Abstract
Mammalian target of rapamycin (mTOR) is aberrantly activated in many cancer types, and two rapamycin derivatives are currently approved by the Food and Drug Administration (FDA) of the United States for treating renal cell carcinoma. Mechanistically, mTOR is hyperactivated in human cancers either due to the genetic activation of its upstream activating signaling pathways or the genetic inactivation of its negative regulators. The tumor suppressor liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11), is involved in cell polarity, cell detachment and adhesion, tumor metastasis, and energetic stress response. A key role of LKB1 is to negatively regulate the activity of mTOR complex 1 (mTORC1). This review summarizes the molecular basis of this negative interaction and recent research progress in this area.
Keywords: Energetic stress, metabolism, bi-allelic inactivation, protein translation
Pharmaceutic reagents that specifically target mammalian target of rapamycin (mTOR) have been approved by the Food and Drug Administration (FDA) of the United States for treating advanced renal cancer, progressive or metastatic pancreatic cancer, and breast cancer in post-menopausal women with estrogen receptor (ER)- or progesterone receptor (PR)-positive and human epidermal growth factor receptor 2 (HER2)-negative lesions. mTOR is aberrantly activated through either the activation of phosphatidylinositide 3-kinases (PI3K)/AKT signaling or the inactivation of LKB1/AMPK/TSC signaling. Here, we focus on the activation of mTOR through the inactivation of LKB1 signaling.
LKB1 as a Serine/Threonine Kinase
The liver kinase B1 (LKB1) gene is located on chromosome 19p13.3 and spans 23 kb. It contains 10 exons, 9 of which are protein-coding, and the gene is transcribed in the telomere-to-centromere direction[1]. The LKB1 transcript is 3.1 kb in length. It is ubiquitously expressed in all fetal and adult tissues, with high expression levels in the pancreas, liver, and skeletal muscle[2]. It encodes a 436-amino acid protein that comprises two nuclear localization sequences, a kinase domain (residues 50–319), and a putative carboxy-terminal regulatory domain. It is evolutionarily conserved with closely related orthologues in mouse, Xenopus[3], Drosophila, and C. elegans (Par4)[4].
LKB1 is a serine/threonine kinase and has at least 13 potential substrates in the AMP-activated protein kinase (AMPK) subfamily, such as AMPK, brain-specific kinase (BRSK), and salt-inducible kinase (SIK)[5]. Although LKB1 is present in both the nucleus and cytoplasm of cells, LKB1 can exert its kinase activities in only the cytoplasm. This is because the phosphorylation of AMPK by LKB1 requires its interactions with two adaptor proteins, Ste20-related adaptor (STRAD) and mouse protein 25 (MO25). STRAD, which has both α and β isoforms, is a pseudokinase that lacks key residues required for catalyzing protein phosphorylation. STRAD is found only in the cytosol, and its binding to LKB1 activates the autophosphorylating kinase activity of LKB1 and targets LKB1 to the cytosol[6]. MO25 (α or β) binds to STRAD and stabilizes the STRAD-LKB1 complex [7]. The LKB1-STRADα-MO25α complex, but not LKB1 alone, can phosphorylate the AMPKα subunit at Thr172[8].
LKB1 kinase activity is also regulated by phosphorylation. Thr189 is usually autophosphorylated, but this modification can be abrogated by introducing a mutation at this site[9]. LKB1 can also be phosphorylated at Ser31, Ser325, Thr336, Thr366, and Ser428 (Ser431 in mice)[2],[10], and mutations at Thr336 and Ser428 impair the ability of LKB1 to suppress cell growth in LKB1 overexpression studies[2],[10],[11]. The aberrant activation of BRAF by V600E mutation and its downstream target ribosomal S6 kinase 2 (RSK2) inactivates LKB1 protein function in melanoma cells through the phosphorylation of LKB1 at Ser324 and Ser428[12],[13].
