CP-751871, a human monoclonal antibody against the IGF-1 receptor, with rapamycin results in a highly effective therapy for xenografts derived from childhood sarcomas. AACR Meeting Abstracts. 2007 Oct;:C172. [Google Scholar]
- 77.Naing A, LoRusso P, Fu S, et al. et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors. Clin Cancer Res. 2012;18:2625–2631. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vlahovic G, Meadows K, Arrowood C, et al. et al. Phase I study of the IGF-1R antibody ganitumab (AMG 479) in combination with everolimus in patients with advanced solid tumors. Mol Cancer Ther. 2011;10:B58. Suppl. 1. [Google Scholar]
- 79.Di Cosimo S, Bendell JC, Cervantes-Ruiperez A, et al. et al. A phase I study of the oral mTOR inhibitor ridaforolimus (RIDA) in combination with the IGF-1R antibody dalotozumab (DALO) in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(suppl):abstr 3008. [Google Scholar]
- 80.Naing A, LoRusso P, Mills G, et al. et al. Phase I study combining an IGFR inhibitor (IMC-A12) and an mTOR inhibitor (temsirolimus) in patients with solid tumors or lymphoma. J Clin Oncol. 2009;27(suppl):abstr e14535. [Google Scholar]
- 81.Khawaja MR, Younger A, Funke JM, et al. et al. Phase I study of everolimus (RAD001) and AMG 479 in patients (pts) with advanced solid tumors and colorectal cancer (CRC) J Clin Oncol. 2011;29(suppl):abstr TPS157. [Google Scholar]
- 82.Hart L, Burris HA, Infante JR, et al. et al. mTOR inhibitor everolimus (EV) and IGFR inhibitor OSI-906 (OSI) for the treatment of patients (pts) with refractory metastatic colorectal cancer (mCRC) J Clin Oncol. 2011;29(suppl):abstr e14054. [Google Scholar]
- 83.Ahmed SR, Cosgrove D, Ball D, et al. et al. A phase I, single-institution, open-label, dose escalation trial with an expansion cohort evaluating the safety and tolerability of AZD6244 and IMC-A12 in subjects with advanced solid malignancies. J Clin Oncol. 2012;30(suppl):abstr 3020. [Google Scholar]
- 84.Higano CS, Yu EY, Whiting SH, et al. et al. A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-I receptor IgG1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2007;25(suppl):abstr 3505. [Google Scholar]
- 85.Atzori F, Tabernero J, Cervantes A, et al. et al. A phase I, pharmacokinetic and pharmacodynamic) study of Dalotuzumab (MK-0646), an insulin-like growth factor-I receptor monoclonal antibody in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 86.Hidalgo M, Tirado Gomez M, Lewis N, et al. et al. A phase I study of MK-0646, a humanized monoclonal antibody against the insulin-like growth factor receptor type 1 (IGF1R) in advanced solid tumor patients in a q2wk schedule. J Clin Oncol. 2008;26(suppl):abstr 3520. [Google Scholar]
- 87.Dong J, Tamraz S, Berquist L, et al. et al. BIIB022, a human antibody targeting human insulin-like growth factor-1 receptor (IGF-1R), enhances the anti-tumor activities of Tarceva in non–small cell lung carcinoma (NSCLC) and Rapamycin in sarcoma cell lines. AACR Meeting Abstracts. 2008 Apr;:4002. [Google Scholar]
- 88.Hariharan K, Dong J, Demarest S, et al. et al. BIIB022, a fully human nonglycosylated gamma-4P antibody targeting IGF-1R for cancer therapy. AACR Meeting Abstracts. 2007 Oct;:B210. [Google Scholar]
- 89.Cartlidge S, Chang Y, Lecomte-Raeber O, et al. et al. MEDI-573: a fully human antibody to IGF-I and IGF-II for the treatment of solid tumor and hematological diseases. AACR Meeting Abstracts. 2009 Apr;:2802. [Google Scholar]
- 90.Carboni JM, Wittman M, Yang Z, et al. et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–3349. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 91.Girnita A, Girnita L, del Prete F, et al. et al. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 92.Cortes J, Paquette R, Talpaz M, et al. et al. Preliminary clinical activity in a phase I trial of the BCR-ABL/IGF-1R/Aurora Kinase Inhibitor XL228 in patients with Ph* leukemias with either failure to multiple TKI therapies or with T315I mutation. American Society of Hematology Annual Meeting, Dec. 2008:A3232. [Google Scholar]
- 93.Ryan CJ, Harzstark AH, Rosenberg J, et al. et al. A pilot dose-escalation study of the effects of nordihydroguareacetic acid on hormone and prostate specific antigen levels in patients with relapsed prostate cancer. BJU Int. 2008;101:436–439. doi: 10.1111/j.1464-410X.2007.07330.x. [DOI] [PubMed] [Google Scholar]
- 94.Youngren JF, Gable K, Penaranda C, et al. et al. Nordihydroguaiaretic acid (NDGA) inhibits the IGF-1 and c-erbB2/HER2/neu receptors and suppresses growth in breast cancer cells. Breast Cancer Res Treat. 2005;94:37–46. doi: 10.1007/s10549-005-6939-z. [DOI] [PubMed] [Google Scholar]
- 95.Olmos D, Postel-Vinay S, Molife LR, et al. et al. Safety, pharmacokinetics, and preliminary activity of the anti–IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]