Abstract
The phosphoinositide 3-kinase-AKT-mammalian target of rapamycin (PI3K-AKT-mTOR) pathway is a frequently hyperactivated pathway in cancer and is important for tumor cell growth and survival. The development of targeted therapies against mTOR, a vital substrate along this pathway, led to the approval of allosteric inhibitors, including everolimus and temsirolimus, for the treatment of breast, renal, and pancreatic cancers. However, the suboptimal duration of response in unselected patients remains an unresolved issue. Numerous novel therapies against critical nodes of this pathway are therefore being actively investigated in the clinic in multiple tumour types. In this review, we focus on the progress of these agents in clinical development along with their biological rationale, the need of predictive biomarkers and various combination strategies, which will be useful in counteracting the mechanisms of resistance to this class of drugs.
Keywords: PI3K-AKT-mTOR, PTEN, RTK, signaling pathways, molecular therapeutics
Despite significant advancements in the management of solid malignancies over the last six decades, the prognosis of advanced solid malignancies is generally limited to months. In this era of personalized medicine, the focus of treatment has been to exploit the principles of oncogenic addiction and synthetic lethality in order to identify optimal targets for various cancers. Over the last two decades, the phosphoinositide 3-kinase (PI3K) pathway has been studied extensively in view of the growing evidence supporting the critical role of this pathway in cancer progression, as it influences metabolism, tumor growth, survival, and the development of metastases [1]–[4]. Consequently, several drugs have been developed to target this pathway to treat human cancers. These agents have shown some preclinical and clinical activity; however, they have largely failed to live up to the expectations of clinicians and scientists. Further significant efforts are therefore required to target this pathway effectively. In this review, we examine the current literature demonstrating the role of the PI3K pathway in the development and progression of cancer. We review various existing inhibitors of this pathway; their preclinical activity and clinical efficacy, along with possible reasons for their failure; and potential treatment strategies for overcoming resistance to inhibitors of this pathway.
The PI3K-AKT-mTOR Pathway in Cancer
The PI3K-AKT-mTOR signaling in cancer cell growth and survival
The family of lipid kinases termed PI3K are regarded as key regulators in many essential cellular processes, including cell survival, growth, and differentiation[1],[5],[6]. The PI3K pathway has several important nodes that play a crucial role in this pathway, resulting in a diversity of functional outcomes. The AKT-mediated activation of downstream targets, including mammalian target of rapamycin (mTOR), stimulates cell proliferation and the regulation of translation in response to growth factors by the phosphorylation of the protein synthesis machinery (Figure 1) [7]. This translational promotion by mTOR includes the phosphorylation of ribosomal protein S6 kinases (S6K) and 4E-binding protein 1 (4E-BP1), the latter of which results in the release of eukaryotic translation initiation factor 4E (eIF4E), which has known anti-apoptotic activities in vitro[8],[9]. These effects are counteracted by the tuberous sclerosis complex-1 (TSC1)-TSC2 complex, which has inhibitory effects on 4E-BP1 and eIF4E [10],[11]. AKT also phosphorylates and inhibits TSC2, which demonstrates the complexity of this pathway. Intriguingly, rapamycin and its analogues can inhibit mTOR, but this can lead to activation of upstream proteins such as AKT, due to the loss of a feedback loop mechanism[12],[13].
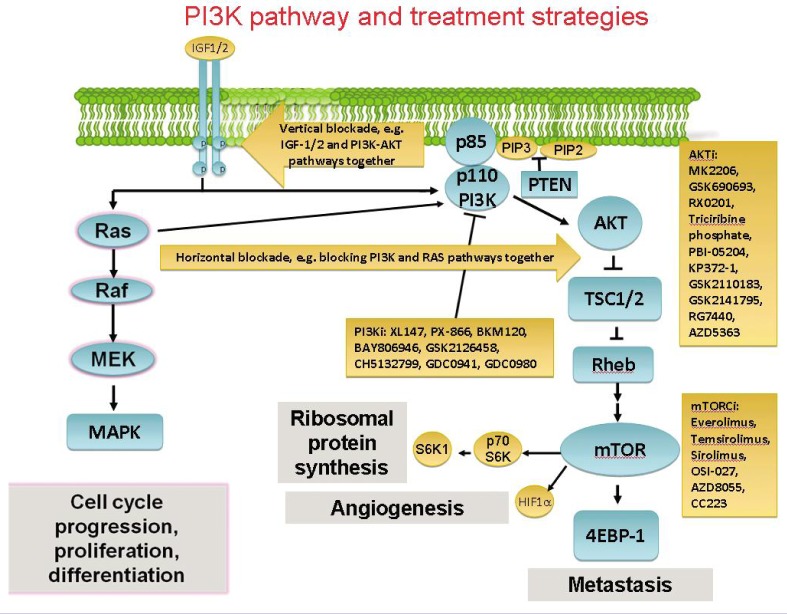
Figure 1. Schematic diagram of signaling through the phosphoinositide 3-kinase-AKT (PI3K-AKT) pathway with current and future treatment strategies.
PI3K signaling impacts on cell growth, survival, differentiation, and proliferation. The pointed arrows represent activation of substrates, while blunt arrows represent inhibition. Activation of membrane kinases, such as insulin-like growth factor-1 (IGF1), by external growth factors can initiate activation of intracellular signaling pathways. AKT is phosphorylated downstream of PI3K with various effects, including the activation of mammalian target of rapamycin (mTOR). mTOR phosphorylates p70S6 and 4E-binding protein 1 (4EBP-1), which then leads to an increased translation of mRNA that encodes several cell cycle regulators. These effects are controlled by the tuberous sclerosis 1 (TSC1)-TSC2 complex. The Ras-Raf-MEK-MAPK pathway has its own distinct downstream effects, but also converges with the PI3K-AKT pathway. Future combination regimens involving targeted agents against this signaling network include the concomitant or sequential blockade of these pathways. PTEN, phosphatase and tensin homolog; HIF-1α, hypoxia-inducible factor-1α; MAPK, mitogen-activated protein kinase.
In addition, the PI3K-AKT pathway interacts with the complex molecular mechanism that controls cellular energy control and glucose metabolism. AKT phosphorylates and inhibits glycogen synthase kinase-3 (GSK3), phospho-diesterase-3B, protein phosphatase 2A, and Raf-1. The PI3K signaling also controls growth, proliferation, senescence, and angiogenesis. These processes are regulated by vascular endothelial growth factor (VEGF) transcriptional activation and hypoxia-inducible factor-1 alpha (HIF-1α) expression[14],[15].
