Abstract
The prognostic value of T category for locoregional control in patients with nasopharyngeal carcinoma (NPC) has decreased with the extensive use of intensity-modulated radiotherapy (IMRT). We aimed to develop a prognostic scoring system (PSS) that incorporated tumor extension and clinical characteristics for locoregional control in NPC patients treated with IMRT. The magnetic resonance imaging scans and medical records of 717 patients with nonmetastatic NPC treated with IMRT at Sun Yat-sen University Cancer Center between January 2003 and January 2008 were reviewed. Age, pathologic classification, primary tumor extension, primary gross tumor volume (GTV-p), T and N categories, and baseline lactate dehydrogenase (LDH) level were analyzed. Hierarchical cluster analysis as well as univariate and multivariate analyses were used to develop the PSS. Independent prognostic factors for locoregional relapse included N2–3 stage, GTV-p ≥26.8 mL, and involvement of one or more structures within cluster 3. We calculated a risk score derived from the regression coefficient of each factor and classified patients into four groups: low risk (score 0), intermediate risk (score >0 and ≤1), high risk (score >1 and ≤2), and extremely high risk (score >2). The 5-year locoregional control rates for these groups were 97.4%, 93.6%, 85.2%, and 78.6%, respectively (P < 0.001). We have developed a PSS that can help identify NPC patients who are at high risk for locoregional relapse and can guide individualized treatments for NPC patients.
Keywords: Nasopharyngeal carcinoma, prognostic scoring system, locoregional control, intensity-modulated radiotherapy
Nasopharyngeal carcinoma (NPC) is the most common cancer in endemic areas of southern China, northern Africa, and Alaska[1]. Radiotherapy is the preferred treatment modality for NPC, which is highly radiosensitive and tends to be inoperable because of the complex anatomy of the region involved. Intensity-modulated radiotherapy (IMRT) has ushered in a new era of treatment for NPC, as it offers excellent target volume coverage and protects the normal tissue adjacent to the target. Several studies have reported that the use of IMRT has improved the 5-year locoregional relapse-free survival of patients with nonmetastatic NPC from 84% (after conventional radiotherapy radiotherapy) to 93%[2]–[4]. Furthermore, some authors have reported that the local relapse-free survival (LRFS) did not significantly differ among NPC patients with T1, T2, and T3 tumors treated with IMRT[5],[6]. Consequently, the excellent locoregional control achieved with IMRT may eliminate the prognostic value of T category for LRFS to a certain extent[3].
Many factors are associated with locoregional control, including age, primary gross tumor volume (GTV-p), T category, parapharyngeal extension, cranial nerve involvement, pathologic classification, and lactate dehydrogenase (LDH) level[7]–[13]. It would be beneficial to combine the above factors in a way that provides effective risk stratification for locoregional relapse and guides individualized treatment, which would thereby further decrease locoregional relapse rates. To the best of our knowledge, only one report has focused on establishing a prognostic scoring system (PSS) for locoregional control in NPC. The results of that study were mainly based on data from three-dimensional conformal radiotherapy[11]. Because prognostic factors of locoregional control are influenced by improvements in diagnostic and treatment modalities, it is questionable whether this PSS is applicable in the era of IMRT.
The purpose of this study therefore was to optimize treatment strategies for NPC patients through the development of a PSS for locoregional control based on clinical characteristics and the degree of tumor extension.
Patients and Methods
Patient selection
Consecutive NPC patients who were newly diagnosed between January 2003 and January 2008 were recruited from Sun Yat-sen University Cancer Center. The inclusion criteria were as follows: (1) had pathologically confirmed NPC; (2) had stage I to IVA-B disease [defined by the 7th edition of the American Joint Commission on Cancer/Union for International Cancer Control staging system (AJCC/UICC)]; (3) underwent a complete patient history inquiry, physical examination, hematologic and biochemical profiles, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal sonography, and acquisition of whole-body bone scans by single photon-emission computed tomography; (4) underwent radical IMRT; and (5) had complete dose-volume parameter records documenting tumor volume.
