Abstract
With improved overall survival of cervical cancer patients, the importance of the quality of life (QOL) is increasingly recognized. This study was conducted to compare the QOL of women with different stage cervical cancer before and after treatment to facilitate improved cervical cancer prevention and treatment. We used the generic Medical Outcomes Study Short Form-36 (MOS SF-36) to collect QOL information. Based on SF-36, we interviewed cervical cancer patients at West China Second Affiliated Hospital and Sichuan Cancer Hospital between May 2010 and January 2011. A total of 92 patients with precancerous lesions, 93 with early cancer, and 35 with advanced cancer responded to our survey. Average physical component summary (PCS) scores were significantly different between the three groups at every time point (P < 0.05). Average mental component summary (MCS) scores were significantly different between the three groups after treatment (P < 0.05). Average PCS and MCS scores increased gradually from the pretreatment to posttreatment period for patients with precancerous lesions. However, they reached the lowest at 1 month after treatment for patients with early and advanced cancers and rebounded between 1 and 6 months after treatment. Our results indicate that patients with precancerous lesions and early cervical cancer show better overall QOL than do those with advanced cervical cancer. Additionally, patients with early cancer recover more quickly than do those with advanced cancer in terms of both physical and mental functions. Thus, early detection and treatment initiatives may improve the QOL for patients with precancerous lesions and cervical cancer.
Keywords: Cervical cancer, clinical stage, quality of life
The incidence of cervical carcinoma ranks as the second highest among malignant tumors in females globally. Approximately 500,000 women are newly diagnosed with cervical cancer, and among them, about 270,000 die of cervical cancer each year[1]. Although the overall incidence of cervical cancer in developed countries has slightly declined, the age of cervical cancer patients has tended to become younger. Notably, a majority of patients gain long-term survival after treatment. In China, the development of clinical treatments for cervical carcinoma, especially radiotherapy, chemotherapy, surgery, and endocrine hormone and alternative complex therapy, has sharply decreased the mortality of cervical cancer, and 70.9% of patients remain alive after 5 years[2]. Hence, the quality of life (QOL) of cervical cancer patients has captivated more and more attention[3]. The morbidity, mortality, and survival rate fail to accurately depict the QOL of cervical cancer patients. In this study, we adopted the internationally recognized Medical Outcomes Study Short Form-36 (SF-36, Chinese version) to measure and compare the QOL of patients with cervical cancer at different clinical stages before and after therapy in order to provide evidence for preventing and treating cervical carcinoma.
Materials and Methods
Patients
A total of 220 patients with cervical cancer at various clinical stages who were admitted to West China Second Affiliated Hospital (West China Women's and Children's Hospital) and Sichuan Cancer Hospital between May 2010 and January 2011 were enrolled in this clinical trial. All participants satisfied the inclusion criteria. Namely, the diagnoses were explicit; the patients were aware of their disease conditions and voluntarily participated in the survey; the patients had no mental disease and consciousness disturbance, and so on. A total of 92 patients had precancerous lesions (CIN2, CIN3/carcinoma in situ), 93 had early cancer (stages I–IIa), and 35 had advanced cancer (stage IIb or higher). The median age of patients in the three groups was 38 years (range: 21 to 53 years), 43 years (range: 24 to 63 years), and 46 years (range: 33 to 66 years), respectively. In the three groups, 10 (10.9%), 14 (15.1%), and 7 (20%) patients self-reported leucorrhoea increase, respectively; 3 (3.3%), 12 (12.9%), and 8 (22.9%) self-reported vaginal contractive bleeding and irregular bleeding, respectively; and 79 (85.9%), 67 (72.0%), and 20(57.1%) were diagnosed with cervical cancer during medical tests, respectively. Referring to biopsy results, body condition, and fertility requirements of patients, patients with precancerous lesions primarily underwent cervical loop electrosurgical excision procedure (LEEP) or radical hysterectomy. Patients with early cervical cancer who were qualified for surgical indications underwent sub-total or radical hysterectomy and pelvic lymphadenectomy (radiochemotherapy was given depending on postoperative examination results). Patients with advanced cervical carcinoma underwent radiotherapy or/and chemotherapy.