This brief summary of the PI3K-AKT pathway involvement in various important cell growth mechanisms highlights the complexity and importance of several nodes within this pathway.
Resistance to anti-cancer therapy
It is well established that inhibition of any of the nodes of the PI3K-AKT pathway can restore vulnerability to chemotherapy, radiotherapy, and hormonal treatment[16],[17]. Several studies have demonstrated a link between drug resistance and altered AKT signaling. Knuefermann et al.[16] showed that the cell lines expressing both HER2 and HER3 in breast adenocarcinoma had a higher phosphorylation level of AKT and were associated with increased resistance to multiple chemotherapeutic agents. Selective inhibition of PI3K or AKT activity leads to induction of apoptosis; therefore, the proposed mechanism for this synergy was the potentiation of apoptosis.
Deregulation of the PI3K-AKT-mTOR pathway in cancer
The PI3K-AKT pathway can be inappropriately activated in various cancers. The two major observed mechanisms of PI3K-AKT activation in human cancers are somatic alterations in specific nodes of the pathway and activation by receptor tyrosine kinases (RTKs). Our understanding of these mechanisms of PI3K pathway activation is crucial for developing effective therapeutic approaches and gaining a clinical benefit from PI3K inhibition.
Somatic alterations of PI3K pathway components in cancer
Genetic mutations/loss of function
Several genetic alterations are known to activate the PI3K-AKT signaling, but the second most common genetic abnormality found in human cancer is inactivation of the PTEN tumor suppressor gene. PI3K signaling is inhibited by PTEN through the dephosphorylation of phophatidylinositol-3,4,5-triphosphate (PIP3), which is the lipid-signaling product of the class I PI3Ks[18]–[20]. The vast majority of these PTEN mutations are protein truncations, whereas missense mutations are also common. Transcriptional repression and epigenetic silencing of PTEN are other observed mechanisms of PTEN inactivation[21]. Preclinical studies have shown that the heterozygous loss of PTEN in mice resulted in neoplasia of multiple epithelia, including the prostate, intestine and mammary gland[22]. Homozygous deletion of PTEN in the prostate epithelium can lead to aggressive prostate carcinoma. It has been shown that cancers with high Gleason scores in primary tumors tend to be associated with PTEN loss in metastases [23],[24]. More recently, Mueller et al. [25] examined the signaling pathways that underlie the pathogenesis of pediatric gliomas and assessed the activation of the PI3K-AKT-mTOR pathway by using immunohistochemistry. They evaluated the downstream signaling molecules phosphorylated p-S6, p-PRAS40, and PTEN, as well as PTEN promoter methylation and the MIB labeling index. They found that the majority (80%) of high-grade gliomas showed activation of the PI3K-AKT-mTOR pathway and that 50% had PTEN promoter methylation. Tumor grade correlated negatively with PTEN expression and positively with p-S6 and p-4EBP1 levels. Trends toward an inverse correlation of PTEN promoter methylation with PTEN protein expression and a direct correlation of p-S6 and p-4EBP1 levels with poor clinical outcomes, as measured by progression-free survival, were also noted. It was concluded that the majority of pediatric gliomas show activation of the PI3K-AKT-mTOR pathway, with PTEN promoter methylation being a common feature of these tumors[25]. Germline mutations in the PTEN gene can result in Cowden disease and Bannayan-Riley-Ruvaslcaba syndrome (associated with macrocephaly, multiple lipomas, and hemangiomata), two conditions that are associated with high risk of malignancies. Unlike other tumor suppressor genes, such as p53, biallelic inactivation is not required for the suppression of PTEN activity; rather, haplo-insufficiency may suffice in promoting tumorigenesis. This suggests that reduced PTEN protein expression without actual mutations may be another mechanism of PTEN hindrance leading to cancer growth.
Genetic amplification of PIK3CA and AKT1/2
Recent studies have shown that somatic mutations in PIK3CA are common in a variety of human tumors, including breast, colon, and endometrial cancers and glioblastoma[4],[26]. The two common mutation regions are clustered in exons 9 and 20, which encode the helical and catalytic domains of p110α, respectively[4]. A small cluster of mutations is also found in the N-terminal p85-interacting domain, which can increase the lipid kinase activity of p110α. However, these mutations do not alter the interaction between p110α and p85α subunits[4]. The PIK3CA mutations increase in vitro PI3K activity, and the expression of p110α mutants in cells confers AKT activation in the absence of growth factor stimulation, which in turn leads to oncogenesis. So far, no other p110 isoform mutations have been identified, indicating that p110α harbors the main oncogenic potential [27],[28]. Preclinical studies have shown that transgenic mice with induction of kinase domain mutant p110α H1047R developed lung adenocarcinoma [29]. Likewise, similar mouse-knockout and transgenic models confirm the tumorigenic potential of hyperactivation of the PI3K pathway.
AKT overexpression
There is now growing evidence that different AKT isoforms have non-overlapping functions in cancer. A single amino acid substitution, E17K, in the lipid-binding PH domain of AKT-1 has been identified in various human cancers including breast, colorectal, endometrial, and ovarian cancers[30]. AKT-2 overexpression has been observed in colorectal cancers and metastases. It is proposed that AKT-2 promotes cellular survival and growth. Interestingly, it was noted that the loss of AKT-1 promoted cellular invasion and metastases, possibly by shifting the balance of signaling through AKT-2[31],[32]. The E17K mutation has been found in some melanomas[33]. Mutations in various AKT isoforms suggest a potential role for AKT inhibitors in therapy, which is discussed below. Notably, in addition to somatic mutations of PTEN, PIK3CA, PIK3R1, and AKT, some cancers have amplifications of AKT-1, AKT-2, and PIK3CA; however, it is not entirely clear if these amplifications have a significant impact on clinical outcome.
Pathway activation by receptor tyrosine kinases (RTKs) and Ras
It is well established that RTK-mediated activation of PI3K is of crucial importance for its oncogenic activity and that it is clearly linked to the RTK signaling. The p85 regulatory subunit is vital in mediating PI3K activation by RTKs. When certain therapies are effective in targeting RTKs, they invariably lead to suppression of PI3K signaling. Examples include PI3K activation by epithelial growth factor receptor (EGFR) in lung cancers harboring somatic activating mutations in EGFR[34] and human epidermal growth factor receptor 2 (HER2) mutations in breast cancers with HER2 amplification [35]. Thus when these cancers are successfully treated, the PI3K signaling is switched off as a result of targeting RTKs. Unfortunately, in some cancers, multiple RTKs activate PI3K signaling, and these cancers tend to be resistant to single RTK-targeted therapies[36]. PI3K is also an effector of Ras-mediated oncogenic signaling, which is a small GTPase that is frequently mutated in human cancers. Studies suggest that a direct link exists between Ras and PI3K. Preclinical studies showed that mutant p110α inhibited K-Ras–induced lung adenocarcinoma in genetically engineered mouse models [37]. This approach has been rationalized in early phase human clinical trials where a combination of MEK and AKT inhibitors has been examined in patients with K-Ras mutated lung adenocarcinoma. However, it remains unclear whether mutated Ras is sufficient to directly activate PI3K and thereby bypass its engagement with phosphotyrosines.