Imaging protocol
MRI was performed using a 1.5-Tesla system (Signa CV/i; General Electric Healthcare, Chalfont St. Giles, United Kingdom). The region from the suprasellar cistern to the inferior margin of the sternal end of the clavicle was examined with a head-and-neck combined coil. T1-weighted, fast spin-echo images on the axial, coronal, and sagittal planes (repetition time, 500–600 ms; echo time, 10–20 ms) and T2-weighted, fast spin-echo images on the axial plane (repetition time, 4,000–6,000 ms; echo time, 95–110 ms) were obtained before injection of contrast material. After intravenous administration of gadopentetate dimeglumine (Gd-DTPA; Magnevist, Schering, Berlin, Germany) at a dose of 0.1 mmol/kg body weight, T1-weighted, spin-echo axial and sagittal sequences and T1-weighted, spin-echo, fat-suppressed coronal sequences were performed sequentially, with the same parameters as those used prior to the Gd-DTPA injection. A section thickness of 5 mm and a matrix size of 512 × 512 were used. All MRI and clinical records were reviewed by two radiologists with more than 10 years of experience with MRI of head and neck cancers. All scans were evaluated independently, and disagreements were resolved by consensus. Details regarding the diagnostic criteria for primary tumor extension have been published previously[14].
Treatment
Radical radiotherapy was implemented. The nasopharynx and upper neck were subjected to IMRT. Target volumes were delineated according to our institutional treatment protocol[15], in agreement with the International Commission on Radiation Units and Measurements Reports 50 and 62. The prescribed doses were 68 Gy at 2.27 Gy/fraction to the planning target volume (PTV) including the GTV-p, 60 Gy to the PTV enclosing the clinical target volume (CTV)-1 (i.e., high-risk regions), 54 Gy to the PTV enclosing the CTV-2 (i.e., low-risk regions and neck nodal regions), and 60–64 Gy to the nodal gross tumor volume (GTV-n) in 30 fractions. Treatment was delivered once daily as 5 fractions per week, and all targets were treated at the same time using the simultaneous integrated boost technique. A separate anterior low-neck field with spinal cord shielding was used for the lower neck and supraclavicular fossa. Detailed information regarding IMRT has been reported previously[15].
Clinical practice guidelines during the study period recommend-ed radiotherapy alone for patients in stages I–IIA, concomitant chemotherapy for those in stage IIB, and both neoadjuvant or adjuvant chemotherapy and concomitant chemotherapy for those in stages III–IV, as defined in the 6th edition of the AJCC/UICC staging system for NPC. Patients with stage III–IV disease were administered neoadjuvant chemotherapy when the waiting time was considered to be longer than acceptable or when it was considered advantageous to downsize bulky tumors. Neoadjuvant or adjuvant chemotherapy consisted of cisplatin with 5-fluorouracil or taxanes every 3 weeks for 2 or 3 cycles. Concurrent chemotherapy consisted of cisplatin given weekly or on weeks 1, 4, and 7 of radiotherapy.
Follow-up
The duration of follow-up was calculated from the first day of treatment to either the day of death or the day of the last examination. Patients were examined at least every 3 months during the first 2 years and every 6 months thereafter, until death. Locoregional failure was diagnosed if a primary site or neck lymph node relapse was detected. Locoregional control was defined as the absence of a primary site or neck lymph node relapse. The date of last follow-up was February 20, 2012.
Hierarchical cluster analysis and statistical analyses
The Statistical Package for Social Sciences, version 16.0 (SPSS, Chicago, IL, USA), was used for statistical analyses. Both clinical characteristics (age, sex, pathologic classification, T and N categories, GTV-p, and LDH level) and anatomic structures with tumor involvement were included to establish the PSS for locoregional control.
GTV-p was delineated with the planning system according to the pretreatment MRI and calculated using the Corvus® inverse IMRT planning system (version 3.0; Peacock® 3.0, Nomos Corp., Deer Park, IL, USA) by the summation-of-area technique, in which the entire area was multiplied by the image reconstruction interval of 3 mm. For patients undergoing induction chemotherapy, GTV-p was contoured according to the primary tumor before induction chemotherapy. In this study, GTV-p included the gross volume of the primary tumor and the enlarged retropharyngeal lymph nodes. Receiver operating characteristic (ROC) curve analysis was used to obtain cut-off points. We defined the ideal cut-off point by maximizing the conditional Youden score (i.e., maximum of sensitivity and specificity). Normal serum LDH levels ranged from 109 to 245 IU/L; therefore, an upper limit of 245 IU/L was selected for the analysis of LDH levels.
