Abstract
For patients with epidermal growth factor receptor (EGFR) mutation-positive lung cancer, the relationship between the dose or duration of treatment with tyrosine kinase inhibitor (TKI) and overall survival remains unclear. Here, we analyzed clinical data of 39 patients who were diagnosed with EGFR mutation-positive non-small cell lung cancer and treated with TKI, but subsequently died. Several parameters were measured in this study: overall survival; first, second, and overall TKI therapy durations; first TKI intensity (actual dose/normal dose); and TKI rate (overall TKI therapy duration/overall survival). The response rate to TKI therapy was 50%, and the median survival was 553 days. After TKI therapy failed, 38.5% patients were re-challenged with TKI. We observed a moderate relationship [r = 0.534, 95% confidential interval (CI) = 0.263 to 0.727, P < 0.001] between overall TKI therapy duration and overall survival. However, we found no relationship between overall survival and first TKI intensity (r = 0.073, 95% CI = -0.380 to 0.247, P = 0.657) or TKI rate (r = 0.0345, 95% CI = -0.284 to 0.346, P = 0.835). Non-small cell lung cancer patients with mutation-positive tumors remained on TKI therapy for, on average, 33% of the overall survival time. These findings suggest that patients with EGFR mutation-positive tumors should not stick to using TKIs.
Keywords: Tyrosine kinase inhibitor, gefitinib, erlotinib, non-small cell lung cancer, epidermal growth factor receptor mutation
Lung cancer is a leading cause of death worldwide. Epidermal growth factor receptor (EGFR) mutations of adenocarcinoma are seen in more than 30% of Asians. Molecular targeting therapy for these patients made dramatic impact in therapy. Patients with non-small cell lung cancer (NSCLC) with EGFR mutation showed superior progression-free survival by first-line tyrosine kinase inhibitor (TKI) treatment than by traditional platinum-doublet chemotherapy in several clinical trials[1]–[4]. Some study groups reported that TKI re-challenge was beneficial for patients who initially responded to TKI[5],[6]. In a previous Japanese study, overall survival increased in patients with EGFR mutation-positive cancer after treatment with gefitinib[7]. However, to the best of our knowledge, the relationship between duration or dose of TKI (including dose reduction and re-challenge) and overall survival has not been investigated. Re-challenge of TKIs after cytotoxic agents or continuation of TKIs after disease progression is frequently seen in practical use. However, it remains unknown whether such administration for disease control benefits survival.
In this retrospective study, we sought to clarify the relationship between total TKI administration and overall survival in patients with EGFR mutation-positive NSCLC.
Materials and Methods
Patients
We analyzed the medical records of 39 patients with EGFR mutation-positive NSCLC who were newly diagnosed at our institute between January 2003 and August 2010, underwent TKI therapy, and died before February 2012. This protocol was approved by the Ethics Committee of Osaka Prefectural Medical Center for Respiratory and Allergic Diseases.
Tumors from patients in this study harbored several EGFR mutations—exon 19 deletion, exon 21 point mutation (L858R), or exon 18 point mutation (G719C, G719S, and G719A)—as determined by direct sequencing or the PNA-LNA PCR Clamp method. Patients with EGFR exon 20 T790M mutation before treatment were excluded from this study. The TKI used in this study was gefitinib or erlotinib.
Parameters
The parameters measured in this study were overall survival; first, second, and overall TKI therapy duration; first TKI intensity; and TKI rate. Overall survival was measured from the date of diagnosis (or confirmed recurrence in postoperative cases) to the date of death. First TKI therapy duration was measured from the start to the end of TKI therapy, or to the switch to another TKI due to disease progression or toxicity. Second TKI therapy duration was calculated from the start of re-challenge to the end of therapy. Overall TKI therapy duration was defined as the first TKI therapy duration plus the second or more TKI therapy duration. First TKI intensity was defined as (actual dose of TKI)/(normal dose of TKI) during first TKI therapy. For example, for a patient who took gefitinib, 250 mg/day, for 100 days and then took it sequentially every other day over 100 days, the first TKI intensity is (250 × 100 + 250 × 100 × 0.5)/(250 × 200) = 0.75. Similarly, for a patient who took erlotinib, 150 mg/day, over 100 days followed by continuous low-dose erlotinib, 100 mg/day, over 100 days, the first TKI intensity is (150 × 100 + 100 × 100)/(150 × 200) = 0.83. To evaluate the contribution of TKI to overall survival, TKI rate was defined as overall TKI therapy duration / overall survival. Response Evaluation Criteria in Solid Tumors[8] were used to evaluate treatment response.