Inhibitors in Clinical Stage Development
There are five major classes of inhibitors designed to target various nodes of the PI3K-AKT-mTOR pathway. Agents in active clinical development are summarized in Table 1.
Table 1. PI3K, AKT, and mTOR inhibitors in clinical development.
| Generic name /code number (trade name) | Company | Stage of development | Target disease | stage | Target and mechanism |
| PI3K inhibitors | ||||
| Pan-PI3K inhibitors | ||||
| XL-147/EXEL6147 | Exelixis / Sanofi-Aventis | PII | Endometrial cancer | PII Breast cancer | PII NSCLC | PII Ovarian cancer | PII Solid tumors | PI Lymphoma | PI Glioblastoma | PI |
Class I PI3K / EGFR inhibitor |
| PX866 | Oncothyreon | PII | Solid tumors | PII Lung cancer | Preclinical Ovarian cancer | Preclinical Glioblastoma | Preclinical Metastatic tumors | PII |
PI3K inhibitor |
| BKM120 | Novartis | PII | Solid tumors | PII Breast cancer | PII Colorectal cancer | PII |
PI3K inhibitor |
| RG7321/GDC0941 | Roche | PI | Breast cancer | PI NSCLC | PI Ovarian cancer | PI Non-Hodgkin's lymphoma | PI |
PI3K inhibitor |
| BAY806946 | Bayer Schering Pharma |
PI | Solid tumors/follicular lymphomas| PI |
PI3K inhibitor |
| GSK2126458 | GlaxoSmithkline | PI | Solid tumors | PI Lymphoma | PI |
PI3K inhibitor |
| CH5132799 | Roche | PI | Solid tumors | PI | PI3K inhibitor |
| ATU027 | Silence Therapeutics |
PI | Solid tumors | PI Pancreatic cancer | Preclinical Gastric cancer | Preclinical Prostate cancer | Preclinical |
PKN3 inhibitor (RNA interference agent) |
| PI3K isoform-specific inhibitors | ||||
| CAL101 | Calistoga Pharmaceuticals |
PII | CLL | PII Non-Hodgkin's lymphoma | PI Allergic rhinitis | Discontinued Inflammation | Discontinued Asthma | Discontinued |
PI3K delta inhibitor |
| BYL719 | Novartis | PI | Neoplasm | PI | PI3K alpha inhibitor |
| AZD6482 | AstraZeneca | Preclinical | Hematologic malignancies | Preclinical | PI3K beta inhibitor |
| GSK2636771 | GlaxoSmithkline | PI/II | Solid tumors | PII | PI3K beta inhibitor |
| AKT inhibitors | ||||
| Allosteric AKT inhibitors | ||||
| MK-2206 | Merck & Co., Inc. | PII | Endometrial cancer | PII Leukemia | PII Ovarian cancer | PII Breast cancer | PII Pancreatic cancer | PII Melanoma | PII Multiple myeloma | PI AML | PI Hematologic tumors | PI CLL | PI Myelodysplastic syndrome | PI |
AKT inhibitor |
| GSK690693 | GlaxoSmithkline | PI | Melanoma | PI Solid tumors | PI |
AKT inhibitor |
| RX0201 (Archexin) | Rexahn Pharmaceuticals |
PII | Lymphoma | PI Pancreatic cancer | PII Solid tumors | PI Renal cell carcinoma | Discontinued |
AKT Antisense |
| ATP-competitive AKT inhibitors | ||||
| Triciribine phosphate | VioQuest Pharmaceuticals / Cahaba Pharmaceuticals |
PII | Leukemia | PII Solid tumors | PII Ovarian cancer | PII Breast cancer | PII Pancreatic cancer | PII Multiple myeloma | PI AML | PI Hematological tumors | PI CLL | PI Myelodysplastic syndrome | PI Melanoma | PI NSCLC | Preclinical |
AKT inhibitor |
| PBI-05204 (oleandrin) | Phoenix Biosciences |
PI | Neoplasm | PI | AKT, FGF-2, NF-κB, and p70S6K inhibitor |
| KP372-1 | Stemgent | Preclinical | Preclinical | AKT, PDK-1, Flt3 |
| AR-42 | Arno | Preclinical | Preclinical | AKT inhibitor |
| GSK2110183 | GlaxoSmithkline | PI | Hematological tumors | PI | AKT inhibitor |
| GSK2141795 | GlaxoSmithkline | PI | Lymphoma | PI Solid tumors | PI Colorectal cancer | PI Endometrial cancer | PI Pancreatic cancer | PI Ovarian cancer | PI |
AKT inhibitor |
| RG7440 | Roche | PI | Solid tumors | PI | AKT inhibitor |
| GDC0068 | Array Biopharma | PI | Solid tumors | PI | AKT inhibitor |
| SR13668 | Preclinical | Preclinical | AKT inhibitor | |
| Pan-AKT inhibitor | ||||
| AZD5363 | Astra Zeneca | PI | Solid tumors | PI | Pan-AKT inhibitor |
| mTOR inhibitors | ||||
| Rapalog mTOR inhibitors | ||||
| Everolimus (Afinitor) | Novartis | Launched | Renal cell carcinoma | Launched Pancreatic cancer | PIII Breast cancer | PIII Gastric cancer | PIII Non-Hodgkin's lymphoma | PIII Liver cancer | PIII AML | PIII GIST | PII Prostate cancer | PII NSCLC | PII Colon cancer | PII Metastatic bone disease | PII Melanoma | PII Sarcoma | PII Myelodysplastic syndrome | PII Thyroid cancer | PII Head & neck cancer | PII Glioblastoma | PII SCLC | PI Macular degeneration | PII ALL | PII Ovarian cancer | PII |
mTOR inhibitor |
| Temsirolimus (TORISEL®) | Pfizer | Launched | Renal cell carcinoma | Launched Non-Hodgkin's lymphoma | Registered Lymphoma | PIII Prostate cancer | PII Multiple myeloma | PII Melanoma | PII CLL | PII Ovarian cancer | PII NSCLC | PII Head & neck cancer | PII Breast cancer | Discontinued |
mTOR inhibitor |
| ridaforolimus | ARIAD Pharmaceuticals/Merck |
PIII | Sarcoma | PIII Glioma | PII Prostate cancer | PII Solid tumors | PII Hematological tumors | PII Endometrial cancer | PII Breast cancer | PI NSCLC | PII |
mTOR inhibitor |
| sirolimus | Celgene | PI | Solid tumors | PI | mTOR inhibitor |
| ABI009 | ||||
| TORC1/2 inhibitors | ||||
| OSI027 | Astellas Pharma | PI | Solid tumors | PI Lymphoma | PI |
TORC1-TORC2 inhibitor |
| AZD8055 | AstraZeneca | PII | Liver cancer | PII Solid tumors | PI |
TORC1-TORC2 inhibitor |
| AZD2014 | AstraZeneca | PI | Solid tumors | PI | TORC1-TORC2 inhibitor |
| INK128 | Intellikine | PI | Solid tumors | PI Multiple myeloma | PI |