Hierarchical cluster analysis (HCA) is an unsupervised agglomerative clustering algorithm that is widely used in the analysis of gene expression data[16] and is beginning to be used in tumor analyses to cluster anatomic structures with primary tumor involvement into several groups. Clusters were gathered by the Jaccard index and between-group linkage using the SPSS 16.0 software. The Jaccard index was chosen to detect similarity in binary variables.
The main end-point was the locoregional control rate, which was calculated from the start of treatment to the emergence of primary or neck lymph node relapse. Actuarial rates were estimated using the Kaplan-Meier method, and differences were compared with the log-rank test. A significance value of 0.1 was used to select the structures that were significantly involved[17], whereas a significance value of 0.05 was used to select other factors. Multivariate analyses using the Cox proportional hazards model were used to calculate the hazard ratio (HR) and to test independent significance by backward elimination of insignificant explanatory variables. The following parameters were included in the model as covariates: age (<50 years vs.≥50 years), pathologic classification, N category (N0–1 vs. N2–3), tumor volume (<26.8 mL vs.≥26.8 mL), LDH level (<245 IU/L vs.≥245 IU/L) and anatomic structures in clusters. The criterion for statistical significance was set at α = 0.05 to test the prognostic variables for locoregional relapse, and P values were based on two-sided tests.
Results
Clinical characteristics
Between January 2003 and January 2008, 749 patients were newly diagnosed with NPC at Sun Yat-sen University Cancer Center. Among them, 717 patients were included in this study. The clinical characteristics are shown in Table 1. Of the 717 patients, 554 were males, and 163 were females. Their median age was 43 years (range, 13–78 years). A pathologic diagnosis of WHO type II or III NPC was observed in 701 (97.8%) patients, whereas only 5 (0.7%) had WHO type I NPC and 11 (1.5%) had basaloid squamous cell carcinoma. According to the 7th AJCC/UICC staging system of NPC, 74 patients were at stage I, 169 at stage II, 272 at stage III, and 202 at stage IVA-B. The median GTV-p was 23.12 mL (range, 0.27–228.05 mL). The cut-off for predicting locoregional relapse was 26.88 mL (sensitivity, 71.11%; specificity, 57.46%; area under the ROC curve, 0.64). Only 39 (5.4%) patients had a baseline LDH level ≥245 IU/L.
Table 1. Clinical characteristics of 717 patients with nasopharyngeal carcinoma (NPC).
| Characteristic | No. of cases (%) |
| Gender | |
| Male | 554 (77.3) |
| Female | 163 (22.7) |
| Age (years) | |
| ≤50 | 529 (73.8) |
| >50 | 188 (26.2) |
| Pathologic classification | |
| WHO I | 5 (0.7) |
| WHO II | 53 (7.4) |
| WHO III | 648 (90.4) |
| BSCC | 11 (1.5) |
| Baseline LDH (IU/L) | |
| <245 | 678 (93.6) |
| ≥245 | 39 (5.4) |
| GTV-p (mL) | |
| <26.8 | 395 (54.6) |
| ≥26.8 | 322 (44.5) |
| T categorya | |
| T1 | 170 (23.5) |
| T2 | 131 (18.1) |
| T3 | 254 (35.1) |
| T4 | 162 (22.4) |
| N categorya | |
| N0-1 | 567 (78.3) |
| N2-3 | 150 (20.7) |
| Clinical stagea | |
| I | 74 (10.2) |
| II | 169 (23.3) |
| III | 272 (37.6) |
| IVA | 155 (21.4) |
| IVB | 47 (6.5) |
WHO, World Health Organization; BSCC, basaloid squamous cell carcinoma; GTV-p, primary gross tumor volume; LDH, lactate dehydrogenase. aAccording to the 7th American Joint Commission on Cancer/Union for International Cancer Control staging system.
Overall, 199 patients were treated with radiotherapy alone, and 518 underwent chemotherapy. Concomitant chemotherapy was given to 490 (67.7%) patients. A combination of induction and concomitant chemotherapy was delivered to 239 (33.3%) patients, and concomitant and adjuvant chemotherapy were delivered to 43 (6.0%) patients. Reasons for intolerance to chemotherapy included age of ≥70 years and organ dysfunction.