Statistical analyses
We evaluated correlation coefficients between overall survival and overall TKI therapy duration, first TKI duration, first TKI intensity, and TKI rate. The correlation coefficients (r) were interpreted as follows: -0.2 ≤ r ≤ 0.2, no relationship; 0.2 < r ≤ 0.4 (-0.4 ≤ r < -0.2), weak positive (or negative) linear relationship; 0.4 < r ≤ 0.7 (-0.7 ≤ r < -0.4), moderate positive (or negative) linear relationship; and 0.7 < r ≤ 1.0 (-1.0 ≤ r < -0.7), strong positive (or negative) linear relationship. The r values were analyzed using Pearson's correlation test. First TKI durations were compared between groups with and without cytotoxic treatment using the Mann-Whitney U test. Survival time from disease diagnosis to death was assessed by Kaplan-Meier survival analysis. P values less than 0.05 were considered significant. All statistical analyses were performed using software R [version 2.13.1, R Development Core Team (2011), R: a language and environment for statistical computing, R Foundation for Statistical Computing; Vienna, Austria].
Results
Of the 39 patients, 18 were males and 21 were females, with a median age of 66 years (Table 1). Of these, 33% received TKIs as first-line chemotherapy. More than three-quarters of the patients took gefitinib as the first TKI. After the first therapy failed, 38.5% patients were re-challenged with TKI. The response rate was 50%, although this objective group included 7 cases that could not be evaluated because toxicity or death reduced the therapeutic period. A variety of EGFR mutations were identified, including 19 exon 19 deletions, 17 exon 21 point mutations, and 3 exon 18 point mutations.
Table 1. Baseline and treatment characteristics of 39 patients with non-small cell lung cancer.
| Category | Item | Number |
| Age (years; median, range) | 66 (45-87) | |
| Duration (days; median, range) | First TKI | 79 (10-639) |
| All TKI | 125 (10-899) | |
| Gender | Men | 18 |
| Women | 21 | |
| Active mutation | Exon 19 | 19 |
| Exon 21 | 17 | |
| Exon 18 | 3 | |
| Stage | IIIB | 8 |
| IV | 23 | |
| postoperative recurrence | 8 | |
| Histopathology | Adenocarcinoma | 36 |
| Adeno-squamous carcinoma | 1 | |
| Squamous cell carcinoma | 2 | |
| First-line chemotherapy | Cytotoxic | 26 |
| TKI | 13 | |
| Response to first chemotherapy | PR | 14 |
| SD | 9 | |
| PD | 5 | |
| (TKI alone) | (11) | |
| First TKI | Gefitinib | 30 |
| Erlotinib | 9 | |
| First TKI response | PR | 19 |
| PD | 13 | |
| NE | 7 | |
| Re-challenge TKI | Yes | 15 |
| No | 24 |
TKI, tyrosine kinase inhibitor; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluated.