TORC1-TORC2 inhibitor |
| CC223 | Celgene | PII | Solid tumors | PII | TORC1-TORC2 inhibitor |
| Dual PI3K-mTOR inhibitors | ||||
| BEZ235 | Novartis | PII | Breast cancer | PI Glioma | PI Prostate cancer | PI Colorectal cancer | PI Renal cell carcinoma | PI |
PI3K-mTOR inhibitor |
| SAR245409/XL765 | Exelixis /Sanofi-Aventis | PII | NSCLC | PII Glioblastoma | PII Breast cancer | PII Solid tumors | PI Colorectal cancer | PI Sarcoma | PI Mesothelioma | PI Prostate cancer | PI Lymphoma | PI |
PI3K-mTOR-ERK inhibitor |
| SF1126 | Semafore Pharmaceuticals |
PI | Solid tumors | PI Prostate cancer | PI Multiple myeloma | PI Non-Hodgkin's lymphoma | PI CLL | PI Renal cell carcinoma |Preclinical Glioma | Preclinical NSCLC | Preclinical |
PI3K-mTOR-Pim inhibitor |
| RG7422 | Roche / Piramal | PI | Non-Hodgkin's lymphoma | PI | PI3K-mTOR inhibitor |
| GDC0980 | Life Sciences | Solid tumors | PI | ||
| PF05212384 | Pfizer | PI | Solid tumors | PI | PI3K-mTOR inhibitor |
| PF4691502 | Pfizer | PI | Solid tumors | PI | PI3K-mTOR inhibitor |
| PP-242 | PI | Solid tumors | PI | PI3K-mTOR inhibitor |
PI3K, phosphatidylinositide 3-kinase; AKT, serine/threonine kinase; mTOR, mammalian target of rapamycin; PI, phase I; PII, phase II; PIII, phase III; NSCLC, non–small cell lung cancer; EGFR, epidermal growth factor receptor; CLL, chronic lymphocytic leukemia; AML, acute myeloid leukemia; EGF-2, epidermal growth factor-2; NF-κB, nuclear factor-kappa B; PDK-1, phosphoinositide-dependent kinase-1; GIST, gastrointestinal stromal tumor; SCLC, small cell lung cancer; ALL, acute lymphocytic leukemia; ERK, extracellular signal-regulated kinases; PKN3, protein kinase N3.
PI3K inhibitors
Pan-PI3K inhibitors
Pan-PI3K inhibitors target all Class IA PI3Ks and are represented by several small molecule drugs, XL147, PX-866, BKM120, GDC-0941, BAY806946, GSK2126458, and CH5132799, and one RNA interfering (RNAi) agent, ATU027. In general, the single agent activities of these pan-PI3K inhibitors have been limited to the prolonging of RECIST stable disease rather than tumor regression, in line with being cytostatic or cytoreduc-tive as monotherapy in preclinical models. Mechanism-based side effects may limit dose escalation further, resulting in suboptimal target engagement and impaired efficacy.
XL147, a selective inhibitor of Class I PI3K isoforms, has a maximally tolerated dose (MTD) of 600 mg in both the 21-day-on/7-day-off schedule and the continuous dosing schedule (CDD)[38]. Dose limiting toxicity (DLT) was grade 3 skin rash. Only 1 partial response in NSCLC (non-PI3K/PTEN mutated) was observed in 68 patients treated in the phase I monotherapy trial.
PX-866 is a derivative of wortmannin and acts as an irreversible pan-PI3K Class 1A and B inhibitors. The MTD for intermittent schedule (once daily on days 1–5 and 8–12 of a 28-day cycle) was 12 mg, and that for continuous schedule was 8 mg, with DLTs including diarrhea and ALT/AST elevation[39],[40]. No objective response was reported in 84 patients treated; however, stable disease was achieved in 22% of the patients[39],[40].
BKM120 is a highly specific pan-Class I PI3K inhibitor without overlapping inhibitory activity against mTOR or Vps34. The MTD for daily dosing schedule was 100 mg with DLTs including hyperglycemia, skin rash, and mood alteration[41]. In the Phase I trial of 35 evaluable patients, there was 1 confirmed and 1 unconfirmed partial response, and 7 patients achieved stable disease for >8 months[42]. Both women with partial responses had breast cancer—one had triple negative breast cancer [estrogen receptor (ER)-, progesterone receptor (PR)-, and HER2-negative] with wild-type PIK3CA and mutant KRAS, without PTEN loss; the other had an ER/PR-positive, HER2-negative tumor with a confirmed PIK3CA mutation (E545K)[41],[42].
GDC-0941 is another pan-Class I PI3K inhibitor that is derived from PI103, an early generation PI3K inhibitor. In phase I trials, two dosing schedules, 3-week-on/1-week-off once daily (QD) or twice a day (BID), were investigated[43]. No MTD has been determined with DLTs consisting of headache, pleural effusion, and decreased diffusion capacity of the lung for carbon monoxide (DLCO). A partial response was observed in 1 patient with breast cancer, with additional anti-cancer signals seen in ovarian cancer.
BAY80-6946 (BAY) is a potent and highly selective reversible pan-Class I PI3k inhibitor. In a phase I trial, it was reported to be tolerated as an infusion at a dose of 0.8 mg/kg on days 1, 8, and 15 in a 28-day cycle[44]. Grade 2/3 hyperglycemia in the first 24 h after receiving a dose was commonly observed at the MTD. In the MTD expansion, 23 patients with solid tumors and 5 patients with follicular lymphoma (FL) were treated with BAY at MTD[45]. All had PI3K mutations. All these patients had grade 2/3 hyperglycemia, as noted in the dose escalation phase. Two patients developed interstitial pneumonitis. Hypertension for less than 24 h was commonly seen in patients with pre-existing hypertension. Three of 4 patients with FL achieved partial response after 2 cycles. Two patients with breast cancer also had partial responses. However, the response to the treatment did not correspond with the PI3K mutation status of these patients[45].