Patterns of failure
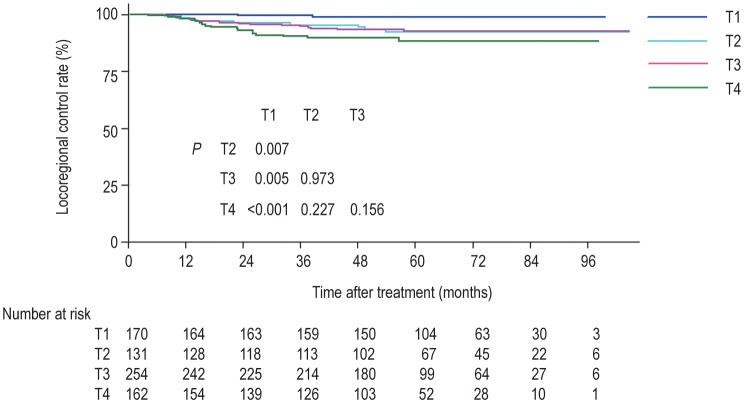
The patients were followed up with a median of 58.4 months (range, 2.5–104.6 months). A total of 45 (6.2%) patients developed locoregional relapse. Distant metastases were developed in 123 (17.0%) patients, and 122 (16.9%) died. The sites of relapse were local in 35 (4.8%) patients, regional in 16 (2.2%) patients, and both local and regional in 6 (0.8%) patients. The median relapse time was 22 months (range, 4–58 months). The 5-year locoregional control rates for T1, T2, T3, and T4 tumors were 98.8%, 93.1%, 93.3%, and 89.5%, respectively (P = 0.004) (Figure 1).
Figure 1. Kaplan-Meier curves of locoregional control rates of nasopharyngeal carcinoma (NPC) at different T categories using the 7th American Joint Commission on Cancer/Union for International Cancer Control (AJCC/UICC) staging system.
Results of hierarchical cluster analysis
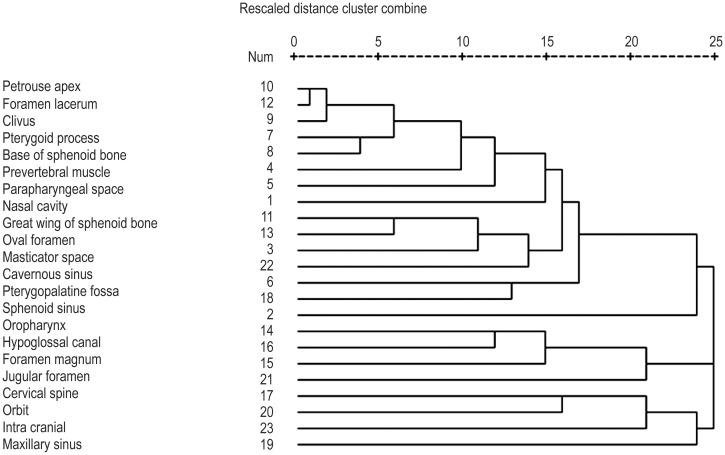
All the superior, inferior, anterior, posterior, lateral, and medial anatomic structures involved by the primary tumor were incorporated into the HCA and were divided into three clusters. Cluster 1 included the nasal cavity, prevertebral muscles, parapharyngeal space, pterygopalatine fossa, pterygoid process, base of the sphenoid bone, petrous apex, clivus, foramen lacerum, oval foramen, sphenoid sinus, greater wing of the sphenoid bone, cavernous sinus, and masticator space. Cluster 2 included the hypoglossal canal, jugular foramen, foramen magnum, and cervical spine. Cluster 3 included the ethmoid sinus, orbit, and intracranium. In addition, the oropharynx and maxillary sinus were incorporated as independent structures and were thus not included in any of the clusters. Figure 2 shows the HCA results of 23 structures.
Figure 2. Results of hierarchical cluster analysis of 23 structures with primary tumor involvement.
Dendrogram was obtained by the Jaccard index and between-group linkage.
Log-rank analyses of the locoregional control rates for the above anatomic structures and clusters were conducted. Meaningful results were obtained for all the structures and clusters, except the oropharynx (P = 0.566), which was eliminated from the multivariate analyses. Table 2 shows the univariate analysis of clinical characteristics and local tumor extension.
