Abstract
The management of postoperative leaks into the mediastinum after esophagectomy remains a challenge. We describe our clinical management of this complication through endoscopic transluminal drainage. Between 2008 and 2011, 4 patients with esophageal squamous cell carcinoma (ESCC) who underwent McKeown-type esophagectomy with two-field lymphadenectomy experienced complicated anastomotic fistulae in the presence of superior mediastinal sepsis. All 4 patients underwent endoscopic transluminal drainage, and all survived. The mean healing period was 50 days (range, 31 to 58 days), the mean stay in the intensive care unit was 7.3 days (range, 1 to 18 days), and the mean hospital stay was 64.5 days (range, 49 to 70 days). Endoscopically guided transluminal drainage should be considered for ESCC patients with superior mediastinal fistulae after esophagectomy.
Keywords: Esophageal squamous cell carcinoma, anastomotic fistulae, transluminal drainage
Patients with localized and/or resectable esophageal squamous cell carcinoma (ESCC) are usually treated with either left thoracotomy or the “three-incision” McKeown-type esophagectomy[1],[2]. Anastomotic fistulae is a serious postoperative complication: it occurs in 12% of patients and is responsible for almost 40% of post-operative deaths[3]–[5]. Although rare, transudate confined to the mediastinum and corroding vessels result in an even higher mortality[6]. The optimal treatment of the latter condition has not yet been determined. In this paper, we describe the use of endoscopic transluminal drainage in 4 patients with superior mediastinal fistulae after esophagectomy.
Case Report
Between August 2008 and November 2011, 4 male patients with ESCC experienced postoperative anastomotic fistulae with mediastinal sepsis after McKeown-type esophagectomy with two-field lymphadenectomy. Their clinicopathologic characteristics are summarized in Table 1.
Table 1. Clinicopathologic characteristics of 4 esophageal cancer patients who underwent endoscopic transluminal drainage for esophageal leaks after esophagectomy.
| Item | Patient No. 1 | Patient No. 2 | Patient No. 3 | Patient No. 4 |
| Age (years) | 56 | 76 | 46 | 49 |
| Weight (kg) | 55 | 72 | 58 | 49 |
| Height (cm) | 165 | 167 | 170 | 170 |
| Incisor distance (cm) | 26-31 | 25-30 | 32-37 | 30-35 |
| Stage (AJCC 7th) | pT3N1M0 | pT2N0M0 | pT3N0M0 | pT2N1M0 |
| Leak site | SM & PS | SM | SM & PS | SM |
| Fistulae occurrencea (days) | 8 | 7 | 3 & 11e | 7 |
| Gemiculture | SA & CA | E. coli | PA& ACB | SA |
| Treatment | ETD & DTP | ETD | ETD & DTP | ETD |
| Time intervalb (days) | 6 | 1 | 12f | 14 |
| Intensive carec (days) | 1 | 1 | 18 | 2 |
| Healing timed (days) | 53 | 31 | 58 | 58 |
| Hospital stay (days) | 63 | 49 | 70 | 76 |
AJCC, American Joint Committee on Cancer; SM, superior mediastinum; PS, pleural spaces; SA, oligotrophic Stenotrophomonas and Aeromonas; CA, Candida albicans; E. coli, Escherichia coli; PA, Pseudomonas aeruginosa; ACB, Acinetobacter calcoaceticus-baumannii; ETD, endoscopic transluminal drainage; DTP, double-tube drainage of pleural cavity. aThe postoperative day on which the fistulae occurred. bPeriod between the diagnosis and treatment of the fistulae. cPeriod in the intensive care unit. dPeriod between diagnosis and healing of the fistulae. eTwo fistulae with bilateral mediastinitis, which were managed by bilateral mediastinal drainage. fThe time from diagnosis of the mediastinal fistulae to bilateral mediastinal drainage.
All 4 patients underwent McKeown-type esophagectomy with two-field lymphadenectomy and cervically located anastomosis. None underwent neoadjuvant therapy. Jejunal feeding and decompression tubes were inserted regularly during surgery. Intravenous hyperali-mentation was performed to increase the recovery of gastrointestinal function after surgery, followed by enteral nutrition when warranted. All patients experienced anastomotic fistulae accompanied by superior mediastinal abscess.