SF-36 survey
A previous study indicated that SF-36 (Chinese version) has been widely recognized by Chinese population regarding its credibility and validity[4]. Thus, we used SF-36 to compile and compare the QOL scores of patients with various clinical stage cervical cancer before treatment and 1, 3, and 6 months after treatment. The first step of survey was performed by professionally trained post-graduates via delivering face-to-face questionnaire surveys to the participants, and the following three steps were conducted via follow-up by telephone. SF-36 consisted of 36 items, 8 scaled sores, and 1 self-evaluation on care outcomes. The 8 scales measured physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH), and these fell into two categories: physical component summary (PCS), including PF, RP, BP and GH; mental component summary (MCS), consisting of VT, SF, RE, and MH[5]. In addition, patient age, name, residence address, contact information, diagnosis date, clinical stage, and other characteristics were also recorded.
Follow-up
One and 3 months after clinical treatment, 81 patients with precancerous lesions, 84 with early cervical cancer, and 32 with advanced cervical cancer were followed up. Six months after treatment, 79 patients with precancerous lesions, 84 with early cervical cancer, and 31 with advanced cervical cancer were still under follow-up. The 6-month follow-up rate was 88%.
Data compilation and analysis
All data were input twice and double checked using EPIDATA3.2 software to guarantee the correctness. QOL scores were assessed according to the scoring rules proposed by Ware et al.[6],[7]. After calculating the raw scores of each scale (reverse items including 1, 6, 7, 8, 9a, 9d, 9e, 9h, 11b, and 11d were subjected to forward conversion when scoring), conversion scores were then calculated using the range method [conversion score = (raw score – lowest possible score)/(highest possible score – lowest possible score) × 100]. Conversion scores ranged from 0 to 100. Higher scores represented more favorable QOL. The conversion scores of PCS and MCS were calculated as follows: PCS = (PF + RP + BP + GH)/4; MCS = (VT + SF + RE + MH)/4.
Statistical analysis
SPSS 18.0 software was used for statistical analysis. After normality tests and homogeneity of variance tests, the QOL scores of each scale obtained after 4-step survey are expressed as mean ± standard deviation (SD). For patients with different stage cervical cancer, the QOL scores of the same scale obtained in the same step survey were subject to pairwise comparison by using ANOVA and SNK. The changing trend of QOL along with different clinical stages was observed and illustrated in tendency charts. The difference between QOL scores before and after treatment was compared among various time points. P values < 0.05 were considered significant.
Results
QOL scores
Prior to clinical treatment, the mean PCS score of patients with precancerous lesions was 82.96 ± 11.68, significantly higher than that of patients with early cervical cancer (77.93 ± 17.64) or advanced cervical cancer (74.06 ± 17.69) (P = 0.008). The mean MCS scores in the three groups did not differ significantly (P = 0.855). The patients in the precancerous lesion group had higher scores than did those in the other two groups in terms of PF, RP, and BP (PPF = 0.001, PRP = 0.003, PBP = 0.009). SF, RE and MH scores in the three groups did not significantly differ (PSF = 0.290, PRE = 0.578, PMH = 0.444), as shown in Table 1.
Table 1. Subscale scores of the patients with different disease stages before treatment.
| Item | Precancerous lesion (n = 92) | Early cancer (n = 93) | Advanced cancer (n = 35) | P |
| PF | 97.28 ± 6.00 | 94.25 ± 6.83 | 92.86 ± 8.25 | 0.001 |
| RP | 81.25 ± 31.15 | 62.90 ± 47.86 | 57.86 ± 49.55 | 0.003 |
| BP | 89.07 ± 17.97 | 82.48 ± 21.23 | 77.26 ± 25.33 | 0.009 |
| GH | 64.24 ± 18.10 | 72.10 ± 17.01 | 68.29 ± 18.98 | 0.012 |
| PCS | 82.96 ± 11.68 | 77.93 ± 17.64 | 74.06 ± 17.69 | 0.008 |
| VT | 61.47 ± 14.48 | 70.32 ± 18.75 | 66.00 ± 22.12 | 0.004 |
| SF | 86.68 ± 19.51 | 87.37 ± 19.98 | 92.50 ± 14.60 | 0.290 |
| RE | 71.38 ± 44.63 | 65.23 ± 47.12 | 63.81 ± 47.40 | 0.578 |
| MH | 66.13 ± 12.43 | 68.90 ± 18.31 | 68.91 ± 17.17 | 0.444 |
| MCS | 71.41 ± 17.81 | 72.96 ± 20.94 | 72.81 ± 20.58 | 0.855 |
All values are presented as mean ± standard deviation. PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; PCS physical component summary; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health; MCS, mental component summary.