Table 2. Univariate analysis of clinical characteristics and local tumor extension of 717 patients with NPC.
| Characteristic | HR | 95% CI | P |
| GTV-p (<26.8 mL vs. ≥26.8 mL) | 3.379 | 1.773-6.440 | <0.001 |
| Baseline LDH (<245 IU/L vs. ≥245 IU/L) | 2.674 | 1.055-6.779 | 0.038 |
| N category (N0-1 vs. N2-3) | 2.108 | 1.134-3.918 | 0.018 |
| Nasal cavity | 2.154 | 1.197-3.879 | 0.011 |
| Oropharynx | 1.286 | 0.544-3.037 | 0.578 |
| Masticator space | 2.335 | 1.278-4.268 | 0.006 |
| Prevertebral muscle | 2.313 | 1.272-4.207 | 0.006 |
| Parapharyngeal space | 5.272 | 1.634-17.018 | 0.005 |
| Pterygopalatine fossa | 2.888 | 1.553-5.369 | 0.001 |
| Pterygoid process | 1.717 | 0.950-3.104 | 0.074 |
| Base of sphenoid bone | 2.145 | 1.174-3.921 | 0.013 |
| Clivus | 2.452 | 1.349-4.454 | 0.003 |
| Petrous apex | 1.720 | 0.958-3.088 | 0.069 |
| Great wing of sphenoid bone | 2.033 | 1.082-3.823 | 0.028 |
| Foramen lacerum | 2.583 | 1.428-4.671 | 0.002 |
| Oval foramen | 1.913 | 0.988-3.704 | 0.054 |
| Hypoglossal canal | 2.817 | 1.394-5.693 | 0.004 |
| Jugular foramen | 4.045 | 1.712-9.556 | 0.001 |
| Foramen magnum | 4.234 | 2.047-8.758 | <0.001 |
| Ethmoid sinus | 4.222 | 1.885-9.458 | <0.001 |
| Sphenoid sinus | 2.261 | 1.187-4.309 | 0.013 |
| Maxillary sinus | 7.015 | 2.172-22.653 | 0.001 |
| Orbit | 4.043 | 1.804-9.060 | 0.001 |
| Cervical spine | 4.480 | 1.765-11.371 | 0.002 |
| Cavernous sinus | 2.730 | 1.468-5.077 | 0.002 |
| Intracranial | 4.068 | 1.816-9.112 | 0.001 |
| Cluster 1with two or more anatomic sites involved | 3.107 | 1.387-6.959 | 0.006 |
| Cluster 2 with one or more anatomic sites involved | 2.344 | 1.187-4.629 | 0.014 |
| Cluster 3 with one or more anatomic sites involved | 3.812 | 1.969-7.383 | <0.001 |
HR, hazard ratio; CI, confidence interval; other abbreviations as in Table 1.
Prognostic scoring system
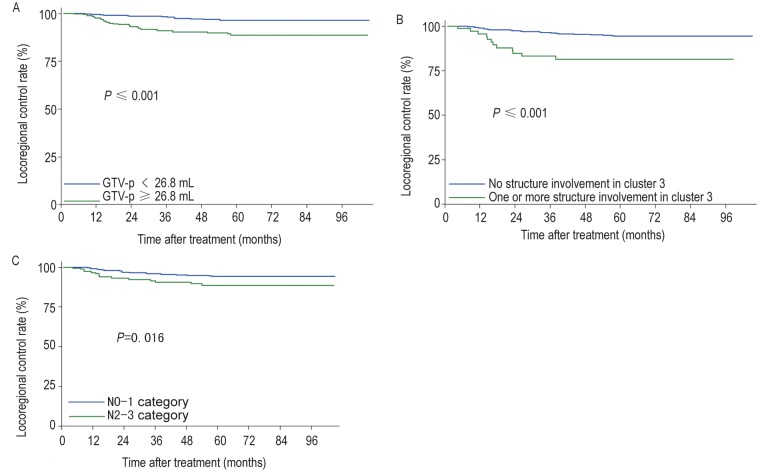
Multivariate analyses using the Cox proportional hazards model revealed that N2–3 category (HR = 2.103, P = 0.019), GTV-p ≥26.8 mL (HR = 2.605, P = 0.007), and involvement of one or more structures within cluster 3 (HR = 2.444, P = 0.013) predicted locoregional relapse (Table 3). Figure 3 shows the 5-year locoregional control rate of these risk factors. Using risk factor analysis, we derived a formula to calculate the risk score for locoregional relapse from the presence or absence of the above risk factors, weighted by regression coefficients[18],[19]:
Table 3. Independent prognostic factors for locoregional control on multivariate analysis.
| Factor | B | HR | 95% (CI) | P |
| GTV-p ≥26.8 mL | 0.958 | 2.605 | 1.305-5.204 | 0.007 |
| Cluster 3 with one or more structures involved | 0.894 | 2.444 | 1.203-4.960 | 0.013 |
| N2-3 category | 0.743 | 2.103 | 1.131-3.909 | 0.019 |
Figure 3. Kaplan-Meier curves of the locoregional control rates according to the presence of risk factors.