Once a fistulae was confirmed, empiric antibiotics were administered immediately, followed by the appropriate antibiotics once culture results were obtained. The presence of a fistulae was confirmed by clinical symptoms, meglumine diatriazoate swallowing, and endoscopy, with a computed tomography (CT) scan of the thorax and abdomen performed if necessary. Three patients were initially treated for mediastinal fistulae by endoscopic transluminal drainage. One was initially treated conservatively due to his mild symptoms and young age, followed by endoscopic transluminal drainage when conservative management failed. Two patients, both with sepsis and fluid within the superior mediastinal space, underwent transluminal drainage alone; the other two also required transthoracic drainage for uncontained fistulae in both the superior mediastinal and pleural spaces. Markers of inflammation, such as white blood cell (WBC) count and C-reactive protein (CRP) concentration, were measured during healing.
Following the diagnosis of superior mediastinal fistulae, a guide wire was passed through the anastomotic defect endoscopically, and a nasogastric suction tube was placed over it and into the mediastinal abscess cavity from inside the esophagus. The decision to add side holes was determined by the abscess size, as evaluated by radiographic/imaging results. The nozzle was placed as close to the bottom of the abscess as possible, with pumping used to ensure the correct position and patency. Continuous suction was maintained with appropriate pressure (usually 30 cm H2O). The space was irrigated intermittently with saline to make the lumen pourable and dilute pus, thus eliminating the infected fluid and inducing the formation of granulation tissue. Two weeks later, meglumine diatriazoate was injected through the suction tube, and contrast imaging was performed weekly thereafter to determine the volume of the abscess cavity. As the abscess collapsed, the tube was gradually withdrawn every three days until withdrawal was complete. At that time, the patient was permitted a liquid diet. All patients underwent clinical, radiologic, and endoscopic follow-up.
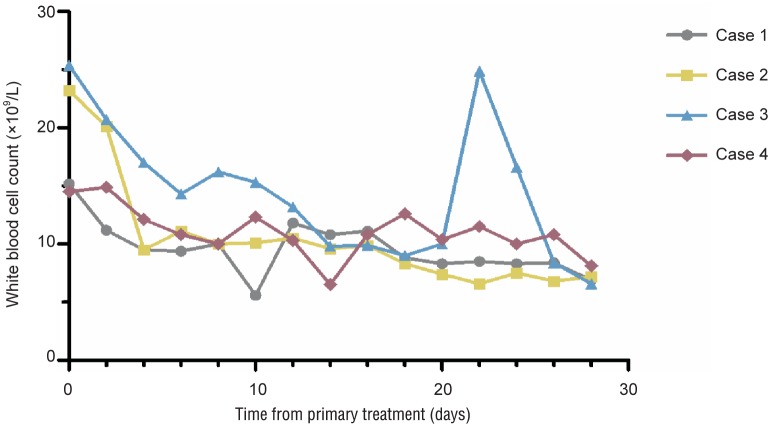
No patients died. All patients presented with hyperpyrexia and leukocytosis to various degrees. At 1 to 2 days after the aspiration of purulent material, the general condition of each patient began to improve, with steady decreases in body temperature and WBC counts (Figure 1). Endoscopy or imaging was performed to monitor the clinical healing process of the fistulae while the patients were in the hospital (Figure 2).
Figure 1. Changes of white blood cell count while managing esophageal leaks by endoscopic transluminal drainage in 4 esophageal cancer patients with superior mediastinal sepsis after esophagectomy.
The white blood cell count decreased after endoscopic transluminal drainage.
Figure 2. The results of clinical examinations before and after treatment.