LKB1 as a Regulator of mTOR
Tremendous progress over the last few years has linked LKB1 to the regulation of mTOR. mTOR is an evolutionarily conserved serine/threonine kinase that plays a central role in the regulation of cell growth and proliferation, which is achieved in part through the regulation of protein translation. Two key downstream targets of mTOR are p70 ribosomal S6 kinase (S6K) and eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1). mTOR promotes translational initiation by phosphorylating S6K, which stimulates the translation of ribosomal proteins, and by phosphorylating 4E-BP1. The phosphorylation of 4E-BP1 releases eIF4E, which is then free to form a complex with eIF4F, promoting cap-dependent translation.
LKB1 is linked to mTORC1 through the sequential activation of AMPK and the tumor suppressor tuberin (TSC2)[14]–[16] (Figure 1). AMPK is a metabolic master regulator that is activated in response to reduced energy availability [high cellular adenosine monophosphate (AMP): adenosine triphosphate (ATP) ratios] or hypoxic stress[17],[18]. AMPK exists as a heterotrimer composed of a catalytic domain (α) and two regulatory domains (β and γ). Phosphorylation of the AMPKα subunit in the activation loop at Thr172 by LKB1 is essential for the catalytic activity of AMPK[8],[14]–[16],[19]. LKB1 acts upstream of AMPK, as indicated by genetic evidence showing that AMPK activation in response to treatment with 5-aminoimidazole-4-carboxamide riboside (AICAR), an AMP analog that increases the perceived AMP:ATP ratio, is compromised in Lkb1−/− mouse embryonic fibroblasts (MEFs) and can be restored following reconstitution of LKB1[8].
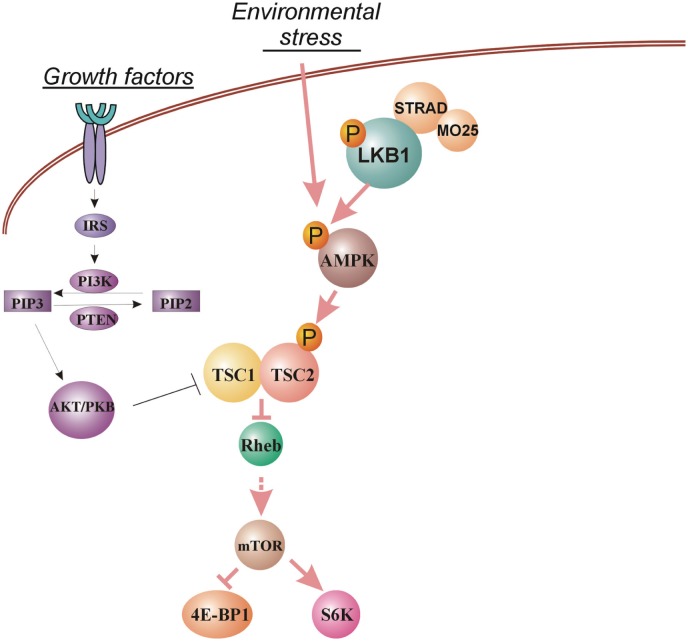
Figure 1. The regulation of mammalian target of rapamycin (mTOR) by the LKB1/AMPK signaling pathway.
Environmental stress leads to the activation of AMP-activated protein kinase (AMPK) by liver kinase B1 (LKB1), which subsequently activates hamartin (TSC1)/tuberin (TSC2) complex. The activation of this pathway down-regulates mTOR activity, which leads to cell growth arrest. mTOR activity can also be regulated by growth factors through the PI3K/PTEN/AKT pathway.