A, GTV-p≥26.8 mL vs. <26.8 mL. B, involvement of one or more structures in cluster 3 vs. no structure involvement. C, N2-3 category vs. N0-1 category.
Score = 0.958 × (0,1) [GTV-p≥26.8 mL] + 0.894 × (0,1) [Cluster 3 ≥1] + 0.743 × (0,1) [N2–3 stage].
The risk scores calculated with this formula were used to divide patients into four groups: a score of 0 indicated low risk (312 patients, 43.5%); a score >0 and≤1 indicated intermediate risk (283 patients, 39.5%); a score >1 and≤2 indicated high risk (108 patients, 15.1%); and a score >2 indicated extremely high risk (14 patients, 1.9%). All patients completed treatment regimens. The median overall treatment time was 43 (range, 32-57) days in the low-risk group, 44 (range, 32-68) days in the intermediate-risk group, 44 (range, 33-77) days in the high-risk group, and 48 (range, 39-55) days in the extremely high-risk group. Reasons for prolonging treatment time included severe acute toxicity, machine breakdown, a public holiday, and the patient's personal affairs. The complete and partial response rates after chemoradiotherapy were 91.3% (211/231) and 8.7% (20/231) in the low-risk group, 81.3% (265/326) and 18.7% (61/326) in the intermediate-risk group, 76.0% (111/146) and 24.0% (35/146) in the high-risk group, and 64.3% (9/14) and 35.7% (5/14) in the extremely high-risk group.
Some patients developed locoregional relapse or were loss to follow-up: 8 (2.6%) and 20 (6.8%) in the low-risk group, 18 (6.4%) and 17 (7.6%) in the intermediate-risk group, 16 (14.8%) and 3 (4.4%) in the high-risk group, and 3 (21.4%) and 1 (10%) in the extremely high-risk group. The 5-year locoregional control rates for the four groups were 97.4%, 93.6%, 85.2%, and 78.6%, respectively (P<0.001) (Figure 4).
Figure 4. Kaplan-Meier curves of locoregional control rates for NPC patients grouped according to a risk score of locoregional recurrence.
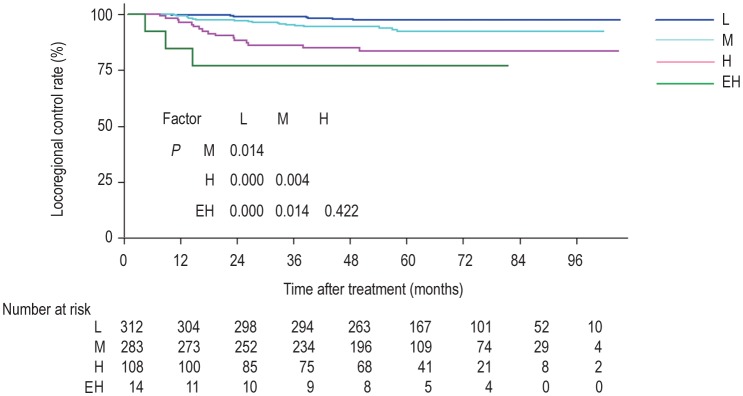
L: low risk (score 0); M: intermediate risk (score >0 and ≤1); H: high risk (score >1 and ≤2); EH: extremely high risk (score >2).
Discussion
Analysis of relapse patterns is crucial for the formulation of appropriate treatment strategies. Many studies have reported that T category is an independent prognostic factor for locoregional control of NPC. However, the emergence of IMRT has significantly improved the outcome of NPC patients, and as a result, T category is no longer a good predictor of locoregional control[3]. We developed a PSS for locoregional control that included tumor volume, local tumor extension, and N category to identify high-risk patients for aggressive therapy with the goal of further decreasing locoregional relapse rates.