ATU027 is a novel RNAi therapeutic directed against protein kinase N3 (PKN3), a protein kinase C–related kinase downstream in the PI3K signaling pathway. Preclinically, systemic administration of ATU027 siRNA results in specific silencing of PKN3 expression, inhibition of tumor growth and lymph node metastasis, and a reduction in lymph vessel density[46],[47]. A phase I trial in cancer patients is ongoing. If successful, ATU027 and other siRNA-based therapeutics will become a novel approach for targeting individual components of the PI3K pathway.
PI3K isoform-specific inhibitors
These PI3K isoform–specific inhibitors selectively inhibit PI3K p110α, β, δ, or γ catalytic subunits and may therefore have an advantage of more complete target inhibition with fewer side effects.
CAL101 is a specific inhibitor of PI3Kδ, the isoform that is predominantly expressed in leukocytes. Early trials generated impressive response rates of 57%, 67%, and 30% in patients with relapse or recurrent indolent non-Hodgkin's lymphoma (NHL), mantle cell lymphoma (MCL), and chronic lymphocytic leukemia (CLL), respectively[48]. The compound is generally well tolerated with reversible spikes in serum transaminases as DLT. Phase Il/III trials in B-cell malignancies are currently ongoing.
AKT inhibitors
There are two main classes of AKT inhibitors in clinical development: ATP-competitive and allosteric AKT inhibitors.
ATP-competitive AKT inhibitors
ATP-competitive AKT inhibitors are pan-AKT kinase inhibitors. Because of the shared homology of the ATP-binding pocket among various kinases, this class of AKT inhibitors often has overlapping activity against other AGC kinases such as p70S6 kinase, protein kinase C (PKC), and Rho kinase. It remains to be determined whether such an inhibitory profile will result in undesirable side effects in clinical settings.
AZD5363 is a potent pan-AKT kinase inhibitor that has pharmacologic properties consistent with AKT inhibition in vivo. It inhibited the growth of a range of human tumor xenografts and caused significant regression in combination with docetaxel, particularly in breast cancer xenografts[54]. AZD5363 is currently being investigated in phase I clinical trials[55].
Allosteric AKT inhibitors
Allosteric AKT inhibitors bind to the PH domain of the AKT enzyme-forming drug-enzyme complexes. Due to conformational changes, translocation of AKT to the plasma membrane, a step essential for AKT activation, is disrupted[51]. Such a conformation-based approach circumvents the issue of kinase selectivity often seen with ATP-competitive AKT inhibitors.
MK-2206 is an allosteric AKT inhibitor that selectively inhibits AKT-1, -2, and -3 isoforms with nano-molar (nM) potency. Two dosing schedules were investigated in phase I monotherapy trials[52],[53]. The MTD for once every other day (QOD) schedule is 60 mg, and the MTD for once every week (QW) is preliminarily determined to be 200 mg. The main DLT was skin rash. No objective responses were observed. Evidence of target inhibition measured by changes in p-AKT before and after treatment was confirmed in tumor biopsies as well as surrogate tissues[52],[53].
Other AKT inhibitors
Triciribine phosphate (TCN-PM) is a potent pan-AKT inhibitor. TCN-PM was administered to subjects whose tumors displayed evidence of increased AKT phosphorylation (p-AKT), as measured by immunohistochemical analysis (IHC), over 30 min on days 1, 8, and 15 of a 28-day cycle[49]. Modest decreases in tumor p-AKT were detected following treatment with TCN-PM. No MTD was determined, and no objective response was observed.
PBI-05204 contains oleandrin, a cardiac glycoside, which inhibits the α-3 subunit Na-K ATPase pump. Oleandrin inhibits the export of fibroblast growth factor-2 (FGF-2), activation of necrosis factor-κB (NF-κB) and phosphorylation of AKT. PBI-05204 also inhibits p70S6K, decreasing mTOR activity. PBI-05240 was given orally for 21 days of a 28-day cycle[50]. No MTD has been determined, with no DLT reported. Only minor responses or stable disease were observed. Western blotting of peripheral blood mononuclear cells (PBMCs) showed that PBI-05204 markedly reduced the phosphorylation of AKT, p70S6K, and S6 in a time-dependent manner, suggesting target engagement.
RX-0201 (Archexin) is a 20-mer oligonucleotide with sequence complementary to Akt-1 mRNA. RX-0201 was administered to patients by up to 2 cycles of continuous infusion; each cycle of infusion lasted for 14 days followed by a 7-day rest[56]. Grade 3 fatigue was DLT. Only stable disease was seen in 2 of 17 patients.
Perifosine is a plasma membrane disrupting agent that inhibits AKT among other membrane-associated kinases by preventing their translocation to the plasma membrane. Perifosine has been evaluated in multiple phase I/Il clinical trials both alone and in combination with various other agents[57],[58]. The most common adverse reactions are fatigue and gastrointestinal toxicity. Like other PI3K-targeting agents, monotherapy activity with perifosine has generally been disappointing, although activity has been observed in patients with sarcoma and Waldenström's macroglobulinemia[57],[58].
mTOR inhibitors
Rapalogs
Rapamycin (sirolimus) and its analogs, temsirolimus, everolimus, and deforolimus, inhibit the mTORC1 kinase by binding to an abundant intracellular protein, FKBP-12, forming a complex that inhibits the mTOR signaling. Monotherapy activity was observed in early trials of these rapalogs, serving as a proof-of-concept for targeting the PI3K-AKT-mTOR pathway[59]. Follow-up studies confirmed clinical efficacy and led to the approval of rapalogs in the treatment of renal cell carcinoma[60]. Of note, everolimus, as compared with placebo, significantly prolonged progression-free survival (PFS) [11.0 months vs. 4.6 months, hazard ratio (HR) = 0.35, 95% confidence interval (CI) = 0.27-0.45, P < 0.001] among patients with progressive advanced pancreatic neuroendocrine tumors[60]. Everolimus has shown significant improvement in PFS in renal cell carcinoma when compared to the best supportive care in previously treated patients (4.9 months vs. 1.9 months)[61]. Other indications with significant clinical activities include mantle cell lymphoma and sarcoma. Combination trials of rapalogs plus hormonal therapy in ER-positive breast cancer have shown the potential of these agents in this patient population too[62].
mTORC1/2 inhibitors
mTOR catalytic site inhibitors directly target the kinase domain of mTOR and therefore impede the activity of both m TORC1 and mTORC2 kinases. A theoretical advantage of dual mTORC1/2 inhibition is to prevent compensatory feedback activation of AKT upon mTORC1 inhibition by rapalogs[63].