TSC1 and TSC2 (also known as hamartin and tuberin, respectively) are tumor suppressor genes involved in tubular sclerosis, a familial cancer syndrome that is associated with benign renal polyps and exhibits some clinical similarities to Peutz-Jeghers syndrome (PJS). TSC2 is a GTPase-activating protein (GAP) for the ras-like GTPase Rheb. GTP-bound Rheb stimulates mTOR activity through an unknown mechanism. The phosphorylation of TSC2 at Thr1227 and Ser1345 by AMPK activates TSC2 GAP activity, shifting the balance toward Rheb-GDP and thus suppressing mTOR activity[20]–[22].
mTOR and the phosphorylation of S6K and 4E-BP1 are rapidly induced by mitogens, insulin, and nutrients. In this context, the activation of mTOR is mediated by the PI3K/AKT pathway. AKT stimulates mTOR through the phosphorylation and inactivation of TSC2. AKT can also phosphorylate mTOR directly (at S2448). Thus, the tumor suppressor complex TSC1/TSC2, together with mTOR, constitutes a key integration point for mitogenic and stress-activated signals. The activation of the LKB1/AMPK/TSC pathway under conditions of low energy or insufficient nutrient availability thus overrides the mitogenic signals transmitted by the PI3K/AKT pathway. In this sense, the LKB1/AMPK/TSC pathway can be thought of as a cellular stress-activated “checkpoint” that prevents energy- or nutrient-consuming processes, such as protein translation or cell division, from occurring under suboptimal conditions. Indeed, Lkb1−/− or Tsc2−/− MEFs undergo apoptosis in response to low glucose, whereas their wild-type counterparts are protected from this event[20],[23].
Loss of LKB1 Kinase Activity Leads to Aberrant mTOR Activation in a Variety of Tissues
LKB1 was originally identified as the causal gene mutation in the inherited cancer syndrome PJS, an autosomal-dominant disease that is characterized by benign hamartomatous polyposis of the gastrointestinal (GI) tract, hyperpigmentation of mucosal membranes, and an increased risk of intestinal malignancies[1],[24]. PJS patients are also at an increased risk of developing other cancers, including cancers of the lung, breast, pancreas, uterus, ovary, cervix, and testis[25]–[28]. It is estimated that PJS patients have a 93% lifetime risk of cancer, with a mean age of cancer diagnosis at 43 years old. Most PJS-associated LKB1 mutations are in the highly conserved kinase domain. Almost all PJS point mutants that have been tested for in vitro kinase activity have been inactive, consistent with the hypothesis that PJS-associated mutations disrupt LKB1 enzymatic function. The intestinal polyps in PJS patients were shown to have up-regulated mTORC1 signaling[29], supporting that the genetic inactivation of LKB1 promotes aberrant activation of mTORC1 signaling in GI polyps. Interestingly, the loss of LKB1 in stromal cells may also contribute to the formation of GI polyps; the loss of murine Lkb1 in mesenchymal cells also leads to GI polyps that are indistinguishable from those in a PJS mouse model[30].
The negative regulation of mTORC1 signaling by LKB1 is not limited to GI polyps. The conditional knockout of Lkb1 in the beta cell compartment of pancreatic islets leads to elevated mTOR activity, increased beta cell mass, and enhanced glucose tolerance[31]. Interestingly, LKB1 controls beta cell size via the mTOR pathway but regulates cell polarity through an mTOR-independent mechanism[32]. Cardiac myocyte-specific Lkb1 knockout leads to decreased AMPK phosphorylation and increased mTOR activity in both the atria and ventricles of Lkb1-deficient mice. These mice display cardiac dysfunction and atrial fibrillation and usually die within 6 months[33]. A somatic testicular cell-specific deletion of Lkb1 leads to germ cell loss, accompanied by defects in Sertoli cell polarity and the testicular junctional complex. Additionally, AMPK is inhibited and mTOR signaling is activated in Lkb1-null testes[34].
Because LKB1 kinase activity requires the adaptor protein STRAD, it might be expected that hereditary STRAD mutations have an effect similar to that of LKB1 mutation. Indeed, homozygous deletion of STRADα leads to a rare human autosomal-recessive disorder called polyhydramnios, megalencephaly, and symptomatic epilepsy (PMSE) syndrome, which is characterized by abnormal brain development, cognitive disability, and intractable epilepsy[35]. Neurons in the cortex of patients with PMSE exhibit abnormal nuclear localization of LKB1, and these patients have high levels of ribosomal S6 phosphorylation in large cells within the frontal cortex, basal ganglia, hippocampus, and spinal cord, consistent with the aberrant activation of mTORC1 signaling in the brain[36].