Information about GTV-p was obtained using treatment planning systems, which have been used extensively worldwide. Numerous studies have confirmed that tumor volume is a significant prognostic predictor in NPC patients treated with IMRT. Wu et al.[12] have shown that GTV-p (<48 mL vs.≥48 mL) was significantly associated with local control. Guo et al.[13] have defined a GTV-p cut-off of 19 mL to predict adverse effects on disease-free survival (DFS) and overall survival (OS). Furthermore, they found that this GTV-p cut-off was a significant predictor of locoregional relapse only in patients with locally advanced NPC. In our study, GTV-p (≥26.8 mL) was an adverse prognostic factor for locoregional relapse, which is a finding that agrees with those of Wu et al. We selected a cut-off value of 26.8 mL to predict locoregional relapse. The survival difference was expected to be more apparent with this cut-off because more patients with early T1–2 tumors were included in the group with GTV-p < 26.8 mL. Generally, a large tumor volume indicates a high tumor burden and tumor hypoxia. In addition, a large tumor volume has a high probability of being close to a critical normal organ, which influences dose escalation. For patients with GTV-p≥26.8 mL, radiotherapy alone or concurrent chemoradiotherapy may not be sufficient to achieve radical control, and more aggressive treatment is recommended.
In our study, HCA showed that cluster 1 mainly included structures with a medium-to-high incidence of tumor invasion; cluster 2 included structures that were infiltrated by the tumor via a posterior route; and cluster 3 included structures infiltrated via a superior route. The maxillary sinus was rarely involved (1.5%, 11/717), which is likely because it is distant from the nasopharynx and separated from it by other anatomic sites. The results of the clustering reflected the pattern of local disease extension, which was different from that reported by Cheng et al.[11]. Liang et al.[20] have reported that local disease spreads in a stepwise manner from proximal sites to more distal sites and that a skip pattern of local extension was unusual. In the present study, when structures in cluster 1 (medium- to high-risk anatomic sites) were involved, the low-risk sites in cluster 3 tended to be involved at an increased rate, from 4.8% (35/717) to 27.8% (32/115). Additionally, the involvement of structures in cluster 3 was constantly associated with large tumor volumes. Consequently, the involvement of cluster 3 structures was found to be a prognostic factor on multivariate Cox regression analysis [HR = 2.444, 95% confidence interval (CI) = 1.203–4.965, P = 0.013].
Advanced N stage has been considered an independent predictor of locoregional relapse-free survival and distant metastasis-free survival (DMFS)[21]. In this study, the 5-year locoregional control rates for patients at N0, N1, N2, and N3 stages were 97.1%, 93.6%, 89.3%, and 91.5%, respectively. We combined N2 and N3 stages, which were found to be significant predictors of locoregional relapse on multivariate analysis (HR = 2.103, 95% CI = 1.131–3.909, P = 0.019) (Figure 3C).
Studies have revealed that the baseline serum LDH level was closely related to survival of NPC patients treated with chemoradiotherapy[10],[22]. For NPC patients undergoing IMRT, Zhou et al.[10] have found that elevated baseline LDH levels were an adverse prognostic factor for OS, DFS, and DMFS, but not for LRFS. In our study, LDH level was found to be a prognostic factor on univariate analysis but not on multivariate analysis. These results are consistent with those from Zhou et al. and may be associated with the enhanced local control achieved by IMRT used in both studies.
This study identified patients in low- and intermediate-risk groups with satisfactory 5-year locoregional control rates of 97.4% and 93.6%, respectively. The prescribed dose of 68 Gy at 2.27 Gy/fraction was sufficient to radically cure the primary tumor in these patients. Intensive chemotherapy regimens should be avoided in NPC patients with low or intermediate risk to decrease chemotherapy-related mortality. However, patients in the high-risk and extremely high-risk groups who were given current treatment strategies had lower 5-year locoregional control rates of 85.2% and 78.6%, respectively. The benefits of systemic chemotherapy and molecular targeting treatments should be explored in these patients.
This study had some limitations. Due to the limited cases of relapse, we could not create a validation set to verify the PSS. In addition, the uneven distribution of patients in the risk groups is difficult to avoid due to the retrospective nature of the study. The large sample size in this study may strengthen the prognostic significance of this PSS, and a prospective, multicenter study is warranted to test this scoring system in the future.
Conclusions
We have developed a PSS for locoregional control in NPC patients treated with radical IMRT. This scoring system is based on tumor volume, tumor involvement of anatomic sites, and N category, and it could screen for patients who are at a high risk of relapse, which would assist in optimizing individualized treatment strategies.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (No. 81071836) and Sun Yat-sen University 5010 projects (No. 050243).