OSI027 is an oral inhibitor of mTORC1 and mTORC2. Three dosing schedules were tested: days 1–3, once weekly, and continuous once daily[64]. DLTs included decreased left ventricular ejection fraction and fatigue. No objective response was achieved in phase I trials. Decreases in the phosphorylation of 4E-BP1 in PBMCs at threonine 37/46, a rapamycin-insensitive, mTOR-dependent phosphorylation site, confirmed TORC2 inhibition in patients[64].
Other mTORC1/2 inhibitors are still in early phase I stage, and it is premature to predict whether this class of inhibitors will have higher clinical efficacy than rapalogs with acceptable tolerance.
Dual PI3K-mTOR inhibitors
The dual PI3K-mTOR inhibitors target the p110 subunit of PI3K as well as mTOR. The potential advantage is more complete inhibition of the PI3K-AKT-mTOR pathway, which may increase clinical efficacy.
BEZ235 is an orally available, potent and highly selective reversible PI3K-mTOR dual inhibitor. No DLTs have been observed in the first 59 treated patients of a phase I trial[65]. Of the 51 evaluable patients, 2 achieved partial responses, including 1 ER-positive, HER2-negative breast cancer patient with unknown PI3K pathway status, and 1 patient with Cowden's syndrome (germline PTEN mutation) who had developed lung cancer. Furthermore, 16 patients achieved minor responses, with 14 achieving stable disease for 4 months or longer. It is of interest to point out that tumors from 6 of these 14 patients showed dysregulation of the PI3K pathway[65].
XL765 is also known as SAR245409, an oral dual inhibitor of PI3K/mTOR. Stable disease in 12 patients for 16 weeks or longer and in 7 patients for 24 weeks or longer was observed among 83 patients in a phase I trial[66]. The most frequently observed toxicities involved elevated liver enzymes, nausea, vomiting, diarrhea, and skin rash. The MTD has been defined as 50 mg twice daily or 90 mg daily.
GDC-0980 is a dual PI3K/mTOR inhibitor that targets all PI3K isoforms and mTOR at low nanomolar concentrations. It was investigated in a phase I trial with daily dosing on a 3-week-on/1-week-off schedule[67]. Early data suggested pharmacodynamic target engagement and preliminary clinical activity. Toxicity included rash, hyperglycemia, mucositis, and pneumonitis, which resolved with drug cessation. GDC-0980 showed antitumor activity in 3 of 33 patients with mesothelioma. Fluorodeoxyglucose positron emitting tomography (PET-FDG) responses were also observed in gastrointestinal stromal tumor (GIST) and adrenal tumors[68].
SF1126 is composed of the pan-PI3K inhibitor LY294002 conjugated to an RGDS-targeting peptide. It is designed to increase solubility and binding to integrins expressed on tumor vasculature. LY294002 inhibits other kinases, including mTOR, DNA-PK, PIM1, PLK1, and CK2, and induces oxidative stress in cancer cells independent of its PI3K inhibition. Stable disease (≥8 weeks) was seen in 19 of 33 (58%) evaluable patients, with durations of 20 weeks for patients with GIST, endometrial cancer, and prostate cancer[69]. MTD was not reached but the maximum administered dose (MAD) was 1,100 mg/m2. Pharmacodynamic studies showed reduced p-AKT and increased apoptosis, indicating inhibition of the PI3K pathway signaling.
Other dual PI3K-mTOR inhibitors in clinical development include the orally administered PF-04691502 and an intravenous agent, PF-05212384.
Common Challenges in the Clinical Development of PI3K-AKT-mTOR Inhibitors
Lack of monotherapy efficacy
Single agent activity has not been widely observed in clinical trials of these PI3K-AKT-mTOR inhibitors. The mTOR inhibitors, rapalogs, are the most advanced agents in clinical development. Single agent activities in renal cell carcinoma led to market approval of everolimus and temsirolimus[62]. Patient benefit in pancreatic neuroendocrine cancer and sarcoma have been noted for everolimus[60] and ridaforolimus[70], respectively. However, monotherapy activity in other cancer types has been modest.
The lack of single agent activity may be due to (1) incomplete inhibition of target (degree and duration), (2) activation of feedback compensatory loops leading to tumor cell resistance, and (3) lack of predictive biomarkers to prospectively select “sensitive” patient subpopulations.
Mechanism-based toxicities
Skin rash
Skin rash has been observed in clinical trials of agents targeting the PI3K-AKT-mTOR pathway. Skin rash was reported as the DLT for the allosteric AKT inhibitor MK-2206[53]. This cutaneous toxicity is also associated with several PI3K-AKT-mTOR inhibitors, including XL147, XL764, BKM120, BEZ335, everolimus, and temsirolimus, suggesting a mechanism-based class adverse effect. Skin rash presents as erythematous maculo-papular rash, often first appearing on truncal areas, in contrast to EGFR inhibitor–associated acneiform facial rash. These non-pustular, non-blistering rashes recover fully upon drug interruption or dose reduction. Clinical symptom management includes the use of anti-histamines, topical steroids, and moisturizing cream. The pathophysiologic mechanism underlying PI3K-AKT-mTOR inhibitor–induced rash is not yet delineated but is suspected to involve local cytokine and chemokine deregulation upon pathway inhibition.
Hyperglycemia and hyperlipidemia
Hyperglycemia and hyperlipidemia have been anticipated as side effects for PI3K-AKT-mTOR pathway inhibitors based on the role of the pathway in regulating the insulin signaling and the physiologic hemo-state of glucose metabolism[71],[72]. Although the exact mechanism of metabolic derangements is not entirely clear, one possibility is that physiologic adaptation to pathway inhibition partially compensates the disrupted insulin-glucose regulatory axis. The pathophysiology of mTOR inhibitors causing dyslipidemia may involve the impaired clearance of lipids from the bloodstream via stimulation of insulin-stimulated lipoprotein lipase (LPL)[73],[74]. However, PI3K and AKT inhibitors are less likely to lead to grade 3 or 4 hyperlipidemia, unlike mTOR inhibitors. To date, only mild and reversible hyperglycemia accompanied by elevated insulin C-peptide levels has been frequently observed in clinical trials, confirming pharmacodynamic effects. The detailed management of metabolic disorders induced by PI3K-AKT-mTOR inhibitors is beyond the scope of this review, but the aim of management should be to avoid acute and sub-acute complications of hyperglycemia, while ensuring that hypoglycemia is avoided[75].