Loss of LKB1 as a Mechanism for mTOR Dysregulation in Human Cancers
Considering the pivotal role of mTOR as an integrator of cell growth and cell stress signals, it stands to reason that acquired mutations that lead to the activation of mTOR survival signaling would provide a selective advantage during tumor progression. Indeed, most major forms of cancer exhibit acquired alterations in the genes that either promote mTOR activation directly or inactivate pathways that negatively regulate mTOR. As noted above, mTOR is positively regulated by mitogenic stimuli through the PI3K/AKT pathway. Growth factors, such as insulin, act through receptor tyrosine kinases to activate PI3K, which catalyzes the conversion of phosphatidylinositol(4,5)-bisphosphate (PIP2) to phosphatidylinositol(3,4,5)-trisphosphate (PIP3). PIP3 stimulates AKT kinase activity, which in turn stimulates mTOR by phosphorylating and inactivating TSC2. The tumor suppressor phosphatase and tensin homolog (PTEN) acts as a negative regulator of this process through its function as a lipid phosphatase that converts PIP3 back to PIP2. Constitutive activation of the PI3K/AKT pathway is common in human cancers and can occur due to activating mutations in PIK3CA (the p110α catalytic subunit of PI3K) or through the inactivation of PTEN by mutation or promoter methylation. In fact, the somatic mutation rates of PIK3CA plus PTEN vary strikingly among cancers; whereas the rate exceeds 30% in many common tumor types (brain, colon, and breast tumors), it is <15% in non-small cell lung cancer (NSCLC)[37]. Although PTEN mutations have been observed in approximately 15% of NSCLC cell lines, they are rarely observed in primary tumors[38]. Likewise, phosphorylated (activated) AKT was observed in less than 20% of NSCLCs[39],[40]. In contrast, although allelic loss at the LKB1 locus (chromosome 19p13.3) is observed in many cancers, somatic mutations in LKB1 are relatively rare in most common tumor types, including colon[41], breast[42], ovarian[43], and brain[44] cancers. However, mutations in LKB1 do occur in a small fraction of malignant melanomas[45],[46], cervical cancers[47],[48], and pancreatic cancers[49],[50]. The exception is in NSCLCs, among which somatic mutations in LKB1 have been initially observed in 30% of adenocarcinomas[51],[52], 15% of large cell carcinomas[53], and 19% of squamous carcinomas[54]. A recent paper suggested that the complete inactivation of the LKB1 gene by either homozygous deletion or loss of heterozygosity due to somatic mutation occurs in 39% of NSCLCs[55]. High-throughput next-generation sequencing analyses have also indicated that LKB1 is frequently inactivated in adenocarcinoma and squamous carcinoma of the lung[56]–[58]. The loss of LKB1 in lung cancer cell lines associates with the losses of the STRADα protein and energetic stress-induced AMPK activation, and the aberrant activation of mTOR signaling[59]–[61]. Hence, the bi-allelic inactivation of LKB1 appears to be a major mechanism for the dysregulation of mTOR in NSCLC.
Rapamycin as a Treatment Option in Lkb1-null Tumor Mouse Models
Because the loss of LKB1 promotes the activation of mTORC1 signaling, rapamycin has been evaluated as a therapeutic option in several conditional Lkb1-knockout mouse models (Table 1). In an Lkb1+/− PJS mouse model, rapamycin was shown to efficiently decrease the tumor burden of existing large polyps[62] and reduce the onset of polyposis[63]. Endometrium-specific deletion of Lkb1 gene by Sprr2f-Cre revealed that bi-allelic inactivation of LKB1 is required for the formation of highly invasive endometrial adenocarcinomas. These tumors exhibit elevated mTOR signaling, and rapamycin treatment not only slowed disease progression but also led to the regression of pre-existing tumors[64]. In mouse urothelium, conditional deletions of Lkb1 or Pten alone failed to reveal any morphologic or growth differences, but the combination of Lkb1 and Pten deletion significantly elevated mTOR activity, EMT transition, and bladder tumor formation. Rapamycin treatment significantly reduced mitosis and tumor formation through the down-regulation of mTOR activity[65].