One month after therapy, patients in the precancerous lesion group had higher scores for each scale compared with their counterparts in the remaining two groups. In addition, their PCS and MCS scores were 84.43 ± 12.51 and 78.46 ± 13.03, significantly higher than those of patients with early cervical cancer (48.32 ± 16.20, 63.29 ± 19.32) and advanced cervical cancer (49.19 ± 22.27, 59.21 ± 24.10) (PPCS < 0.001, PMCS < 0.001), as illustrated in Table 2.
Table 2. Subscale scores of the patients with different disease stages at 1 month after treatment.
| Item | Precancerous lesion (n = 81) | Early cancer (n = 84) | Advanced cancer (n = 32) | P |
| PF | 90.68 ± 8.97 | 63.45 ± 25.31 | 63.75 ± 24.46 | <0.001 |
| RP | 86.11 ± 27.67 | 9.23 ± 27.65 | 17.19 ± 36.72 | <0.001 |
| BP | 94.01 ± 14.02 | 61.07 ± 21.92 | 60.81 ± 28.26 | <0.001 |
| GH | 66.91 ± 15.26 | 59.52 ± 17.69 | 55.00 ± 21.33 | 0.002 |
| PCS | 84.43 ± 12.51 | 48.32 ± 16.20 | 49.19 ± 22.27 | <0.001 |
| VT | 60.62 ± 8.89 | 52.92 ± 18.81 | 53.28 ± 21.58 | 0.006 |
| SF | 93.36 ± 14.93 | 79.61 ± 20.24 | 74.61 ± 23.44 | <0.001 |
| RE | 88.89 ± 29.81 | 54.76 ± 48.99 | 48.96 ± 50.08 | <0.001 |
| MH | 70.96 ± 9.81 | 65.86 ± 15.80 | 60.00 ± 21.41 | 0.002 |
| MCS | 78.46 ± 13.03 | 63.29 ± 19.32 | 59.21 ± 24.10 | <0.001 |
Footnotes as in Table 1.
Three months after treatment, PCS score in the precancerous lesion group was 89.08 ± 9.96, significantly higher than those in the early cervical cancer (61.38 ± 17.71) and advanced cervical cancer groups (58.93 ± 16.47) (both P < 0.001). The mean MCS score in the precancerous lesion group (82.01 ± 7.29) was significantly higher than those in the early cervical cancer (72.95 ± 15.51) and in advanced cervical cancer groups (64.43 ± 22.76) (both P < 0.001). The scores for the eight scales for patients with precancerous lesions were higher than those for patients with early and advanced cervical carcinoma. Except VT, a significant difference was noted when comparing the scores of the remaining scales among the three groups (Table 3).
Table 3. Subscale scores of the patients with different disease stages at 3 months after treatment.