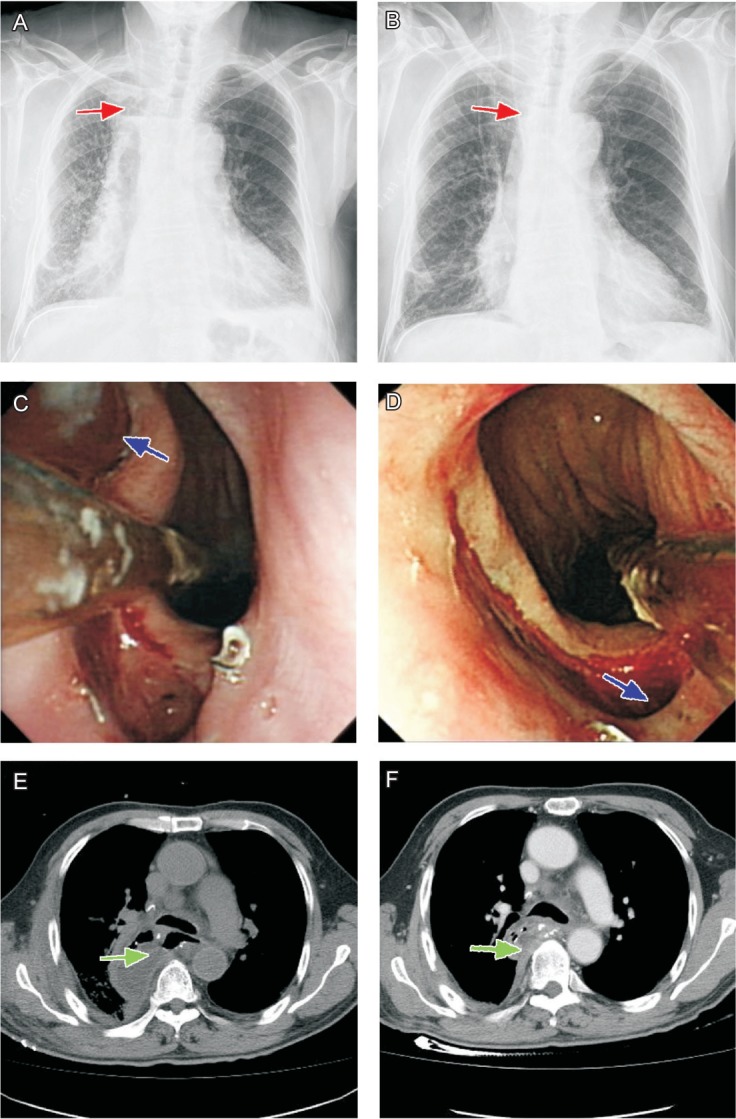
A, chest X-ray radiography shows the gas-fluid level (red arrow) in the right superior mediastinum caused by the fistulae. B, the encapsulated effusion disappeared after transluminal drainage (red arrow). C, endoscopy shows that a transluminal drainage tube (blue arrow) was inserted into the fistulae for irrigation and section. D, the fistulae was healed after removal of the drainage tube (blue arrow). E, chest computed tomography shows partial abscess (green arrow) in the right chest before transluminal drainage. F, the abscess decreased markedly after transluminal drainage (green arrow).
Patient 1 showed displacement of the intrathoracic drain tube 12 days after primary treatment. After repositioning the tube, the increased WBC count returned to normal gradually. No other morbidity occurred until discharge.
Patient 2 had a relatively short healing time (49 days), despite his old age. He suffered anastomotic stenosis during follow-up and was managed by bougienage.
Patient 3 experienced fistulae with bilateral mediastinitis. Severe sepsis and surgical strike resulted in cardiopulmonary insufficiency, which was managed by the placement of two endoscopic drains and prolonged inotropic cardiovascular support and ventilator treatment for 18 days. On day 22 in the hospital, he had a urinary infection (Figure 2) and was managed by bladder irrigation with nitrofurazone. Oral intake was initiated before complete healing of the suture line in this patient because the residual cavity was so small that it could be ignored, and the patient was closely monitored during this period. When monitoring showed a normal presentation, he was discharged. No obvious morbidity occurred during follow-up.
Conservative therapy in patient 4 resulted in a marked delay in performing transluminal drainage and prolonged the healing period to 76 days. Meglumine diatriazoate was aspirated during a regular checkup while in the hospital.