Table 1. The effect of rapamycin treatment in various Lkb1-deficient mouse models.
| Mouse model | Effect of rapamycin treatment | Reference |
| Lkb1+/− PJS | Tumor burden is decreased. | [62] |
| Polyposis onset is reduced. | [63] | |
| Lkb1−/− endometrial cancer | Disease progression is slowed; pre-existing tumor is regressed. | [64] |
| Lkb1−/− Pten−/− urothelium cancer | Tumor formation is reduced. | [65] |
| Lkb1−/− prostate cancer | mTOR is down-regulated by a LKB1-independent mechanism. | [66] |
| Kras (G12D) Lkb1−/− lung cancer | mTOR is up-regulated. | [54] |
| Cancer metastasis occurs through an mTOR independent mechanism. | [67] |
It is important to note that several Lkb1-null induced tumors are not driven by elevated mTOR activity. For example, the conditional knockout of Lkb1 in murine prostate lobes leads to 100% atypical hyperplasia and 83% prostate intraepithelial neoplasia (PIN) at the anterior prostate within 4 months[66]. However, these PIN lesions feature increased cytoplasmic phosphorylated AMPK and a loss of nuclear phosphorylated mTOR, suggesting that an alternative mechanism accounts for the activation of AMPK and suppression of mTOR and that aberrant activation of mTOR signaling is not related to the formation of these lesions.
LKB1 mutations are frequently observed in NSCLC, but Lkb1 inactivation alone in mice was insufficient to induce pulmonary neoplasia[54]. Currently, the most relevant mouse lung cancer model is the inactivation of Lkb1 in a mutant Kras background, which leads to the formation of adeno-, squamous, and large-cell carcinomas of the lung and promotes tumor metastasis[54]. In these Lkb1-null tumors, the Nedd9 protein level is elevated and is essential for tumor metastasis[67]. LKB1 negatively regulates Nedd9 gene expression through its substrate SIK2 but not the AMPK-mTOR axis. Consistent with this mechanism, rapamycin treatment fails to alter NEDD9 protein levels in this mouse model[54].
Conclusions
In summary, LKB1 negatively regulates mTOR signaling through its substrate AMPK, and the loss of LKB1 leads to the aberrant activation of mTOR in a variety of tissues. LKB1 loss-associated mTOR activation is the driving force in several mouse models of tumorigenesis, such as the formation of PJS polyps, endometrial adenocarcinomas, and bladder cancers, and rapamycin treatment appears to be effective in these disease models. However, LKB1 has other downstream targets, and the inactivation of LKB1 can promote tumorigenesis in an mTOR-independent manner in other tumor types. In these tumors, the targeted inhibition of mTOR is unlikely to be a viable therapeutic option.
Acknowledgments
We would like to thank Anthea Hammond for manuscript editing. This work was supported by NIH Grant R01-CA140571 (to W.Z.) and P01-CA116676 (to W.Z. and P.V.). W.Z. is an Anise McDaniel Brock Scholar, Georgia Cancer Coalition Scholar, and American Cancer Research Scholar.