| Item | Precancerous lesion (n = 81) | Early cancer (n = 84) | Advanced cancer (n = 32) | P |
| PF | 98.27 ± 4.48 | 80.42 ± 19.16 | 83.13 ± 10.91 | <0.001 |
| RP | 95.06 ± 20.32 | 27.08 ± 40.18 | 26.56 ± 43.40 | <0.001 |
| BP | 94.95 ± 12.77 | 73.67 ± 22.06 | 66.97 ± 24.16 | <0.001 |
| GH | 68.02 ± 14.33 | 64.35 ± 15.42 | 59.06 ± 18.94 | 0.022 |
| PCS | 89.08 ± 9.96 | 61.38 ± 17.71 | 58.93 ± 16.47 | <0.001 |
| VT | 61.23 ± 8.04 | 60.23 ± 15.64 | 57.03 ± 20.27 | 0.358 |
| SF | 96.91 ± 8.51 | 86.46 ± 15.91 | 77.34 ± 22.32 | <0.001 |
| RE | 97.53 ± 13.72 | 75.00 ± 41.35 | 58.33 ± 49.37 | <0.001 |
| MH | 72.35 ± 8.40 | 70.10 ± 11.36 | 65.00 ± 18.09 | 0.012 |
| MCS | 82.01 ± 7.29 | 72.95 ± 15.51 | 64.43 ± 22.76 | <0.001 |
Footnotes as in Table 1.
Six months after treatment, the PCS (91.71 ± 4.16) and MCS (84.22 ± 3.97) scores in the precancerous lesion group were the highest, and the PCS (71.61 ± 17.04) and MCS scores (75.73 ± 16.83) in the advanced cancer group were the lowest among the three groups (PPCS < 0.001, PMCS = 0.002). In the precancerous lesion group, PF, RP, BP, SF, and RE scores were significantly higher than those in the other two groups (Table 4).
Table 4. Subscale scores of the patients with different disease stages at 6 months after treatment.
| Item | Precancerous lesion (n = 79) | Early cancer (n = 84) | Advanced cancer (n = 31) | P |
| PF | 99.56 ± 2.43 | 90.42 ± 13.31 | 92.10 ± 8.04 | <0.001 |
| RP | 98.73 ± 11.25 | 66.07 ± 44.19 | 51.61 ± 47.41 | <0.001 |
| BP | 97.35 ± 8.59 | 88.44 ± 16.34 | 79.52 ± 18.20 | <0.001 |
| GH | 71.20 ± 8.78 | 73.45 ± 14.08 | 63.23 ± 15.25 | 0.001 |
| PCS | 91.71 ± 4.16 | 79.60 ± 15.44 | 71.61 ± 17.04 | <0.001 |
| VT | 63.04 ± 9.62 | 71.25 ± 13.71 | 67.26 ± 16.27 | <0.001 |
| SF | 99.05 ± 3.89 | 92.26 ± 14.11 | 87.90 ± 18.39 | <0.001 |
| RE | 100.00 ± 0.00 | 88.89 ± 28.02 | 77.42 ± 40.72 | <0.001 |
| MH | 74.78 ± 6.67 | 79.48 ± 10.03 | 70.32 ± 15.21 | <0.001 |
| MCS | 84.22 ± 3.97 | 82.97 ± 13.12 | 75.73 ± 16.83 | 0.002 |
Footnotes as in Table 1.
These results suggest that the scores for each scale for patients with precancerous lesions were higher 6 months after treatment than before treatment. The scores for most scales (except PF) for patients with early cervical cancer 6 months after treatment exceeded those before treatment. For subjects with advanced cervical cancer, PF, RP, GH, and SF scores 6 months after treatment failed to recover to the levels before treatment. During the course of rehabilitation, the VT score of the precancerous lesions group was lowest among all scales; for those with early and advanced cancer, the RP score was lowest.