Lack of predictive biomarkers
There is strong preclinical evidence that germline loss or acquired somatic mutations of PTEN or mutations within exon 9 (E542K and E545K) or exon 20 (H1047R) of PIK3CA lead to uncontrolled activity of the PI3K enzyme and thus promote oncogenesis[76]. There is also strong preclinical evidence suggesting the predictability of the response to targeted therapies in patients harboring these mutations[76], but this is yet to be evaluated in a clinical setting. Other preclinical data suggest that efficacy correlates with protein biomarkers such as pS6, pEIF4, rictor, raptor, pAKT and total AKT[77], but again, this is yet to be validated in prospective human studies. The lack of predictive biomarkers in the clinical setting remains a challenge at this stage, hampering future development of these targeting agents.
Future Perspectives
The PI3K-AKT-mTOR pathway is a well established driver of cancer in humans, and therefore blocking different nodes of the pathway is a relevant treatment strategy for human malignancies. There are several targeting agents in clinical development, but most of them are currently in phase I trial, suggesting that in the near future more data will be available to examine the antitumor properties of PI3K/AKT inhibitors. The activity of these agents in early phase clinical trials has been limited, and it is likely that cancer cells acquire resistance via different feedback loop and crosstalk mechanisms. The future development of these promising inhibitors should therefore focus on combination regimens, including the concomitant or sequential blockade of signaling pathways, e.g., horizontal blockade of the PI3K-AKT-mTOR and RAS-RAF-MEK pathways or vertical blockade of the PI3K/AKT/mTOR and IGF pathways. Indeed, this strategy is now being evaluated in several clinical trials. Finally, there is an urgent need to develop robust predictive biomarkers to increase the efficacy of these drugs by prospectively identifying patients who are most likely to benefit.
Acknowledgments
The Drug Development Unit of the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research is supported in part by a program grant from Cancer Research U.K. Support was also provided by the Experimental Cancer Medicine Centre (to The Institute of Cancer Research) and the National Institute for Health Research Biomedical Research Centre (jointly to the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research). TY is recipient of the 2011 Scott Minerd Prostate Cancer Foundation Young Investigator Award.
References
- 1.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 2.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 3.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3:1221–1224. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 4.Samuels Y, Wang Z, Bardelli A, et al. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 5.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 6.Bader AG, Kang S, Zhao L, et al. et al. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 7.Wendel HG, De Stanchina E, Fridman JS, et al. et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 8.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 9.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 10.Dan HC, Sun M, Yang L, et al. et al. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 11.Hengstschlager M, Rosner M, Fountoulakis M, et al. et al. Tuberous sclerosis genes regulate cellular 14-3-3 protein levels. Biochem Biophys Res Commun. 2003;312:676–683. doi: 10.1016/j.bbrc.2003.10.170. [DOI] [PubMed] [Google Scholar]
- 12.Harrington LS, Findlay GM, Gray A, et al. et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Cicchetti G, Onda H, et al. et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl JA, Cheng M, Roussel MF, et al. et al. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and Subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skinner HD, Zheng JZ, Fang J, et al. et al. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J Biol Chem. 2004;279:45643–45651. doi: 10.1074/jbc.M404097200. [DOI] [PubMed] [Google Scholar]
- 16.Knuefermann C, Lu Y, Liu B, et al. et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Cerniglia GJ, Mick R, et al. et al. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys. 2003;56:846–853. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Yen C, Liaw D, et al. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 19.Teng DH, Hu R, Lin H, et al. et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 20.Haas-Kogan D, Shalev N, Wong M, et al. et al. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Huang J, Homma T, et al. et al. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer Res. 2009;29:1739–1743. [PubMed] [Google Scholar]
- 22.Podsypanina K, Ellenson LH, Nemes A, et al. et al. Mutation of Pten/Mrnac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Gao J, Lei Q, et al. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 25.Mueller S, Phillips J, Onar-Thomas A, et al. et al. PTEN promoter methylation and activation of the PI3K/Akt/mTOR pathway in pediatric gliomas and influence on clinical outcome. Neuro Oncol. 2012;14:1146–1152. doi: 10.1093/neuonc/nos140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, et al. et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Shayesteh L, Lu Y, Kuo WL, et al. et al. PIK3CA is implicated as an Oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 30.Bleeker FE, Felicioni L, Buttitta F, et al. et al. AKT1 (E17K) in human solid tumours. Oncogene. 2008;27:5648–5650. doi: 10.1038/onc.2008.170. [DOI] [PubMed] [Google Scholar]
- 31.Rychahou PG, Kang J, Gulhati P, et al. et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci USA. 2008;105:20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irie HY, Pearline RV, Grueneberg D, et al. et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies MA, Stemke-Hale K, Tellez C, et al. et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–1268. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelman JA, Chen L, Tan X, et al. et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eichhorn PJ, Gili M, Scaltriti M, et al. et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelman JA, Zejnullahu K, Mitsudomi T, et al. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 37.Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Edelman G, Bedell C, Shapiro G, et al. et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28:abstr 3004. [Google Scholar]
- 39.Hong DS, Bowles DW, Falchook GS, et al. et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–4182. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 40.Jimeno A, Herbst RS, Falchook GS, et al. et al. Final results from a phase I, dose-escalation study of PX-866, an irreversible, pan-isoform inhibitor of PI3 kinase. J Clin Oncol. 2010;28:abstr3089. [Google Scholar]
- 41.Baselga J, Jonge MJD, Rodon J, et al. et al. A first-in-human Phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28:abstr 3003. [Google Scholar]