References
- 1.Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 2.Collins SP, Reoma JL, Gamm DM, et al. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J. 2000;345 Pt 3:673–680. [PMC free article] [PubMed] [Google Scholar]
- 3.Su JY, Rempel RE, Erikson E, et al. Cloning and characterization of the Xenopus cyclin-dependent kinase inhibitor p27XIC1. Proc Natl Acad Sci U S A. 1995;92:10187–10191. doi: 10.1073/pnas.92.22.10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemphues KJ, Priess JR, Morton DG, et al. Identification of genes required for cytoplasmic localization in early c. Elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 5.Lizcano JM, Goransson O, Toth R, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baas AF, Boudeau J, Sapkota GP, et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22:3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudeau J, Baas AF, Deak M, et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karuman P, Gozani O, Odze RD, et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001;7:1307–1319. doi: 10.1016/s1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 10.Sapkota GP, Boudeau J, Deak M, et al. Identification and characterization of four novel phosphorylation sites (Ser31, Ser325, Thr336 and Thr366) on LKB1/STK11, the protein kinase mutated in Peutz-Jeghers cancer syndrome. Biochem J. 2002;362:481–490. doi: 10.1042/0264-6021:3620481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapkota GP, Kieloch A, Lizcano JM, et al. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 12.Esteve-Puig R, Canals F, Colome N, et al. Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS ONE. 2009;4:e4771. doi: 10.1371/journal.pone.0004771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng B, Jeong JH, Asara JM, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley SA, Davison M, Woods A, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 15.Stein SC, Woods A, Jones NA, et al. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345 Pt 3:437–443. [PMC free article] [PubMed] [Google Scholar]
- 16.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 18.Mu J, Brozinick JT Jr, Valladares O, et al. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 19.Hong SP, Leiper FC, Woods A, et al. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corradetti MN, Inoki K, Bardeesy N, et al. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Brugarolas J, Kaelin WG., Jr Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 25.Boardman LA, Thibodeau SN, Schaid DJ, et al. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med. 1998;128:896–899. doi: 10.7326/0003-4819-128-11-199806010-00004. [DOI] [PubMed] [Google Scholar]
- 26.Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316:1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- 27.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 28.Lim W, Hearle N, Shah B, et al. Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer. 2003;89:308–313. doi: 10.1038/sj.bjc.6601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shackelford DB, Vasquez DS, Corbeil J, et al. mTOR and HIF-1alpha-mediated tumor metabolism in an Lkb1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci U S A. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katajisto P, Vaahtomeri K, Ekman N, et al. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nat Genet. 2008;40:455–459. doi: 10.1038/ng.98. [DOI] [PubMed] [Google Scholar]
- 31.Fu A, Ng AC, Depatie C, et al. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10:285–295. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Granot Z, Swisa A, Magenheim J, et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009;10:296–308. doi: 10.1016/j.cmet.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda Y, Sato K, Pimentel DR, et al. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem. 2009;284:35839–35849. doi: 10.1074/jbc.M109.057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanwar PS, Kaneko-Tarui T, Zhang L, et al. Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of sertoli cell polarity and spermatogenesis. Hum Mol Genet. 2012;21:4394–4405. doi: 10.1093/hmg/dds272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puffenberger EG, Strauss KA, Ramsey KE, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 36.Orlova KA, Parker WE, Heuer GG, et al. STRADalpha deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J Clin Invest. 2010;120:1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forgacs E, Biesterveld EJ, Sekido Y, et al. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17:1557–1565. doi: 10.1038/sj.onc.1202070. [DOI] [PubMed] [Google Scholar]