QOL score changes
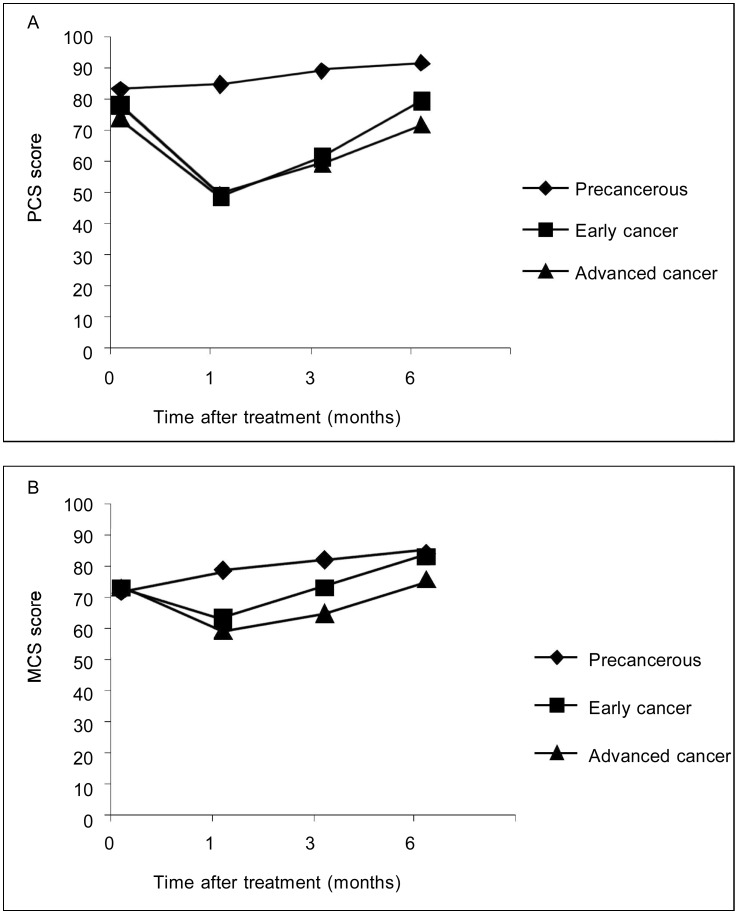
In the precancerous lesion group, both the PCS and MCS scores steadily increased over time (Figure 1). Trends in PCS and MCS scores in early cancer group were similar to those in advanced cancer group: lowest 1 month after treatment, with relative recovery between 1 and 6 months after treatment. However, the PCS score of patients with advanced cervical cancer (71.61 ± 17.04) did not recover to the pre-treatment level (74.06 ± 17.69).
Figure 1. Physical component summary (PCS) and mental component summary (MCS) of the patients in different disease stages over time.
Higher scores indicate better well-being. A, PCS of SF-36 by clinical stage over time; B, MCS of SF-36 by clinical stage over time.
The pre-treatment scores were deemed as baseline, and the PCS and MCS scores were determined by longitudinal comparison. One month after treatment, the PCS and MCS scores in the precancerous lesion group were greater than 0. The PCS and MCS scores differed significantly among the three groups (PPCS < 0.001, PMCS < 0.001), whereas no significant difference was noted between the early and advanced cancer groups. Three months after treatment, the PCS score in the precancerous lesion group was above 0, significantly higher than those in the early and advanced cancer groups (P < 0.001). Pairwise comparison revealed that the MCS score was significantly different between the three groups (P = 0.001). Six months after treatment, the PCS score was significantly different between the three groups (P = 0.028), whereas the difference in the MCS score was not significant between the three groups (P= 0.156) (Table 5).
Table 5. Variation in PCS and MCS at different time points after treatment compared with before treatment in the patients with different disease stages.
| Time after treatment | PCS |
P | MCS |
P | ||||
| Precancerous lesion | Early cancer | Advanced cancer | Precancerous lesion | Early cancer | Advanced cancer | |||
| 1 month | 0.62 | –29.65 | –24.38 | <0.001 | 6.14 | –8.80 | –13.54 | <0.001 |
| 3 months | 5.27 | –16.59 | –14.63 | <0.001 | 9.69 | 0.86 | –8.32 | 0.001 |
| 6 months | 7.81 | 1.63 | –1.34 | 0.028 | 12.03 | 10.88 | 3.20 | 0.156 |
Footnotes as in Table 1.
Over the course of the study, the overall QOL in the precancerous lesion and early cervical cancer groups was better than that in the advanced cervical cancer group. The mental and physical functions of patients with early cancer recovered more rapidly than those of patients with advanced cervical cancer.
Correlation between age and QOL
In this study, the median ages in the precancerous lesion, early cancer, and advanced cancer groups were 38, 43, and 46 years, respectively, and were significantly different (P < 0.001). To evaluate whether age affects QOL, we calculated the multiple correlation coefficient between age and the PCS and MCS scores at various time points. The results showed that there was no significant association between age and QOL (Table 6).