- 42.Bendell JC, Rodon J, Burris HA, et al. et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 43.Hoff DDV, LoRusso P, Tibes R, et al. et al. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J Clin Oncol. 2010;28:abstr 2541. [Google Scholar]
- 44.Patnaik A MJ, Ramanathan RK, Beeram M, et al. et al. A first-inhuman phase I study of intravenous PI3K inhibitor BAY 80-6946 in patients with advanced solid tumors: results of dose-escalation phase. J Clin Oncol. 2011;29:abstr3035. [Google Scholar]
- 45.MT L. Phase I study of intravenous PI3K inhibitor BAY 80-6946: Activity in patients (pts) with advanced solid tumors and non-Hodgkin lymphoma treated in MTD expansion cohorts. J Clin Oncol. 2012;30:abstr3019. [Google Scholar]
- 46.Aleku M, Schulz P, Keil O, et al. et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68:9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 47.Santel A, Aleku M, Roder N, et al. et al. Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin Cancer Res. 2010;16:5469–5480. doi: 10.1158/1078-0432.CCR-10-1994. [DOI] [PubMed] [Google Scholar]
- 48.Furman RR, Byrd JC, Flinn IW, et al. et al. Interim results from a phase I study of CAL-101, a selective oral inhibitor of phosphatidylinositol 3-kinase p110d isoform, in patients with relapsed or refractory hematologic malignancies. J Clin Oncol. 2010;28:abstr 3032. [Google Scholar]
- 49.Garrett CR, Coppola D, Wenham RM, et al. et al. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs. 2011;29:1381–1389. doi: 10.1007/s10637-010-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bidyasar S, Kurzrock R, Falchook GS, et al. et al. A first-in-human phase I trial of PBI-05204 (oleandrin), an inhibitor of Akt, FGF-2, NF-Kb, and p70S6K in advanced solid tumor patients. J Clin Oncol. 2009;27:abstr3537. [Google Scholar]
- 51.Cherrin C, Haskell K, Howell B, et al. et al. An allosteric Akt inhibitor effectively blocks Akt signaling and tumor growth with only transient effects on glucose and insulin levels in vivo. Cancer Biol Ther. 2010;9:493–503. doi: 10.4161/cbt.9.7.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yap TA, Patnaik A, Fearen I, et al. et al. First-in-class phase I trial of a selective Akt inhibitor, MK2206 (MK), evaluating alternate day (QOD) and once weekly (QW) doses in advanced cancer patients (pts) with evidence of target modulation and antitumor activity. J Clin Oncol. 2010;28:abstr 3009. [Google Scholar]
- 53.Yap TA, Yan L, Patnaik A, et al. et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 54.Davies BR, Greenwood H, Dudley P, et al. et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 55.Davies BR, Greenwood H, Dudley P, et al. et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 56.Marshall J, Posey J, Hwang J, et al. et al. A phase I trial of RX-0201 (AKT anti-sense) in patients with an advanced cancer. J Clin Oncol. 2007;25:abstr 3564. [Google Scholar]
- 57.Ghobrial IM, Roccaro A, Hong F, et al. et al. Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia. Clin Cancer Res. 2010;16:1033–1041. doi: 10.1158/1078-0432.CCR-09-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unger C, Berdel W, Hanauske AR, et al. et al. First-time-in-man and pharmacokinetic study of weekly oral perifosine in patients with solid tumours. Eur J Cancer. 2010;46:920–925. doi: 10.1016/j.ejca.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 59.Luan FL, Ding R, Sharma VK, et al. et al. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63:917–926. doi: 10.1046/j.1523-1755.2003.00805.x. [DOI] [PubMed] [Google Scholar]
- 60.Yao JC, Shah MH, Ito T, et al. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motzer RJ, Escudier B, Oudard S, et al. et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 62.Yap TA, Garrett MD, Walton Ml, et al. et al. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 63.O'Reilly KE, Rojo F, She QB, et al. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan DS, Dumez H, Olmos D, et al. et al. First-in-human phase I study exploring three schedules of OSI-027, a novel small molecule TORC1/TORC2 inhibitor, in patients with advanced solid tumors and lymphoma. J Clin Oncol. 2010;28:abstr 3006. [Google Scholar]
- 65.Burris H, Rodon J, Sharma S, et al. et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28:abstr 3005. [Google Scholar]
- 66.Brana I, LoRusso P, Baselga J, et al. et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28:abstr 3030. [Google Scholar]
- 67.Dolly S, Wagner AJ, Bendell JC, et al. et al. A first-in-human, phase I study to evaluate the dual PI3K/mTOR inhibitor GDC-0980 administered QD in patients with advanced solid tumors or non-Hodgkin's lymphoma. J Clin Oncol. 2010;28:abstr 3079. [Google Scholar]
- 68.Wagner AJ, Bendell JC, Dolly S, et al. et al. A first-in-human phase I study to evaluate GDC-0980, an oral PI3K/mTOR inhibitor, administered QD in patients with advanced solid tumors. J Clin Oncol. 2011;29:abstr 3020. [Google Scholar]
- 69.Mahadevan D, Chiorean EG, Harris WB, et al. et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer. 2012;48:3319–3327. doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blay JY. Updating progress in sarcoma therapy with mTOR inhibitors. Ann Oncol. 2010;22:280–287. doi: 10.1093/annonc/mdq307. [DOI] [PubMed] [Google Scholar]
- 71.Di Paolo S, Teutonico A, Leogrande D, et al. et al. Chronic inhibition of mammalian target of rapamycin signaling downregulates insulin receptor substrates 1 and 2 and AKT activation: a crossroad between cancer and diabetes? J Am Soc Nephrol. 2006;17:2236–2244. doi: 10.1681/ASN.2006030196. [DOI] [PubMed] [Google Scholar]
- 72.Crouthamel MC, Kahana JA, Korenchuk S, et al. et al. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin Cancer Res. 2009;15:217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- 73.Hoogeveen RC, Ballantyne CM, Pownall HJ, et al. et al. Effect of sirolimus on the metabolism of apoB100-containing lipoproteins in renal transplant patients. Transplantation. 2001;72:1244–1250. doi: 10.1097/00007890-200110150-00011. [DOI] [PubMed] [Google Scholar]
- 74.Kraemer FB, Takeda D, Natu V, et al. et al. Insulin regulates lipoprotein lipase activity in rat adipose cells via wortmannin-and rapamycin-sensitive pathways. Metabolism. 1998;47:555–559. doi: 10.1016/s0026-0495(98)90239-6. [DOI] [PubMed] [Google Scholar]
- 75.Busaidy NL, Farooki A, Dowlati A, et al. et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 2012;30:2919–2928. doi: 10.1200/JCO.2011.39.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mueller A, Bachmann E, Linnig M, et al. et al. Selective PI3K inhibition by BKM120 and BEZ235 alone or in combination with chemotherapy in wild-type and mutated human gastrointestinal cancer cell lines. Cancer Chemother Pharmacol. 2012;69:1601–1615. doi: 10.1007/s00280-012-1869-z. [DOI] [PubMed] [Google Scholar]
- 77.Kwei KA, Baker JB, Pelham RJ. Modulators of sensitivity and resistance to inhibition of PI3K identified in a pharmacogenomic screen of the NCI-60 human tumor cell line collection. PLoS One. 2012;7:e46518. doi: 10.1371/journal.pone.0046518. [DOI] [PMC free article] [PubMed] [Google Scholar]