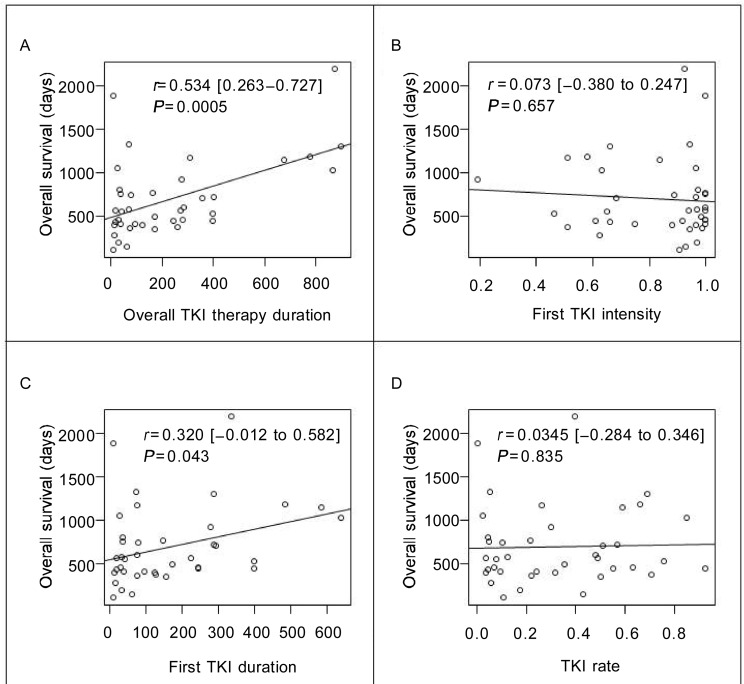
The median overall TKI therapy duration was 125 days. We found a moderate relationship [r = 0.534, 95% confidential interval (CI) = 0.263 to 0.727, P < 0.001] between overall survival and overall TKI therapy duration (Figure 1A). For first TKI intensity, the median was 0.928, the mean was 0.831, and the standard deviation was 0.2. Notably, one-third of patients (13/39) had TKI intensity less than 0.8. We found no relationship (r = 0.073, 95% CI = -0.380 to 0.247, P = 0.657) between first TKI intensity and overall survival (Figure 1B). The median first TKI duration was 79 days. As shown in Figure 1C, there was a weak relationship between overall survival and first TKI therapy duration (r = 0.32, 95% CI = -0.012 to 0.582, P = 0.043). There was no significant difference between first TKI duration and cytotoxic agent use duration (159 days vs. 174 days, P = 0.93). For TKI rate, the median was 0.266, the mean was 0.329, and the standard deviation was 0.268. We observed no relationship (r = 0.0345, 95% CI = -0.284 to 0.346, P = 0.835) between TKI rate and overall survival (Figure 1D). The estimated median survival time was 553 days (95% CI = 444 to 750 days).
Figure 1. The linear correlation between overall survival (OS) and parameters of tyrosine kinase inhibitor (TKI) therapy.
The values were analyzed by using the Pearson's correlation test. A, the relationship between OS and overall TKI therapy duration. B, the relationship between OS and first TKI intensity. One-third of patients had TKI intensity less than 0.8. C, the relationship between OS and the first TKI therapy duration. D, the relationship between OS and TKI rate. No significant relationship was found between OS and TKI rate. r. correlation coefficients [95% confidence interval].
Discussion
This study showed that there is no obvious relationship between overall survival and first TKI intensity or TKI rate in EGFR mutation-positive NSCLC patients. These patients took TKI for one-third of the overall survival time in the mean. If the total duration of TKI therapy, including the time after first progression while on TKI, affects overall survival, physicians need to consider the use of TKI, even beyond disease progression. Many patients need dose reduction due to side effects during TKI therapy. For example, to reduce the dose of gefitinib, drug administration should be changed from everyday to once every 2 or 3 days. However, the effectiveness of such an approach has not yet been proven. If there is a clear relation between TKI dose intensity and overall survival, dose reduction can be accordingly monitored.
We found that overall survival increased as the overall TKI therapy duration increased, but there were insufficient data to determine a causal relationship between these parameters. In contrast, we observed no relationship between overall survival and first TKI intensity. In a post-hoc analysis of large phase III study, Satoh et al.[9] reported that low-dose gefitinib was superior to the usual dose of gefitinib. Likewise, low-dose erlotinib (25 mg/day) was found to be effective in cell lines and in clinical practice[10]. Our finding that TKI intensity and overall survival were not significantly related agreed that reduced doses of TKI did not directly affect overall survival.
The effect of EGFR mutations on patient response to cytotoxic agents remains controversial[11],[12]. In this study, we found no relationship between overall survival and TKI rate. Anticancer agents, except for TKI, appear to increase the survival of cancer patients. We previously reported that long-term chemotherapy might extend survival in responders to first-line chemotherapy[13]. These data suggest EGFR mutation-positive patients should not stick to using TKIs.