Table 6. Multiple correlation coefficients between age and PCS or MCS at different time points.
| Item | Time after treatment (months) |
|||
| 0 | 1 | 3 | 6 | |
| PCS | 0.013(P = 0.857) | –0.095(P = 0.122) | –0.123(P = 0.049) | –0.042(P = 0.553) |
| MCS | 0.103(P = 0.173) | –0.026(P = 0.729) | –0.118(P = 0.108) | –0.049(P = 0.536) |
Footnotes as in Table 1.
Discussion
Significance of QOL of cervical cancer patients
Both domestic and international scholars have performed long-term investigation of etiology, auxiliary diagnosis tools, screening methods, and clinical treatments for cervical carcinoma in an attempt to prolong patient survival. However, the clinical efficacy of available treatments is undesirable for two reasons: (1) advanced cervical cancer is hardly curable, and (2) severe adverse events occur, significantly affecting patient's QOL[8]. Therefore, how to improve QOL, in addition to clinical treatment, has captivated much attention. QOL not only refers to how long patients survive but also stresses social function and mental status, emphasizes subjective feelings and functional status, and reflects individual or population health condition from multiple scales[9]. QOL consideration plays a significant role in exploring pertinent precautions and in evaluating the quality of medical health service. It also meets the novel medical goal proposed by WHO: prevent and treat disease, prolong survival, increase QOL, reduce death rate, and promote mental and physical health.
SF-36, which was used in this study, properly integrates the physical, mental, functional, and subjective feelings of cancer patients. It has been widely recognized due to its desirable conciseness, easy management, credibility and validity, and other favorable factors[10]. This form can be used not only for assessing healthy populations but also for assessing cancer patients.
Comparison of QOL of patients with different clinical stage cervical cancer
In our study, SF-36 outcomes indicated that PCS scores differed significantly among the three groups (P < 0.05). The PCS score in the precancerous lesion group was higher than those in the other two groups, indicating that physical condition of patients with early and advanced cervical cancer was significantly affected by disease symptoms and adverse events. However, the pretreatment MCS score did not significantly differ among the three groups (P > 0.05), probably because the mental function of patients with various clinical stage cervical cancer was influenced when informed of the diagnosis[11]. At the following time points, the MCS score significantly differed among the three groups (P < 0.05).
During the period between pretreatment and 6 months posttreatment, the PCS and MCS scores of the patients with precancerous lesions steadily increased over time. The score of each scale 6 months after treatment exceeded that before treatment. The PCS and MCS scores in early cervical cancer group followed similar trends to those in advanced cancer group; the lowest scores were observed 1 month after treatment, suggesting that the QOL was significantly influenced by related clinical therapy[12]. Scores for these patients then recovered between 1 and 6 months after treatment. Six months after treatment, the scores for most scales (except PF) were higher than the levels before treatment for the patients with early cervical cancer, whereas PF, RP, GH, and SF failed to recover to pretreatment levels for the patients with advanced cervical cancer, suggesting that the QOL, especially physical health condition, of patients with advanced cacner recovered more slowly than that for the patients with early cervical cancer or precancerous lesions. However, the mental status of advanced cervical cancer patients was relatively stable. With pretreatment scores deemed as baseline, longitudinal comparison of the PCS and MCS scores produced results consistent with the conclusions above. This may be associated with the fact that the patients with early cervical cancer underwent operations, whereas those with advanced cervical cancer underwent radiotherapy, thus suffering from more severe damage. Vistad et al.[3] systematically reviewed the QOL of cervical carcinoma patients measured by 23 studies and validated the conclusion as drawn in our study.
During subsequent follow-up, we found that many patients reduced the frequency of sex in case of increasing the recurrence of cervical cancer, which negatively affected their mental life. In addition, some young patients concerned that disease or treatment might damage reproductive capacity. After consulting doctors, patients relieved their mental stress, and they became more optimistic about their future.
The relationship between age and QOL greatly varied among previous studies. In the present study, the median ages in the three groups were 38, 43, and 46 years, which were significantly different. Multiple correlation analysis revealed no association between patient age and QOL, which was consistent with the results reported by Greimel et al.[13]. These results indicate that the QOL of patients with various clinical stage cervical cancer was not affected by age.