Discussion
A postesophagectomy anastomotic fistulae is a radiologically or clinically apparent esophagectomy anastomotic dehiscence. The therapeutic options include surgical repair or resection or conservative management, including the cessation of oral intake and antibiotic therapy. Over the past two decades, various conser-vative management regimens have been designed for patients with anastomotic fistulae, including the implantation of a self-expanding metallic stent, endoscopic clipping, and the application of fibrin glue or biodegradable fistulae plugs[7]–[10].
Despite these therapeutic modalities, treating an intrathoracic fistulae, especially a mediastinal fistulae, remains challenging[2],[5],[11],[12]. The unapproachability of the site makes sufficient drainage almost impossible. In addition, large blood vessels, such as the aorta, may rupture at any time due to corrosion by pus, particularly when fistulae is confined to the superior mediastinum. Moreover, the rarity, occultness, and difficult exposure of this condition often delay its diagnosis, by which time the affected esophageal wall may have become edematous and friable, and fibrinous mediastinitis may have developed, making primary surgical repair almost impossible[13]–[15]. Thus, despite the recently reported muscle onlay approach, which yielded satisfactory results[16], we preferred using more conservative interventional therapy.
Because the leading cause of death in patients with postoperative anastomotic fistulae is an uncontrollable intrathoracic or mediastinal abscess, the key to management is early and adequate drainage[12],[14],[17]. The timely management of our 4 patients resulted in rapid recovery and early discharge, suggesting that this method is effective and feasible, even in elderly individuals.
Compared with other conservative management regimens, such as endoscopic clipping and the application of fibrin glue or biodegradable fistulae plugs, endoscopic transluminal drainage achieve adequate drainage rather than simple closure of the fistulae. When diagnosis is combined with treatment, placing a suction tube into the abscess becomes easy and valid, especially for patients with unapproachable superior mediastinal abscesses. Intermittent irrigation with saline, which rinses the area and suppresses local inflammation, enhances drainage. The practicality and convenience of this approach may enhance its popularity. Similar approaches yielding satisfactory results confirm the effectiveness of our method[18],[19]. Our patients showed improvement in their general condition, including a decreased WBC count, within 1 to 2 days after the insertion of drainage tubes. In addition, this approach eliminated the need for a prolonged stay in the intensive care unit, unless a serious morbidity occurred.
Complications after endoscopic stenting including stent migration, which is observed in 3% to 58% of patients, unplanned endoscopic reintervention, which is observed in 19% to 59%, and intestinal obstruction, which is observed in up to 16%[8],[18],[20],[21]. Endoscopic transluminal drainage was more effective, resulting in spontaneous healing with little manual work. Replacing a displaced tube is no longer difficult. Contrast imaging can be used to assess abscess size by the depth intubated and the amount of meglumine diatriazoate injected, making tube withdrawal more objective and effective. The conservative features of this approach make it suitable for many types of patients, especially those in poor general condition.
The length of hospitalization of our patients, which ranged from 49 to 76 days, was longer than that of patients in a United Kingdom-wide audit, in which patients were hospitalized for 25 to 55 days. The latter results, however, were obtained from European patients with esophagogastric cancer, most of whom underwent less invasive transhiatal esophagectomy and developed fistulae that were common but not fatal, such as cervical fistulae.
CT scans of 2 of our patients showed encapsulated abscesses, allowing these patients to be managed without chest tube drainage. Because a chest tube could not reach the occult abscess, transluminal irrigation combined with suction was the most direct and effective management.
Most mediastinal fistulae are not inherent. Rather, they are usually due to the development of an ignored fistulae that descends to the mediastinal space. Abnormal but nonspecific symptoms, including fever, dysphoria, arrhythmia, and tachycardia, may be due to fistulae development. Timely detection and verification are required to prevent mediastinal fistulae.
Conclusions
In summary, mediastinal fistulae remain a challenge for treatment. Due to its rarity, no standard treatment method has yet been developed. Early detection and adequate drainage are important in preventing morbidity and mortality in these patients. Endoscopically guided transluminal drainage should be considered for these patients. Additional studies with greater numbers of patients are warranted.