References
- 1.Nielsen NH, Mikkelsen F, Hansen JP. Nasopharyngeal cancer in Greenland. The incidence in an Arctic Eskimo population. Acta Pathol Microbiol Scand A. 1997;85:850–858. [PubMed] [Google Scholar]
- 2.Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–293. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Kuang WL, Zhou Q, Shen LF, et al. Outcomes and prognostic factors of conformal radiotherapy versus intensity-modulated radiotherapy for nasopharyngeal carcinoma. Clin Transl Oncol. 2012;14:783–790. doi: 10.1007/s12094-012-0864-5. [DOI] [PubMed] [Google Scholar]
- 4.Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Tham IW, He SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy—the National Cancer Centre Singapore experience. Int J Radio Oncol Biol Phys. 2009;75:1481–1486. doi: 10.1016/j.ijrobp.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Mao YP, Xie FY, et al. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognos-tically useful for patient treatment with intensity-modulated radiotherapy from an endemic area in China. Radio Oncol. 2012;104:331–337. doi: 10.1016/j.radonc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Yap ML, Choo BA, Chan YH, et al. Outcomes following treatment for patients with cranial nerve involvement from nasopharyngeal cancer. J Med Imaging Radiat Oncol. 2012;56:548–553. doi: 10.1111/j.1754-9485.2012.02391.x. [DOI] [PubMed] [Google Scholar]
- 8.Sham JS, Cheung YK, Choy D, et al. Cranial nerve involvement and base of the skull erosion in nasopharyngeal carcinoma. Cancer. 1991;68:422–426. doi: 10.1002/1097-0142(19910715)68:2<422::aid-cncr2820680235>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Nasr Ben Ammar C, Kochbati L, Lejri N, et al. Prognostic value of parapharyngeal extension in nasopharyngeal carcinoma. Tunis Med. 2009;87:814–817. [PubMed] [Google Scholar]
- 10.Zhou GQ, Tang LL, Mao YP, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 2012;82:359–365. doi: 10.1016/j.ijrobp.2011.06.1967. [DOI] [PubMed] [Google Scholar]
- 11.Cheng SH, Tsai SY, Horng CF, et al. A prognostic scoring system for locoregional control in nasopharyngeal carcinoma following conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:992–1003. doi: 10.1016/j.ijrobp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Zeng RF, Su Y, et al. Prognostic significance of tumor volume in patients with nasopharyngeal carcinoma undergoing intensity-modulated radiation therapy. Head Neck. 2013;35:689–694. doi: 10.1002/hed.23010. [DOI] [PubMed] [Google Scholar]
- 13.Guo R, Sun Y, Yu XL, et al. Is primary tumor volume still a prognostic factor in intensity-modulated radiation therapy for nasopharyngeal carcinoma? Radiat Oncol. 2012;104:294–299. doi: 10.1016/j.radonc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 14.King AD, Bhatia KS. Magnetic resonance imaging staging of nasopharyngeal carcinoma in the head and neck. World J Radiol. 2010;2:159–165. doi: 10.4329/wjr.v2.i5.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, Han F, Lu LX, et al. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004;23:1532–1537. [in Chinese] [PubMed] [Google Scholar]
- 16.Gollub J, Sherlock G. Clustering microarray data. Methods Enzymol. 2006;411:194–213. doi: 10.1016/S0076-6879(06)11010-1. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Gao J, Tao YL, et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer. 2012;31:197–206. doi: 10.5732/cjc.011.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Chen NY, Cui RX, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 2012;13:633–641. doi: 10.1016/S1470-2045(12)70102-X. [DOI] [PubMed] [Google Scholar]
- 19.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 20.Liang SB, Sun Y, Liu LZ, et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: improvement of clinical target volume delineation. Int J Radiat Oncol Biol Phys. 2009;75:742–750. doi: 10.1016/j.ijrobp.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 21.Palazzi M, Guzzo M, Bossi P, et al. Regional advanced nasopharyngeal carcinoma: long-term outcome after sequential chemotherapy and radiotherapy. Tumori. 2004;90:60–65. doi: 10.1177/030089160409000114. [DOI] [PubMed] [Google Scholar]
- 22.Turen S, Ozyar E, Altundag K, et al. Serum lactate dehydrogenase level is a prognostic factor in patient with locoregional advanced nasopharyngeal carcinoma treated with chemoradiotherapy. Cancer Invest. 2007;25:315–321. doi: 10.1080/07357900701209103. [DOI] [PubMed] [Google Scholar]