Suggestions for prevention and treatment of cervical cancer
This study included cervical cancer patients with different clinical stage cervical cancer and analyzed QOL before treatment and 1, 3, and 6 months after treatment. The overall QOL of patients with precancerous lesions and early cervical carcinoma were better than that of patients with advanced cervical cancer. In addition, the QOL of patients with precancerous lesions and early cervical carcinoma at 6 months after treatment even exceeded that before treatment, indicating that early diagnosis and active treatment are essential in elevating the QOL of cervical cancer patients. Effective screening and early diagnosis and treatment among high risk populations will enhance the QOL of cancer patients.
The survey results also indicate that different precautions should be applied depending upon the clinical stages of cervical cancer. For patients with precancerous lesions, doctors should provide patients with psychological counseling, help patients to cultivate healthy attitudes towards diseases, and encourage patients to have a normal sex life. For those with early cervical carcinoma, patients should be encouraged to perform ordinary physical activities and return to daily living and work. For those with advanced cancer, patients should be guided to emphasize the recovery of physiological function, to participate in community activities, and so on. Multiple investigations revealed that the more information patients acquire, the higher level of response they have[5],[9],[12]. To sum up, the clinicians should not only treat diseases and improve patient physical and physiological functions, but also ameliorate their mental disorders and social function by providing proper mental counseling and alleviation therapy.
This study preliminarily evaluated and compared the QOL of female patients with different clinical stage cervical cancer. More investigation with larger sample size and longer follow-up should be performed to evaluate the QOL and its influential factors on patients with different clinical stage cervical cancer, and to provide more evidence for extending the screening of cervical carcinoma.
References
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 2.Lu QR, Wang YL, Zhao XH. Study on the quality of life of cervical cancer patients. Chin Nurs Res. 2010;24:946–948. [in Chinese] [Google Scholar]
- 3.Vistad I, Fossa SD, Dahl AA. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol Oncol. 2006;102:563–572. doi: 10.1016/j.ygyno.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Liu CJ, Li NX, Ren XH, et al. et al. Feasibility of using short-form 36 in Chinese population. Huaxi Yike Daxue Xuebao. 2001;32:39–42. [in Chinese] [PubMed] [Google Scholar]
- 5.Wenzel L, DeAlba I, Habbal R, et al. et al. Quality of life in longterm cervical cancer survivors. Gynecol Oncol. 2005;97:310–317. doi: 10.1016/j.ygyno.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 7.Ware JE, Snow KK, Kosinski M, et al. et al. Boston: New England Medical Center, the Health Institute; 1993. SF-36 health survey manual and interpretation guide; pp. 1–12. [Google Scholar]
- 8.Huang Y, Wan CH, Lu YB. Overview of quality of life of cervical cancer patients. Chin J Behavioral Med Sci. 2002;11:109–110. [in Chinese] [Google Scholar]
- 9.Klee M, Thranov I, Machin D. Life after radiotherapy: the psychological and social effects experienced by women treated for advanced stages of cervical cancer. Gynecol Oncol. 2000;76:5–13. doi: 10.1006/gyno.1999.5644. [DOI] [PubMed] [Google Scholar]
- 10.Ware J, Gandek B. Overview of SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 11.Visser M, van Lanscott JJ, van der Velden J, et al. et al. Quality of life in newly diagnosed cancer patients waiting for surgery is seriously impaired. J Surg Oncol. 2006;93:571–577. doi: 10.1002/jso.20552. [DOI] [PubMed] [Google Scholar]
- 12.Greimel E, Thiel I, Peintinger F, et al. et al. Prospective assessment of quality of life of female cancer patients. Gynecol Oncol. 2002;85:140–147. doi: 10.1006/gyno.2002.6586. [DOI] [PubMed] [Google Scholar]
- 13.Greimel ER, Freidl W. Functioning in daily living and psychological well-being of female cancer patients. J Psychosom Obstet Gynaecol. 2000;21:25–30. doi: 10.3109/01674820009075605. [DOI] [PubMed] [Google Scholar]