References
- 1.Safranek PM, Cubitt J, Booth MI, et al. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg. 2010;97:1845–1853. doi: 10.1002/bjs.7231. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Luketich JD. Resection for esophageal cancer: strategies for optimal management. Ann Thorac Surg. 2008;85:S751–S756. doi: 10.1016/j.athoracsur.2007.11.078. [DOI] [PubMed] [Google Scholar]
- 3.Postlethwait RW. Complications and deaths after operations for esophageal carcinoma. J Thorac Cardiovasc Surg. 1983;85:827–831. [PubMed] [Google Scholar]
- 4.Junemann-Ramirez M, Awan MY, Khan ZM, et al. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg. 2005;27:3–7. doi: 10.1016/j.ejcts.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Muller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845–857. doi: 10.1002/bjs.1800770804. [DOI] [PubMed] [Google Scholar]
- 6.Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634–640. doi: 10.1016/s0002-9610(99)80238-4. [DOI] [PubMed] [Google Scholar]
- 7.Toussaint E, Eisendrath P, Kwan V, et al. Endoscopic treatment of postoperative enterocutaneous fistulas after bariatric surgery with the use of a fistula plug: report of five cases. Endoscopy. 2009;41:560–563. doi: 10.1055/s-0029-1214606. [DOI] [PubMed] [Google Scholar]
- 8.Leers JM, Vivaldi C, Schafer H, et al. Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc. 2009;23:2258–2262. doi: 10.1007/s00464-008-0302-5. [DOI] [PubMed] [Google Scholar]
- 9.Rodella L, Laterza E, De Manzoni G, et al. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy. 1998;30:453–456. doi: 10.1055/s-2007-1001307. [DOI] [PubMed] [Google Scholar]
- 10.Lippert E, Klebl FH, Schweller F, et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis. 2011;26:303–311. doi: 10.1007/s00384-010-1104-5. [DOI] [PubMed] [Google Scholar]
- 11.Lang H, Piso P, Stukenborg C, et al. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol. 2000;26:168–171. doi: 10.1053/ejso.1999.0764. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein LA, Thompson WR. Esophageal perforations: a 15 year experience. Am J Surg. 1982;143:495–503. doi: 10.1016/0002-9610(82)90202-1. [DOI] [PubMed] [Google Scholar]
- 13.Keszler P, Buzna E. Surgical and conservative management of esophageal perforation. Chest. 1981;80:158–162. doi: 10.1378/chest.80.2.158. [DOI] [PubMed] [Google Scholar]
- 14.Richardson JD, Martin LF, Borzotta AP, et al. Unifying concepts in treatment of esophageal leaks. Am J Surg. 1985;149:157–162. doi: 10.1016/s0002-9610(85)80026-x. [DOI] [PubMed] [Google Scholar]
- 15.Brinster CJ, Singhal S, Lee L, et al. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004;77:1475–1483. doi: 10.1016/j.athoracsur.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Kotzampassakis N, Christodoulou M, Krueger T, et al. Esophageal leaks repaired by a muscle onlay approach in the presence of mediastinal sepsis. Ann Thorac Surg. 2009;88:966–972. doi: 10.1016/j.athoracsur.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Infante M, Valente M, Andreani S, et al. Conservative management of esophageal leaks by transluminal endoscopic drainage of the mediastinum or pleural space. Surgery. 1996;119:46–50. doi: 10.1016/s0039-6060(96)80212-1. [DOI] [PubMed] [Google Scholar]
- 18.Williams RN, Hall AW, Sutton CD, et al. Management of esophageal perforation and anastomotic leak by transluminal drainage. J Gastrointest Surg. 2011;15:777–781. doi: 10.1007/s11605-011-1472-3. [DOI] [PubMed] [Google Scholar]
- 19.Ahrens M, Schulte T, Egberts J, et al. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42:693–698. doi: 10.1055/s-0030-1255688. [DOI] [PubMed] [Google Scholar]
- 20.Freeman RK, Ascioti AJ, Wozniak TC. Postoperative esophageal leak management with the Polyflex esophageal stent. J Thorac Cardiovasc Surg. 2007;133:333–338. doi: 10.1016/j.jtcvs.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Blackmon SH, Santora R, Schwarz P, et al. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg. 2010;89:931–937. doi: 10.1016/j.athoracsur.2009.10.061. [DOI] [PubMed] [Google Scholar]