Two prospective studies described the efficacy of TKI re-challenge. Asahina et al.[5] reported that the response rate and progression-free survival was 0% and 2.5 months, respectively, when gefinitib was re-administered after cytotoxic chemotherapy. They suggested that this was the most efficient treatment option. Koizumi et al.[6] reported that the response rate and progression-free survival was 15% and 2 months, respectively, after gefitinib readministration. In our study, 15 patients (38.5%) underwent TKI re-challenge, and we observed a moderate relationship between overall survival and overall TKI therapy duration. These results suggest that TKI re-challenge may contribute to survival in selected cases.
Our study has some limitations. First, it was retrospective and conducted on a small population at a single institution. EGFR mutation-positive patients who transferred to the palliative care unit or local hospitals were excluded from this study. Second, only patients who died were followed; long-term survivors were excluded. Third, the method of dose reduction was not unified. It remains unknown whether the two TKIs (gefitinib and erlotinib) showed the same clinical effect or whether their serum concentrations differed among mutation-positive patients. Hughes et al.[14] reported that the plasma concentrations of erlotinib were affected by smoking status. Thus, further investigation is needed to determine the relationship between overall survival and TKI administration.
In conclusion, we found no relationship between overall survival and first TKI intensity or TKI rate in the treatment of patients with EGFR mutation-positive NSCLC. EGFR mutation-positive patients should not stick to using TKIs. A prospective study to define the most effective duration of TKI therapy and to reduce adverse effects is needed.
References
- 1.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012;13:456–475. doi: 10.1016/S1470-2045(12)70117-1. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Asahina H, Oizumi S, Inoue A, et al. Phase II study of gefitinib readministration in patients with advanced non-small cell lung cancer and previous response to gefitinib. Oncology. 2012;79:423–429. doi: 10.1159/000326488. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi T, Agatsuma T, Ikegami K, et al. Prospective study of gefitinib readministration after chemotherapy in patients with advanced non-small-cell lung cancer who previously responded to gefitinib. Clin Lung Cancer. 2012;13:458–463. doi: 10.1016/j.cllc.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Takano T, Fukui T, Ohe Y, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol. 2008;26:5589–5595. doi: 10.1200/JCO.2008.16.7254. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Satoh H, Inoue A, Kobayashi K, et al. Low-dose gefitinib treatment for patients with advanced non-small cell lung cancer harboring sensitive epidermal growth factor receptor mutations. J Thorac Oncol. 2011;6:1413–1417. doi: 10.1097/JTO.0b013e31821d43a8. [DOI] [PubMed] [Google Scholar]
- 10.Yeo WL, Riely GJ, Yeap BY, et al. Erlotinib at a dose of 25 mg daily for non-small cell lung cancers with EGFR mutations. J Thorac Oncol. 2010;5:1048–1053. doi: 10.1097/JTO.0b013e3181dd1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotta K, Kiura K, Toyooka S, et al. Clinical significance of epidermal growth factor receptor gene mutations on treatment outcome after first-line cytotoxic chemotherapy in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2007;2:632–637. doi: 10.1097/JTO.0b013e318074bc0d. [DOI] [PubMed] [Google Scholar]
- 12.Kalikaki A, Koutsopoulos A, Hatzidaki D, et al. Clinical outcome of patients with non-small cell lung cancer receiving front-line chemotherapy according to EGFR and K-RAS mutation status. Lung Cancer. 2010;69:110–115. doi: 10.1016/j.lungcan.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Hirashima T, Suzuki H, Kobayashi M, et al. Long-term chemotherapy may prolong survival in advanced non-small-cell lung cancer among responders to first-line chemotherapy. Med Oncol. 2012;29:1629–1637. doi: 10.1007/s12032-011-0034-6. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AN, O'Brien ME, Petty WJ, et al. Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers. J Clin Oncol. 2012;27:1220–1226. doi: 10.1200/JCO.2008.19.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]