- 38.Hosoya Y, Gemma A, Seike M, et al. Alteration of the PTEN/MMAC1 gene locus in primary lung cancer with distant metastasis. Lung Cancer. 1999;25:87–93. doi: 10.1016/s0169-5002(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 39.Massion PP, Taflan PM, Shyr Y, et al. Early involvement of the phosphatidy-linositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med. 2004;170:1088–1094. doi: 10.1164/rccm.200404-487OC. [DOI] [PubMed] [Google Scholar]
- 40.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004 doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 41.Avizienyte E, Roth S, Loukola A, et al. Somatic mutations in LKB1 are rare in sporadic colorectal and testicular tumors. Cancer Res. 1998;58:2087–2090. [PubMed] [Google Scholar]
- 42.Bignell GR, Barfoot R, Seal S, et al. Low frequency of somatic mutations in the LKB1/Peutz-Jeghers syndrome gene in sporadic breast cancer. Cancer Res. 1998;58:1384–1386. [PubMed] [Google Scholar]
- 43.Wang ZJ, Churchman M, Campbell IG, et al. Allele loss and mutation screen at the Peutz-Jeghers (LKB1) locus (19p13.3) in sporadic ovarian tumours. Br J Cancer. 1999;80:70–72. doi: 10.1038/sj.bjc.6690323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobottka SB, Haase M, Fitze G, et al. Frequent loss of heterozygosity at the 19p13.3 locus without LKB1/STK11 mutations in human carcinoma metastases to the brain. J Neurooncol. 2000;49:187–195. doi: 10.1023/a:1006442024874. [DOI] [PubMed] [Google Scholar]
- 45.Rowan A, Bataille V, MacKie R, et al. Somatic mutations in the Peutz-Jeghers (LKB1/STK11) gene in sporadic malignant melanomas. J Invest Dermatol. 1999;112:509–511. doi: 10.1046/j.1523-1747.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 46.Guldberg P, thor Straten P, Ahrenkiel V, et al. Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma. Oncogene. 1999;18:1777–1780. doi: 10.1038/sj.onc.1202486. [DOI] [PubMed] [Google Scholar]
- 47.Wingo SN, Gallardo TD, Akbay EA, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCabe MT, Powell DR, Zhou W, et al. Homozygous deletion of the STK11/LKB1 locus and the generation of novel fusion transcripts in cervical cancer cells. Cancer Genet Cytogenet. 2010;197:130–141. doi: 10.1016/j.cancergencyto.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahin F, Maitra A, Argani P, et al. Loss of Stk11/Lkb1 expression in pancreatic and biliary neoplasms. Mod Pathol. 2003;16:686–691. doi: 10.1097/01.MP.0000075645.97329.86. [DOI] [PubMed] [Google Scholar]
- 50.Su GH, Hruban RH, Bansal RK, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- 52.Carretero J, Medina PP, Pio R, et al. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004;23:4037–4040. doi: 10.1038/sj.onc.1207502. [DOI] [PubMed] [Google Scholar]
- 53.Zhong D, Guo L, de Aguirre I, et al. LKB1 mutation in large cell carcinoma of the lung. Lung Cancer. 2006;53:285–294. doi: 10.1016/j.lungcan.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 55.Gill RK, Yang SH, Meerzaman D, et al. Frequent homozygous deletion of the LKB1/STK11 gene in non-small cell lung cancer. Oncogene. 2011;30:3784–3791. doi: 10.1038/onc.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammerman PS, Hayes DN, Wilkerson MD, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carretero J, Medina PP, Blanco R, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–1625. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- 60.Eggers CM, Kline ER, Zhong D, et al. STE20-related kinase adaptor protein alpha (STRADα) regulates cell polarity and invasion through PAK1 signaling in LKB1-null cells. J Biol Chem. 2012;287:18758–18768. doi: 10.1074/jbc.M111.316422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong D, Liu X, Schafer-Hales K, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008;7:809–817. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 62.Wei C, Amos CI, Zhang N, et al. Suppression of Peutz-Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin Cancer Res. 2008;14:1167–1171. doi: 10.1158/1078-0432.CCR-07-4007. [DOI] [PubMed] [Google Scholar]
- 63.Wei C, Amos CI, Zhang N, et al. Chemopreventive efficacy of rapamycin on Peutz-Jeghers syndrome in a mouse model. Cancer Lett. 2009;277:149–154. doi: 10.1016/j.canlet.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Contreras CM, Akbay EA, Gallardo TD, et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Model Mech. 2010;3:181–193. doi: 10.1242/dmm.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shorning BY, Griffiths D, Clarke AR. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder. PLoS One. 2011;6:e16209. doi: 10.1371/journal.pone.0016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearson HB, McCarthy A, Collins CM, et al. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68:2223–2232. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- 67.Feng Y, Wang Y, Wang Z, et al. The CRTC1-NEDD9 signaling axis mediates lung cancer progression caused by LKB1 loss. Cancer Res. 2012;72:6502–6511. doi: 10.1158/0008-5472.CAN-12-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]